Abstract
Human serum albumin (HSA) is a mixture of mercaptalbumin (HMA, reduced form) and nonmercaptalbumin (HNA, oxidized form), i.e., a protein-thiol redox couple in the extracellular fluid (ECF), and it might have antioxidant properties. Forty-two patients with orthopedic disorders participated in this study and were divided into two groups according to their age (young and older groups). By using HPLC to separate HSA into HMA and HNA, we analyzed the percentages of HMA and HNA in serum and lumbar cerebrospinal fluid (CSF). We also examined the redox activity of cultured normal human astrocytes, aortic endothelial cells, and dermal fibroblasts for HSA-thiol. The mean HMA value from the serum of the older group was significantly lower than that of the young group, whereas that from CSF was not significantly different between the two groups; CSF albumin is almost completely in the reduced form, and no age-related differences were observed. Cultured astrocytes and aortic endothelial cells showed conversion of HNA to HMA, whereas dermal fibroblasts showed no such redox activity. From the results obtained from in-vivo and in-vitro studies, HMA is considered to participate in redox regulation in the ECF, for example in the CSF that surrounds the central nervous system (CNS), and in blood serum.
Keywords: Albumin, Astrocyte, Cerebrospinal fluid (CSF), Protein thiol, Redox state
Introduction
Serum albumin is synthesized in the liver and is exported as a simple, non-glycosylated protein containing about 580 amino acids. This protein is the most abundant protein in the circulatory system and is also present in the interstitial fluids of body tissues [1]. One of the most important features of its structure is the presence of a highly reactive Cys-34 thiol group, called mercaptalbumin (the reduced form; in humans: human mercaptalbumin, HMA) in which the thiol group is in the free state [2]. In contrast, albumin in which the thiol group is bound to thiol-containing compounds is called nonmercaptalbumin (the oxidized form; in humans: human nonmercaptalbumin, HNA) [2]. The major HNA compound is a mixed disulfide with cystine or oxidized glutathione (the reversible form of HNA; tentatively called HNA-1) whereas others include oxidation products higher than the mixed disulfide, for example the sulfenic (–SOH), sulfinic (–SO2H), and sulfonic (–SO3H) states (the irreversible forms of HNA; called HNA-2) [3]. In living systems, human serum albumin (HSA) is known to be a mixture of HMA and HNA, i.e., a major part of the protein-thiol redox potential in the extracellular milieu, and it might have antioxidant properties because of the relatively high concentrations of protein-thiol in the body tissues [4].
We have developed a convenient HPLC method for separation of HSA into HMA and HNA [5, 6] and have extensively studied the dynamic changes of the HSA-thiol redox state under various physiologic [7–10] and pathophysiologic states, for example hepatic [11, 12], renal [13–18], diabetic [19, 20], and other diseases [21–23].
Cerebrospinal fluid (CSF) is a modified extracellular fluid (ECF) present in the central nervous system (CNS). The protein content and concentration of the CSF is very different from that of the serum, because transport of substances from the blood into the CSF is highly restricted by the blood–CSF barrier [24]. The major CSF protein is albumin, which has a relatively low molecular mass compared with other serum proteins, for example immunoglobulins [24]. Although quantitative determination of CSF albumin in normal and diseased brain tissues has been described in several papers [25–27], there are no reports concerning the redox state of CSF albumin-thiol. Our HPLC method has been proved to be a useful technique for analysis of the reduced and oxidized albumin (HMA and HNA) fractions in ECF, especially when the sample size is small and the concentration is low, as is the case for CSF albumin in the cerebrospinal space [24].
In this study we have successfully obtained important evidence concerning the maintenance of the redox pool in the CSF, even in the elderly, as monitored via the CSF albumin-thiol redox state. Moreover, in an in-vitro study it was found that both normal human astrocytes and aortic endothelial cells actively convert HNA to HMA, whereas dermal fibroblasts had no such redox activity.
Materials and methods
Subjects
Forty-two patients with orthopedic disorders, for example lumbar disc herniation, lumbar spondylosis, and lumbar spinal cord stenosis participated in this study (26 men and 16 women with ages ranging from 16 to 80 years (mean ± SD: 56.7 ± 17.5 years)), as selected by the Department of Orthopedics, Gifu University Graduate School of Medicine, Gifu, Japan. They showed no signs of systemic diseases, for example diabetes mellitus or hepatic or renal dysfunction, other than their orthopedic disorders. Patients who showed signs of inflammation or damage related to blood–brain barrier function were excluded from the study. The local ethics committee approved this study protocol, and informed consent was obtained from all patients. All procedures complied with the Declaration of Helsinki. When the patients underwent a myelography for diagnostic purposes, approximately 1 ml of lumbar CSF was tapped at the beginning of the operation through a lumbar puncture, with special care taken to avoid blood contamination. Approximately 5 ml blood was drawn just before or after the myelographic examination, and serum was collected by centrifugation at 3,000 rpm for 15 min at 4°C. The specimens were immediately stored at −80°C until analysis.
HPLC system for measurement of the albumin redox state
Measurement of the albumin-thiol redox state was performed by use of a previously reported HPLC method [18]. Briefly, the HPLC system consisted of an AS-8010 autosampler (injection volume: 2 μl for serum and 100 μl for CSF) and a CCPM double-plunger pump in conjunction with an SC-8020 system controller (all from Tosoh, Tokyo, Japan). The chromatograph was obtained with a Finnigan UV6000LP photodiode-array detector (detection range: 200–600 nm in 1-nm steps; Thermo Electron, Waltham, MA, USA). A Shodex-Asahipak ES-502 N 7C column (10 × 0.76 cm I.D., DEAE-form for ion-exchange HPLC; Showa Denko, Tokyo, Japan; column temperature: 35 ± 0.5°C) was used in this study. Elution was performed with a linear gradient with ethanol (at the concentrations: 0 to 1 min: 0%, 1 to 50 min: 0 → 10%, 50 to 55 min: 10 → 0%, 55 to 60 min: 0%) for specimens in a 0.05 M sodium acetate and 0.40 M sodium sulfate mixture (pH 4.85) at a flow rate of 1.0 ml/min. The buffer solution was deaerated by bubbling helium through it.
The HPLC profiles obtained from these procedures were subjected to numerical curve fitting with simulation software (PeakFit, version 4.05, SPSS Science, Chicago, IL, USA); each peak shape was approximated by a Gaussian function. The values for the fractions of HMA [f(HMA)], HNA-1 [f(HNA-1)], and HNA-2 [f(HNA-2)] (%) were obtained by use of the following equations:
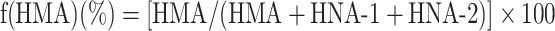 |
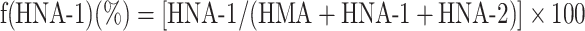 |
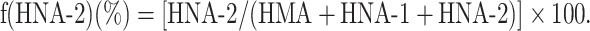 |
Cell culture
Normal human astrocytes (donor: unknown, CC-2565, lot 4F0755), normal human aortic endothelial cells (54-year-old male, CC-2535, lot 7F3087), and normal human dermal fibroblasts (33-year-old female, CC-2511, lot 6F3305) were obtained from Lonza Walkersville (Walkersville, MD, USA). HSA (A1653, lot 36K7545) was obtained from Sigma–Aldrich (St Louis, MO, USA) and used without further purification.
The cells were maintained in the growth media supplied by the manufacturer (astrocytes: AGM BulletKit, aortic endothelial cells: EGM-2 BulletKit, dermal fibroblasts: FGM-2 BulletKit) under a 5% CO2 atmosphere. Trypsinized cells were subcultured in 24-well plates at densities of 1 or 2 × 105 cells/well on day 0. HSA was dissolved in each growth medium immediately before being added to the wells at a final concentration of 10 mg/ml and filter-sterilized. On the next day, the medium was aspirated, and fresh medium with or without HSA was added to the wells. HSA-containing medium was also added to the wells without cells, as a control. Aliquots of the medium were subjected to measurement at time 0. At the indicated times (3, 6, 9, and 24 h), 500 μl supernatant was aspirated and frozen at −80°C until the HPLC analysis. Using independent cultures, the data are presented as the means ± SE from a representative experiments performed in triplicate. Experiments were repeated twice, and essentially the same results were obtained.
Statistical analysis
In the clinical study, values are expressed as means ± SD unless otherwise stated. We used Stat View 5.0 statistical software (SAS Institute, Cary, NC, USA). The Mann–Whitney’s U test was used for comparisons between the young and older groups, while the Wilcoxon signed-rank test was used for comparisons within groups. P values less than 0.05 were considered to be significant. To measure the magnitude of correlation, we used Pearson’s correlation coefficient (R). The correlation was determined to be significant when the P value was less than 0.05 with Fisher’s Z transformation.
Results
Redox state of lumbar CSF and serum albumin
In the relationship between the age of the 42 subjects and the values (%) for HMA fraction (f(HMA)) of albumin from the serum and lumbar CSF, the f(HMA) values were correlated significantly not with that for the CSF (open triangles) (R = 0.086, P = 0.59) but with that for the serum (R = −0.635, P < 0.0001), as detected by linear-regression analysis (Fig. 1). Based on this result, subjects were divided into two groups according to their age (young group: 16–36 years (29.1 ± 6.6 years, n = 10), older group: 51–80 years (65.4 ± 8.3 years, n = 32)), and we compared the fractions of HMA and HNA of serum albumin, together with those of lumbar CSF albumin. The HPLC results are summarized in Table 1. According to the results given in Table 1, the statistical significance of the values (%) for HMA and HNA (f(HMA) and f(HNA)) are shown in Fig. 2a and b, respectively.
Fig. 1.
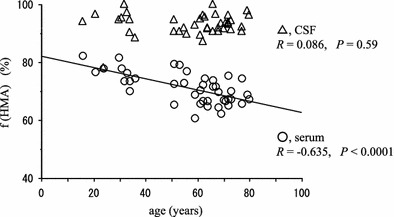
Correlation between f(HMA) values of albumin and age of 42 subjects (serum, open circles; CSF, open triangles). There is a significant negative correlation between age and f(HMA) value from the serum (R = −0.635, P < 0.0001), however, no correlation is observed between age and f(HMA) value from the CSF (R = 0.086, P = 0.59)
Table 1.
Values (%) for f(HMA), f(HNA-1), and f(HNA-2) of serum and lumbar CSF albumins from patients with orthopedic disorders
| Subjects (average age) | n | f(HMA) (%) | f(HNA-1) (%) | f(HNA-2) (%) | |
|---|---|---|---|---|---|
| Serum | Young group (29.1 ± 6.6) | 10 | 76.4 ± 3.7 | 22.0 ± 3.9 | 1.6 ± 0.4 |
| Older group (65.4 ± 8.3) | 32 | 69.5 ± 4.7 | 28.6 ± 4.5 | 1.9 ± 0.6 | |
| CSF | Young group (29.1 ± 6.6) | 10 | 93.0 ± 6.1 | 6.7 ± 6.1 | 0.3 ± 0.7 |
| Older group (65.4 ± 8.3) | 32 | 93.1 ± 2.8 | 5.8 ± 2.2 | 1.1 ± 0.9 |
Fig. 2.
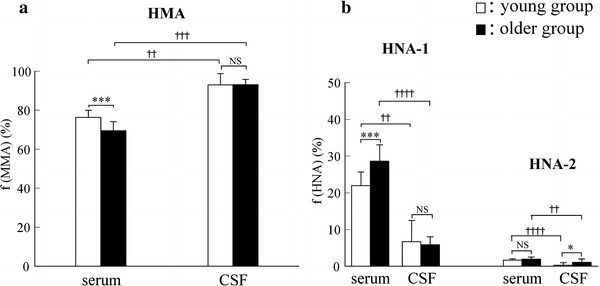
Values (%) of a f(HMA) and b f(HNA) (f(HNA-1) and f(HNA-2)) of albumin in serum and CSF from the young group (n = 10, open columns) and older group (n = 32, closed columns). Each column and bar represents the mean value and SD. Mann–Whitney’s U test was used for comparisons between groups (***P < 0.001, *P < 0.05, NS: not significant), and the Wilcoxon signed-rank test was used for comparisons within groups (†††† P < 0.0001, ††† P < 0.001, †† P < 0.01)
As shown in Fig. 2a, the value (%) for f(HMA) from the serum of the older group (69.5 ± 4.7%, closed column) was significantly lower than that from the serum of the young group (76.4 ± 3.7%, open column) (P < 0.001, Mann–Whitney’s U test). The mean f(HMA) value of 76.4% in young group is in accordance with that previously reported for healthy young subjects (74.6%) [9], and that of 69.5% in the older group is also in accordance with that previously reported for elderly patients with senile cataracts (66.4%) [22]. In the serum, f(HMA) decreases with increasing age, indicating that redox capacity is likely to decrease age-dependently in the ECF. These phenomena agree well with many reports of age-related increases of oxidative states in the elderly. On the other hand, the f(HMA) values from the CSF were 93.0 ± 6.1% for the young group and 93.1 ± 2.8% for the older group, i.e. there is no significant difference between them. Furthermore, it is intriguing that the albumin in the CSF is almost all in the reduced form and that this does not differ with age.
Figure 2b shows the value (%) for oxidized albumin (HNA). With regard to the oxidized albumin, we further analyzed the values (%) for HNA-1 (the “reversibly” oxidized form) and HNA-2 (the “irreversibly” oxidized form) fractions in the serum and CSF. For the values of HNA-1, there was a significant difference in the serum between the two groups (P < 0.001, Mann–Whitney’s U test), whereas there was no significant difference in the CSF between the two groups. For the HNA-2 values, there were no significant differences in the serum, whereas a significant difference was observed in the CSF (P < 0.05, Mann–Whitney’s U test).
Conversion of HNA to HMA in three kinds of normal human cultured cells
In order to clarify which tissue regulates or maintains the redox state of albumin-thiol in the ECF, a commercial HSA product was incubated with three kinds of normal human cultured cells; astrocytes, aortic endothelial cells, and dermal fibroblasts. To examine whether conversion of HNA to HMA occurs in these cultured cells, a very low HMA content of HSA product (Sigma–Aldrich; A1653, lot 36K7545) was used in this study (HMA: 12.0%, HNA-1: 47.8%, HNA-2: 40.2%): usually one cannot obtain a high HMA content of HSA product because of auto-oxidation during the processes of manufacturing HSA from large-scale pooled blood.
Figure 3 shows the time-dependent changes in the f(HMA) value (%) of HSA incubated at 37°C with (a) astrocytes, (b) aortic endothelial cells, or (c) dermal fibroblasts (closed circles: cell (+), open circles: cell (−)). As shown in the figures, the HMA content of the astrocytes (11.9%) and aortic endothelial cells (11.1%) gradually increased during the first 9 h and then reached a plateau until 24 h (around 20%). However, in the absence of these cells, the HMA content decreased because of auto-oxidation. In the case of dermal fibroblasts, however, the HMA content was almost unchanged with and without the cells.
Fig. 3.
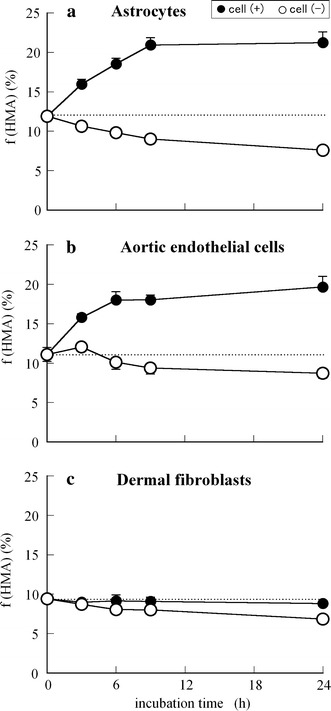
Time course of f(HMA) values (%) of HSA incubated at 37°C in each growth medium in the absence (open circles) or presence (closed circles) of cell culture (2 × 105 cells/well): normal human a astrocytes, b aortic endothelial cells, and c dermal fibroblasts. Using independent cell cultures, the data presented are the means ± SE obtained from a representative experiment performed in triplicate
Figures 4 and 5 show the time-dependent changes in f(HNA-1) and f(HNA-2) values (%) of HSA incubated at 37°C with (a) astrocytes, (b) aortic endothelial cells, or (c) dermal fibroblasts (closed circles: cell (+), open circles: cell (−)). As shown in Fig. 4, the amounts (%) of HNA-1, i.e., the reversibly oxidized albumin in astrocytes and aortic endothelial cells decreased from 47–49% to 40–42% in a time-dependent manner, whereas almost no change was observed in that in fibroblasts. It can be seen from Fig. 5 that there were almost no changes in HNA-2 content (%) among the three types of cells, because HNA-2 includes highly oxidized species, for example the sulfenic (–SOH), sulfinic (–SO2H), or sulfonic (–SO3H) states of oxidized albumin, i.e., the “irreversibly” oxidized form. However, in the case of astrocytes, HNA-2 content decreased slightly from 41 to 38%, indicating that the ability of astrocytes to convert the HNA-2 to HMA might be stronger than that of other two kinds of cells.
Fig. 4.
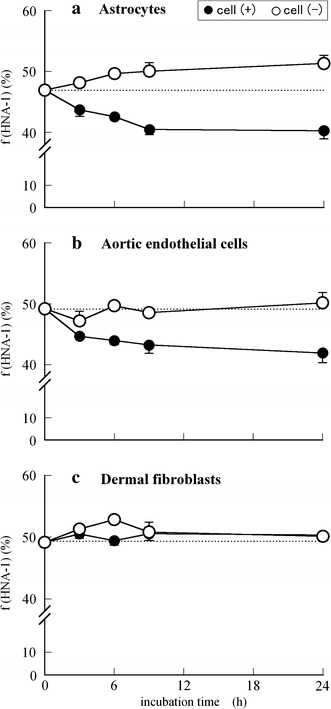
Time course of f(HNA-1) values (%) of HSA incubated at 37°C in each growth medium in the absence (open circles) or presence (closed circles) of cell culture (2 × 105 cells/well): normal human a astrocytes, b aortic endothelial cells, and c dermal fibroblasts. Using independent cell cultures, the data presented are the means ± SE obtained from a representative experiment performed in triplicate
Fig. 5.
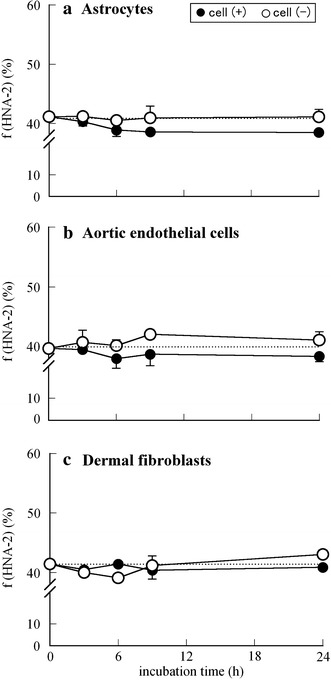
Time course of the f(HNA-2) values (%) of HSA incubated at 37°C in each growth medium in the absence (open circles) or presence (closed circles) of cell culture (2 × 105 cells/well): normal human a astrocytes, b aortic endothelial cells, and c dermal fibroblasts. Using independent cell cultures, the data presented are the means ± SE obtained from a representative experiment performed in triplicate
Each growth medium usually contains various kinds of supplemental growth factors and other active substances. However, in the absence of the cells, no increase in f(HMA) (Fig. 3) nor any decrease in f(HNA) (Figs. 4 and 5) was observed, suggesting that there were no active substances in the growth medium that affected the HMA ↔ HNA conversion of HSA.
From the set of in vitro results shown in Figs. 3, 4, and 5, it is assumed that normal human astrocytes and aortic endothelial cells, especially astrocytes, actively convert the oxidized form of albumin to the reduced form, whereas dermal fibroblasts have no such redox activity.
Discussion
Even in normal circumstances, small amounts of reactive oxygen radicals and other free radicals are produced, resulting in cellular damage associated with peroxidation of membrane lipids. In this regard, animals have developed many defenses to protect themselves from free radicals in the intracellular and extracellular environment [28]. Both ICF and ECF contain low-molecular mass antioxidants such as vitamin C, vitamin E, and thiols, which are actively involved in defense against reactive oxygen species (ROS) [28]. Moreover, with regard to thiols, and especially protein cysteinyl thiols, the oxidation of protein thiols to mixed disulfides and their reduction back to thiols might be an effective antioxidant system in both fluids [29]. Redox-dependent signaling events involving the post-translational modification of proteins are now accepted as an important regulatory process under physiological conditions [30, 31]. In cells, Cohen’s group demonstrated in their review that the functions or signaling mechanisms of intracellular proteins such as sarco/endoplasmic reticulum Ca2+ ATPase and p21ras are significantly affected by irreversible oxidation of their key thiols in disease [32]. On the other hand, with regard to extracellular protein thiols, albumin, with its single thiol group (Cys-34), is the most abundant protein in the ECF including the interstitial fluid; it is, hence, responsible for the largest fraction of reactive thiol in the ECF. Therefore, thiol-disulfide exchange reactions of albumin, i.e., dynamic changes in the redox state of albumin, might be considered to participate in the maintenance of a constant redox potential, and the f(HMA) value for HSA might reflect the redox buffer capacity of the human body.
The CSF is a modified ECF in the cerebrospinal space, which makes up the internal environment of the CNS. Because the volume of the CSF is relatively small and the albumin concentration is also very low in the CSF, there have been no studies of the functional role or analysis of the redox state of human albumin in CSF. As can be seen in Table 1 and Figs. 1 and 2, it is interesting to note that the values for f(HMA) from the CSF are significantly different from the corresponding serum values both in the young and older groups: the albumin in the CSF is almost all in the reduced form and this does not change with age. As already mentioned, to protect cell functions against ROS, antioxidant substances such as vitamin C and E, uric acids, glutathione, and thiol-containing amino acids are present in tissues [28]. From the literature, the mean concentrations of vitamin E in the serum and CSF of a normal control group (60.8 years old, n = 78) were 33.2 μM and 30.1 nM, indicating that the vitamin E level in CSF is much lower than that in serum [33]. On the other hand, mean concentrations of vitamin C in the serum and CSF were 42.8 and 163.8 μM in the normal control group (n = 63), indicating that the vitamin C concentration in the CSF is approximately four times the corresponding serum value, by means of facilitative transport through the choroid plexus [34]. Compared with other organs, because of the presence of an effective antioxidant system, the brain is able to defend itself against ROS that are continuously generated during oxidative metabolism. With regard to the defense system in brain tissue, some researchers emphasize in their reviews that glutathione is one of the most important detoxifying substances for ROS in the brain, i.e., metabolic interaction of glutathione occurs between astrocytes and neurons [35, 36]. Glutathione also regulates many protein functions by glutathiolation and vice versa; some proteins, for example protein disulfide isomerase and thioredoxin, regulate glutathione metabolism by a reversible thiol–disulfide exchange reaction [37]. From this point of view, it is possible to say that albumin may also function in the regulation of glutathione metabolism, because the Cys-34 of HMA can bind reversibly to the oxidized form of glutathione or cystine in the ECF.
From the existence of strong antioxidant biomolecules in the CNS demonstrated above, together with our result concerning the high level of reduced human albumin in the CSF (Fig. 2a), it is necessary to explore the relationship between neural cell functions and albumin-thiol redox state. Astrocytes, the main glial cells in the CNS, play a major role in supporting the development of neurons because of the production of growth factors and cytokines; hence, we examined how astrocytes correlate with the dynamics of the redox regulation of albumin-thiol by using cultured normal human astrocytes and comparing them with that of aortic endothelial cells and dermal fibroblasts.
As shown in Figs. 3, 4, and 5, the values for f(HMA) of HSA incubated both in astrocytes and aortic endothelial cells increased gradually and maintained high f(HMA) values until 24 h. In contrast, the values for f(HNA-1) decreased both in astrocytes and aortic endothelial cells. These results indicate that both types of cell actively generate the thiol-disulfide exchange reaction, i.e., the HNA-1 → HMA conversion of human albumin. Moreover, this conversion effect was larger in astrocytes than in the aortic endothelial cells. It is interesting to note that astrocytes can also convert HNA-2 to HMA (Fig. 5a). As already mentioned, HNA-2 is composed of highly oxidized species, for example the sulfenic, sulfinic, and sulfonic states of oxidized albumin, i.e., “irreversibly” oxidized albumin. However, it is reported for HNA-2 that HSA sulfenic acid (HSA-SOH) is generated by the reaction of HMA with hydrogen peroxide and peroxynitrite, and it has been proposed that this serves as an intermediate in the formation of low-molecular-mass disulfides [38]. Very recently, it was reported that HSA-SOH can react rapidly with plasma thiols and is a central intermediate in redox modulation; the role of stable HSA-SOH was also discussed [39]. Therefore, it might be reasonable to classify HSA-SOH in HNA-2, because it can be converted to HMA by the strong redox effect present in astrocytes (Fig. 5a). Glial cells, especially astrocytes, constantly provide substantial amounts of cysteine and/or glutathione to maintain their levels in the local environment surrounding neurons [35, 36, 40]. Moreover, astrocytes can internalize albumin by receptor-mediated endocytosis, and albumin in cells regulates the synthesis of the neurotrophic factor oleic acid [41]. From this evidence, together with our current results, it can be speculated that the albumin in the CSF participates in redox regulation between astrocytes and neurons via thiol-disulfide exchange reactions with cysteine and/or glutathione.
Albumin, hemoglobin, and fibrin were the first proteins of the body to be studied. Among them, albumin is probably the most studied because of its being the most abundant soluble protein and the most prominent protein in sera. On the basis of a number of physicochemical studies on the structure and functions of bovine and human albumins from the 1960s, the HMA ↔ HNA conversion of HSA is well known to occur in the non-enzymatic process [42], and, hence, HMA and HNA can be obtained by ion-exchange chromatography on DEAE-Sephadex A-50 [43] and by affinity chromatography on activated thiol Sepharose 4B [44]. However, as has recently been reviewed [30], enzymes such as glutaredoxin, thioredoxin, and peroxiredoxin are potent antioxidants and multifunctional enzymes in protein thiolation/dethiolation processes in cells and plasma; further study oriented toward discovery of enzymes involved in this conversion in cultured cells is needed, using new methods such as RNAi.
In summary:
this study presents the first evidence that the redox state of human CSF albumin has significantly high f(HMA) and low f(HNA) values, compared to the corresponding values in serum from patients with orthopedic disorders. Although further studies comparing the results from age-matched healthy subjects are also needed, studies in humans are limited, especially studies of normal human CSF. This is, of course, because of ethical concerns. Our data on cultured cells show that astrocytes have a strong ability to convert oxidized albumin to reduced albumin, which may act as an effective antioxidative system in the brain, together with the high levels of vitamin C [34] and glutathione [35, 36] in the CSF;
from the study on cultured aortic endothelial cells it is speculated that during circulation through the body in normal circumstances, a high level of HSA-thiol is constantly maintained by the reduction activity of the endothelial cells of whole blood vessels, but not by dermal fibroblasts in connective tissues.
Abbreviations
- HSA
Human serum albumin
- HMA
Human mercaptalbumin
- HNA
Human nonmercaptalbumin
- ROS
Reactive oxygen species
References
- 1.Peters T., Jr . All about albumin. Biochemistry, genetics, and medical applications. New York: Academic Press; 1996. pp. 9–75. [Google Scholar]
- 2.King TP. On the sulfhydryl group of human plasma albumin. J Biol Chem. 1961;236:PC5. [PubMed] [Google Scholar]
- 3.Janatova J, Fuller JK, Hunter MJ. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem. 1968;243:3612–3622. [PubMed] [Google Scholar]
- 4.Cha M-K, Kim I-H. Glutathione-linked thiol peroxidase activity of human serum albumin: a possible antioxidant role of serum albumin in blood plasma. Biochem Biophys Res Commun. 1996;222:619–625. doi: 10.1006/bbrc.1996.0793. [DOI] [PubMed] [Google Scholar]
- 5.Sogami M, Nagaoka S, Era S, Honda M, Noguchi K. Resolution of human mercapt- and nonmercaptalbumin by high-performance liquid chromatography. Int J Pept Protein Res. 1984;24:96–103. doi: 10.1111/j.1399-3011.1984.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 6.Sogami M, Era S, Nagaoka S, Kuwata K, Kida K, Miura K, Inouye H, Suzuki E, Hayano S, Sawada S. HPLC-studies on nonmercapt-mercapt conversion of human serum albumin. Int J Pept Protein Res. 1985;25:398–402. doi: 10.1111/j.1399-3011.1985.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 7.Era S, Hamaguchi T, Sogami M, Kuwata K, Suzuki E, Miura K, Kawai K, Kitazawa Y, Okabe H, Noma A, Miyata S. Further studies on the resolution of human mercapt- and nonmercaptalbumin and on human serum albumin in the elderly by high-performance liquid chromatography. Int J Pept Protein Res. 1988;31:435–442. doi: 10.1111/j.1399-3011.1988.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 8.Era S, Kuwata K, Imai H, Nakamura K, Hayashi T, Sogami M. Age-related change in redox state of human serum albumin. Biochim Biophys Acta. 1995;1247:12–16. doi: 10.1016/0167-4838(94)00166-e. [DOI] [PubMed] [Google Scholar]
- 9.Imai H, Hayashi T, Negawa T, Nakamura K, Tomida M, Koda K, Tajima T, Koda Y, Suda K, Era S. Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn J Physiol. 2002;52:135–140. doi: 10.2170/jjphysiol.52.135. [DOI] [PubMed] [Google Scholar]
- 10.Imai H, Era S, Hayashi T, Negawa T, Matsuyama Y, Okihara K, Nakatsuma A, Yamada H. Effect of propolis supplementation on redox state of human serum albumin during high-intensity kendo training. Adv Exerc Sports Physiol. 2005;11:109–113. [Google Scholar]
- 11.Sogami M, Era S, Nagaoka S, Kuwata K, Kida K, Shigemi J, Miura K, Suzuki E, Muto Y, Tomita E, Hayano S, Sawada S, Noguchi K, Miyata S. High-performance liquid chromatographic studies on non-mercapt mercapt conversion of human serum albumin. II. J Chromatogr. 1985;332:19–27. doi: 10.1016/S0021-9673(01)83283-0. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima H, Miwa Y, Shiraki M, Gomi I, Toda K, Kuriyama S, Nakamura H, Wakahara T, Era S, Moriwaki H. Oral branched-chain amino acid supplementation improves the oxidized/reduced albumin ratio in patients with liver cirrhosis. Hepatol Res. 2007;37:765–770. doi: 10.1111/j.1872-034X.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumano K, Yokota S, Go M, Suyama K, Sakai T, Era S, Sogami M. Quantitative and qualitative changes of serum albumin in CAPD patients. Adv Perit Dial. 1992;8:127–130. [PubMed] [Google Scholar]
- 14.Soejima A, Kaneda F, Manno S, Matsuzawa N, Kouji H, Nagasawa T, Era S, Takakuwa Y. Useful markers for detection decreased serum antioxidant activity in hemodialysis patients. Am J Kidney Dis. 2002;39:1040–1046. doi: 10.1053/ajkd.2002.32787. [DOI] [PubMed] [Google Scholar]
- 15.Soejima A, Matsuzawa N, Hayashi T, Kimura R, Ootsuka T, Fukuoka K, Yamada A, Nagasawa T, Era S. Alteration of redox state of human serum albumin before and after hemodialysis. Blood Purif. 2004;22:525–529. doi: 10.1159/000082524. [DOI] [PubMed] [Google Scholar]
- 16.Terawaki H, Yoshimura K, Hasegawa T, Matsuyama Y, Negawa T, Yamada K, Matsushima M, Nakayama M, Hosoya T, Era S. Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int. 2004;66:1988–1993. doi: 10.1111/j.1523-1755.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 17.Terawaki H, Matsuyama Y, Era S, Matsuo N, Ikeda M, Ogura M, Yokoyama K, Yamamoto H, Hosoya T, Nakayama M. Elevated oxidative stress measured as albumin redox state in continuous ambulatory dialysis patients correlates with small uraemic solutes. Nephrol Dial Transplant. 2007;22:968. doi: 10.1093/ndt/gfl635. [DOI] [PubMed] [Google Scholar]
- 18.Terawaki H, Nakayama K, Matsuyama Y, Nakayama M, Sato T, Hosoya T, Era S, Ito S. Dialyzable uremic solutes contribute to enhanced oxidation of serum albumin in regular hemodialysis patients. Blood Purif. 2007;25:274–279. doi: 10.1159/000101986. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki E, Yasuda K, Takeda N, Sakata S, Era S, Kuwata K, Sogami M, Miura K. Increased oxidized form of human serum albumin in patients with diabetes mellitus. Diabetes Res Clin Pract. 1992;18:153–158. doi: 10.1016/0168-8227(92)90140-M. [DOI] [PubMed] [Google Scholar]
- 20.Kawai K, Yoh M, Hayashi T, Imai H, Negawa T, Tomida M, Sogami M, Era S. Effect of diabetic retinopathy on redox state of aqueous humor and serum albumin in patients with senile cataract. Tokai J Exp Clin Med. 2001;26:93–99. [PubMed] [Google Scholar]
- 21.Hayakawa A, Kuwata K, Era S, Sogami M, Shimonaka H, Yamamoto M, Dohi S, Hirose H. Alteration of redox state of human serum albumin in patients under anesthesia and invasive surgery. J Chromatogr B. 1997;698:27–33. doi: 10.1016/S0378-4347(97)00274-0. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Era S, Kawai K, Imai H, Nakamura K, Onda E, Yoh M. Observation for redox state of human serum and aqueous humor albumin from patients with senile cataract. Pathophysiology. 2000;6:237–243. doi: 10.1016/S0928-4680(99)00022-X. [DOI] [Google Scholar]
- 23.Tomida M, Ishimaru JI, Hayashi T, Nakamura K, Murayama K, Era S. The redox states of serum and synovial fluid of patients with temporomandibular joint disorders. Jpn J Physiol. 2003;53:351–355. doi: 10.2170/jjphysiol.53.351. [DOI] [PubMed] [Google Scholar]
- 24.Møllgård K, Jacobsen M, Jacobsen GK, Clausen PP, Saunders NR. Immunohistochemical evidence for an intracellular localization of plasma proteins in human foetal choroid plexus and brain. Neurosci Lett. 1979;14:85–90. doi: 10.1016/0304-3940(79)95349-7. [DOI] [PubMed] [Google Scholar]
- 25.Rao ML, Böker D-K. Cerebrospinal fluid and serum levels of albumin, IgG, IgA and IgM in patients with intracranial tumors and lumbar disc herniation. Eur Neurol. 1987;26:241–245. doi: 10.1159/000116343. [DOI] [PubMed] [Google Scholar]
- 26.Reiber H. Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189–203. doi: 10.1016/0022-510X(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 27.Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K. A population study on blood-brain barrier function in 85-year-olds. Relation to Alzheimer’s disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- 28.Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1:441–445. [PubMed] [Google Scholar]
- 29.Thomas JA, Poland B, Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- 30.Di Simplicio P, Frosali S, Priora R, Summa D, Di Simplicio FC, Di Giuseppe D, Di Stefano A. Biochemical and biological aspects of protein thiolation in cells and plasma. Antioxid Redox Signal. 2005;7:951–963. doi: 10.1089/ars.2005.7.951. [DOI] [PubMed] [Google Scholar]
- 31.Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 32.Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bustos F, Jiménez-Jiménez FJ, Molina JA, Esteban J, Guerrero-Sola A, Zurdo M, Ortí-Pareja M, Tallón-Barranco A, Gómez-Escalonilla C, Ramirez-Ramos C, Arenas J, Salamanca RE. Cerebrospinal fluid levels of alpha-tocopherol in amyotrophic lateral sclerosis. J Neural Transm. 1998;105:703–708. doi: 10.1007/s007020050089. [DOI] [PubMed] [Google Scholar]
- 34.Reiber H, Ruff M, Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin Chim Acta. 1993;217:163–173. doi: 10.1016/0009-8981(93)90162-W. [DOI] [PubMed] [Google Scholar]
- 35.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 36.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 37.Ghezzi P. Regulation of protein function by glutathiolation. Free Radic Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 38.Carballal S, Radi R, Kirk MC, Barnes S, Freeman BA, Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42:9906–9914. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 39.Turell L, Botti H, Carballal S, Ferrer-Sueta G, Souza JM, Durán R, Freeman BA, Radi R, Alvarez B. Reactivity of sulfenic acid in human serum albumin. Biochemistry. 2008;47:358–367. doi: 10.1021/bi701520y. [DOI] [PubMed] [Google Scholar]
- 40.Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 41.Tabernero A, Velasco A, Granda B, Lavado EM, Medina JM. Transcytosis of albumin in astrocytes activates the sterol regulatory element-binding protein-1, which promotes the synthesis of neurotrophic factor oleic acid. J Biol Chem. 2002;277:4240–4246. doi: 10.1074/jbc.M108760200. [DOI] [PubMed] [Google Scholar]
- 42.Peters T., Jr . All about albumin. Biochemistry, genetics, and medical applications. New York: Academic Press; 1996. pp. 301–305. [Google Scholar]
- 43.Noel JK, Hunter MJ. Bovine mercaptalbumin and non-mercaptalbumin monomers. Interconversions and structural differences. J Biol Chem. 1972;247:7391–7406. [PubMed] [Google Scholar]
- 44.Carlsson J, Svenson A. Preparation of bovine mercaptalbumin by means of covalent chromatography. FEBS Lett. 1974;42:183–186. doi: 10.1016/0014-5793(74)80781-7. [DOI] [PubMed] [Google Scholar]


