Abstract
It is well known that Parkinson’s disease (PD) is the second most common neurodegenerative disorder in humans. In this regard, the neuroprotective effect of Althaea officinalis (AO) has already been reported. Therefore, this study examined whether administration of AO extract would improve behavioral, biochemical and structural abnormalities in an experimental animal model of PD in rats. For this purpose, we induced hemi-Parkinsonism by unilateral intranigral injection of 6-hydroxydopamine (6-OHDA, 8 μg/5 μl saline-ascorbate). The rats were pretreated i.p. with AO extract (10 mg/kg) started 6 days before surgery and continued until the 3rd day post-surgery. Regarding oxidative stress, brain MDA concentration (as a lipid peroxidation marker) increased significantly in the 6-OHDA-administered group in comparison with rats pretreated with AO extract. It was found that AO treatment attenuated rotational behavior in the 6-OHDA-administered group and protected the neurons of substantia nigra pars compacta against 6-OHDA toxicity. Overall, AO extract administration indicated neuroprotective effects against 6-OHDA-induced hemi-Parkinsonism in rats.
Keywords: Althaea officinalis, Parkinson’s disease, 6-Hydroxydopamine
Introduction
Parkinson’s disease (PD) is the most prevalent neurodegenerative disorder after Alzheimer’s disease in humans [1]. In this disease the basal ganglia cells and substantia nigra cells are destroyed and then the level of dopamine is decreased [2]. Prevalence of PD is 1/100 and this disease usually appears in those who are 60 years old [3]. Oxidative stress not only destroys the dopaminergic neurons, but it also compromises mitochondrial oxidative phosphorylation, leading to decreased energy output by these organelles and eventually to secondary death of the cells [4]. There is also an increasing amount of evidence that the neurotoxicity of 6-OHDA for modeling of PD is mainly due to its oxidation, resulting in generation of cytotoxic free radicals, which are believed to play a pivotal role in degeneration of the nigrostriatal dopaminergic system [2]. Although great advances have been made in development of agents to treat PD, none yet address the underlying problem associated with it, i.e. the progressive loss of dopaminergic neurons [5].
Presently, most treatments for PD are aimed at controlling the symptoms. Although dopamine replacement therapy using agents like levodopa can effectively relieve symptoms, it does not prevent disease progression. In addition, there is a progressive increase in the prevalence of drug-related motor fluctuations and dyskinesia. Run-down of effectiveness, on–off effect, end of dose deterioration, and peak-dose dyskinesia are the most common side effects and sometimes are quite difficult to deal with [6]. Because dopamine metabolism increases oxidative stress and metabolites of levodopa are thought to be toxic, long-term use of levodopa may be harmful to dopaminergic neurons and may endanger the patient’s health [7].
As mentioned above, searches for neuroprotection-based strategies with emphasis on natural products have received much attention in recent years. Using plants from the Malvaceae family for herbal therapy is very common in the Middle East, especially Althaea officinalis and Althaea rosea. Althaea officinalis (AO) is native to Asia, Europe and the United States of America. It is widely used traditionally for the treatment of irritation of oral and pharyngeal mucosa and associated dry cough, mild gastritis, skin burns and for insect bites. It is also used in catarrh of the mouth and throat, gastrointestinal tract and urinary tract complaints, as well as for inflammation, ulcers, abscesses, burns, constipation and diarrhea [8]. AO contains several antioxidants and polyphenols that possess many biological activities including antioxidant and anti-inflammation properties [9]. In addition, AO is rich in phenolics with a strong neuroprotective and antineurodegenerative activity which protects neuronal cells (PC12) from cell-damaging oxidative stress in a dose-dependent manner [10, 11]. On this foundation, this study was designed to investigate, for the first time, the possible neuroprotective effects of Althaea officinalis aqueous extract in 6-hydroxydopamine-induced hemi-Parkinsonism with emphasis on behavioral, biochemical and histochemical evidence in a rat model.
Materials and methods
Plant material collection
The leaves of Althaea officinalis L. were authenticated in the botany department of an agricultural organization (Lorestan province, Khorram Abad, Iran). The plant was shed dried at Razi Herbal Medicines Research Center (Lorestan University of Medical Sciences, Khorram Abad, Iran) for a week and pulverized mechanically using a grinder.
Althaea officinalis extract and its composition
One hundred grams of plant leaf was poured into 1 l boiling water in a beaker and kept at room temperature for 2 h. After that the solution was purified and kept in a bain-marie. One hundred grams of Althaea officinalis gave 10 g of extract, as described previously [12]. Many compounds were extracted from the leaves of Althaea officinalis, these included pectins 11 %, starch 25–35 %, mono- and di-saccharide saccharose 10 %, mucilage 5 %, flavonoids (hypolaetin-8-glucoside, isoquercitrin, kaempferol, caffeic acid, p-coumaric acid), coumarins, scopoletin, phytosterols, tannins, asparagines and many amino acids [33].
Animals
Adult male Wistar rats (200–250 g; n = 32) (Pasteur’s Institute, Tehran, Iran) were housed three to four per cage in a temperature-controlled colony room under a light/dark cycle with food and tap water available ad libitum. All rats were treated humanely and in compliance with the recommendations of the Animal Care Committee for Lorestan University of Medical Sciences (Khorram Abad, Iran). The animals were held in the colony room for at least 1 week before being tested. Only rats not showing any biased rotational behavior (net rotations <30/h) following intraperitoneal injection of apomorphine hydrochloride (0.5 mg/kg) (Sigma Chemical, St. Louis, Mo., USA) were selected for the present study. The animals (n = 30 rats) were randomly divided into three equal groups (n = 10 rats per group): a sham-operated group, a lesioned group (6-OHDA) and an AO-pretreated lesioned group (6-OHDA+AO). Unilateral intranigral injection (left side) of 6-OHDA (Sigma Chemical, St. Louis, Mo., USA) was performed through a 5-μl Hamilton syringe on anesthetized rats (ketamine 100 mg/kg and xylazine 5 mg/kg, i.p.) using a stereotaxic apparatus (Stoelting, USA) at the coordinates: ML −2 mm and AP −4.8 mm from bregma and DV −8.3 mm from the surface of the skull according to the Paxinos and Watson atlas [13]. The lesioned group received a single injection of 8 μg of 6-hydroxydopamine-HCl/5 μl of saline and 0.2 % ascorbic acid (W/V) at a rate of 1 μl/min at 0.0 day. The sham group received an identical volume of ascorbate-saline solution at 0.0 day as well. The 6-OHDA+AO group received the neurotoxin (6-OHDA 8 μg/5 μl saline and 0.2 % ascorbic acid (W/V)) at 0.0 day and administration of leaf aqueous extract of AO at a dosage of 10 mg/kg body weight (i.p.) for 10 consecutive days. The AO administration started 6 days before neurotoxin administration and continued until the 3rd day post-surgery. The intraperitoneal administrations were applied every day between 8–9 a.m. for prevention of circadian rhythm changes among days. The therapeutic dose of extract was calculated according to a previous report, which demonstrated a dose dependency for Althaea officinalis extract in guinea pigs with ovalbumine-induced airways inflammation [12].
Behavioral testing
The animals were tested for rotational behavior by apomorphine hydrochloride (0.5 mg/kg, i.p.) 1 week before (baseline) and 1 week after the surgery. The rotations were measured according to the method as described previously [14]. Briefly, the animals were allowed to habituate for 10 min and then 1 min after the injection of drugs, full rotations were counted in a cylindrical container (a diameter of 33 cm and a height of 35 cm) at 10-min intervals for 60 min in a quiet isolated room. Net number of rotations was defined as the positive scores minus the negative scores.
Histological examination
At the end of behavioral experiments, the rats were deeply anesthetized with a high dose of ketamine (150 mg/kg) and perfused through the ascending aorta with 50–100 ml of 0.9 % saline followed by 100–200 ml of fixative solution containing 4 % paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) followed by 100 ml of 0.1 M PB containing 10 % sucrose. Following perfusion, the brains were removed from the skull, the blocks of forebrain and brainstem were prepared, and after the final steps of preparation (30 % sucrose for 2–3 days), sections were cut at a thickness of 5 μm on a freezing microtome (Leica, Germany) and collected in PB (0.1 M). Every second section was Nissl-stained with 0.1 % cresyl violet (Sigma).
Neuronal counting
For each animal, mesencephalic sections (interaural 2.9–4.2 mm) were examined using the method as described previously [15]. Briefly, Nissl-stained neurons of the SNc were counted manually (light microscopy; ×400) using a superimposed grid to facilitate the procedure. At least two sections representative of each of four Paxinos-Watson planes (4.2, 3.7, 3.2, 2.97; interaural) were examined by scanning the entire extent on each side. Counting was done blind to the treatments received.
Protein measurement and lipid peroxidation
Forebrain and brainstem (1 g) was thawed and manually homogenized in cold phosphate buffer (pH 7.4) containing 5 mM EDTA on liquid nitrogen, and debris was removed by centrifugation at 2,000 × g for 5 min as described by Alirezaei et al. [16]. Supernatants were recovered and used for protein measurement and MDA concentration. Protein content of tissue homogenates was determined using a Lowry colorimetric method with bovine serum albumin as a standard [17].
The amount of lipid peroxidation was indicated by the content of thiobarbituric acid reactive substances (TBARS) in the brain. Tissue TBARS were determined by following the production of thiobarbituric acid reactive substances as described previously [18]. In short, 40 μl of homogenate was added to 40 μl of 0.9 % NaCl and 40 μl of deionized H2O, resulting in a total reaction volume of 120 μl. The reaction was incubated at 37º C for 20 min and stopped by the addition of 600 μl of cold 0.8 M hydrochloride acid, containing 12.5 % trichloroacetic acid. Following the addition of 780 μl of 1 % TBA, the reaction was boiled for 20 min and then cooled at 4º C for 1 h. In order to measure the amount of TBARS produced by the homogenate, the cooled reaction was spun at 1,500 × g in a microcentrifuge for 20 min and the absorbance of the supernatant was spectrophotometrically (S2000 UV model; WPA, Cambridge, UK) read at 532 nm, using an extinction coefficient of 1.56 × 105/M cm. The blanks for all of the TBARS assays contained an additional 40 μl of 0.9 % NaCl instead of homogenate as just described. TBARS as indicated in our results by malondialdehyde concentration (MDA) were expressed as nanomoles per milligram of tissue protein (nmol/mg protein).
Statistical analysis
All data were expressed as mean ± SEM. For analysis of 6-OHDA-induced rotations, number of neurons on the left side of SNc and MDA (as a lipid peroxidation marker), one-way ANOVA followed by Tukey’s post hoc test was used. Previously, all variables were tested for normal and homogeneous variances by Levene’s statistic test and a calculated P value of <0.05 was considered statistically significant.
Results
All animals tolerated surgical operations well and there was no mortality due to treatments. There was also no significant change in weight of animals in each group.
The beneficial effect of AO extract was evaluated on apomorphine-induced rotations for a period of 1 h (Fig. 1). There were no significant differences among the groups at baseline (before surgery). Statistical analysis of the total net number of rotations made over a 60-min period 1 week after the surgery showed that apomorphine caused a very significant contralateral turning in the rats of the 6-OHDA group (P < 0.0001) and induced less significant rotations in the 6-OHDA+AO group (P < 0.001) in comparison with the sham group. Moreover, the 6-OHDA+AO group showed a significant reduction of rotations when compared to the 6-OHDA group (F = 18.74; P = 0.0026).
Fig. 1 .
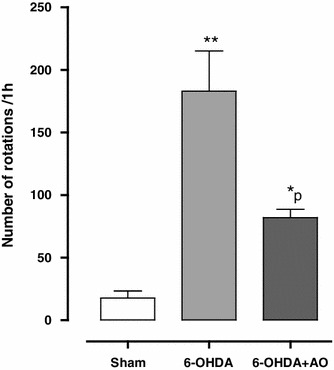
Total number of rotations (mean ± SEM) induced by apomorphine (0.5 mg/kg, i.p.) 1 week following the surgery over a period of 60 min under experimental groups (n = 10 rats per group). Note that the positive values indicate turns contralateral to the side of lesion. *P < 0.001, **P < 0.0001 versus the sham group, ρ; P < 0.05 versus 6-OHDA group. 6-OHDA 6-hydroxydopamine, 6-OHDA+AO 6-hydroxydopamine plus Althaea officinalis extract
The results of histochemical studies regarding the number of Nissl-stained neurons on the left side of the SNc (Figs. 2, 3) showed a significant reduction in the 6-OHDA group (P < 0.01) and no such reduction was obtained for the 6-OHDA+AO group when compared to the sham group. In addition, the number of Nissl-stained neurons on the left side of SNc was significantly higher in 6-OHDA+AO-treated rats versus the 6-OHDA group (F = 26.45; P = 0.0011).
Fig. 2 .
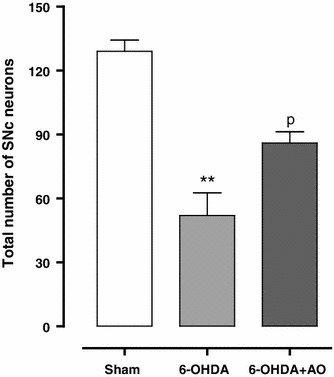
Total number of Nissl-stained neurons (mean ± SEM) on the left side of substantia nigra pars compacta in experimental groups 2 weeks after the surgery (n = 10 rats per group). **Demonstrates P < 0.01 in comparison with the sham group and ρ indicates P < 0.05 in comparison with 6-OHDA group. There is no significant difference between the sham and 6-OHDA+AO groups (P > 0.05). 6-OHDA 6-hydroxydopamine, 6-OHDA+AO 6-hydroxydopamine plus Althaea officinalis extract
Fig. 3.
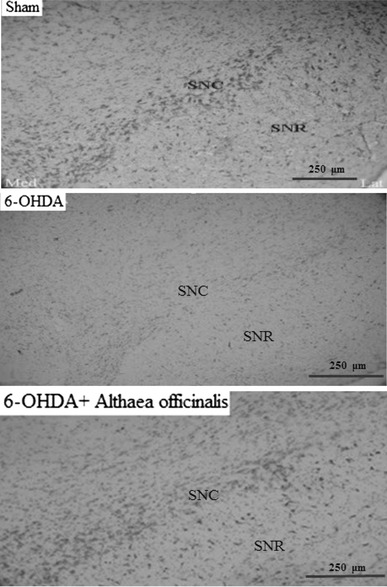
Photomicrograph of coronal sections through the midbrain showing Nissl-stained neurons in experimental groups. A severe reduction in the number of neurons in the SNc was observed in the 6-OHDA-lesioned group, but no such marked decrease was noted in the 6-OHDA+AO group in comparison with the sham group. Scale bar 250 μm (SNc and SNR substantia nigra pars compacta and pars reticulate, respectively). 6-OHDA 6-hydroxydopamine, 6-OHDA+AO 6-hydroxydopamine plus Althaea officinalis extract. ×400
Regarding lipid peroxidation, pretreatment of rats with AO extract significantly decreased MDA concentration in 6-OHDA+AO-treated rats when compared to the 6-OHDA group (F = 8.708; P = 0.0168). However, the concentration of MDA in the 6-OHDA+AO group was slightly higher compared with the sham group (P > 0.05; Fig. 4).
Fig. 4.
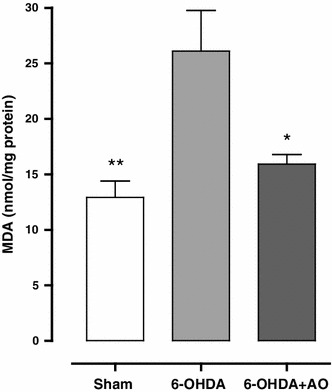
Comparison of malondialdehyde (MDA) concentration among the sham and treated groups (n = 10 rats per group). Values represent mean ± SEM of MDA (nanomoles per milligram protein of brain tissue). **, * Indicate significant difference in comparison with 6-OHDA group (P < 0.05). There is no significant difference between the sham and 6-OHDA+AO groups (P > 0.05). 6-OHDA 6-hydroxydopamine, 6-OHDA+AO 6-hydroxydopamine plus Althaea officinalis extract
Discussion
To the best of our knowledge, the present research indicates therapeutic effects of Althaea officinalis extract on a model of hemi-Parkinsonism, for the first time. The unilateral damage to the nigrostriatal dopaminergic system through intrastriatal injection of 6-OHDA is followed by a reduction in Nissl-stained neurons in the left side of the brain. These changes produce a prominent functional and motor asymmetry that can be evaluated by direct-acting (apomorphine) and indirect-acting (amphetamine) dopaminergic agonists [19]. These rotations, especially those induced by apomorphine, are considered to be reliable indicators of nigrostriatal dopamine depletion [20]. In the present study, a significant attenuation of the apomorphine-induced rotational behavior was observed in AO extract pretreated rats in the 6-OHDA+AO group after 2 weeks. The observed attenuation of rotational behavior in AO extract pretreated rats in this study could be attributed to the possible protective effects of AO extract against nigral neurodegeneration and maintenance of striatal dopamine at a level that is not accompanied by marked turning behavior. On the other hand, nigrostriatal neurons within the SNc were mainly preserved against neurodegenerative effects induced by the neurotoxin 6-OHDA. In this respect, it has been reported that reactive oxygen species (ROS) are involved in the toxicity of 6-OHDA-induced nigrostriatal lesions which are used as an experimental model of unilateral Parkinsonism [21].
There is abundant evidence for oxidative stress in the PD nigra [22–24]. It is well known that oxidative stress is an important factor that could affect the survival of dopaminergic neurons in PD. Neuronal cells mostly depend on energy produced by mitochondria and are simultaneously faced with high levels of ROS as well as increased levels of free iron, which can promote hydroxyl radical formation [25]. Overload of free radical formation may lead to cell death. In addition, auto-oxidation of dopamine or levodopa overdosing produces semiquinone and then polymerization, with the production of ROS [15]. Dopamine can also be metabolized by monoamine oxidase to produce hydrogen peroxide (H2O2) which can then, in the presence of iron, be converted by the Fenton reaction to produce the highly reactive hydroxyl radical [24]. The potential pro-oxidant actions of levodopa have added to the debate over the role of oxidative stress in PD and its role in disease progression [26–28]. Formation of species such as semiquinones and other free radicals could especially damage nucleic acids, proteins, and membrane lipid components, subsequently inducing lipid peroxidation [25]. In the present study, it seems that AO extract can exert antioxidant effects in a pretreated group, indicated by a decrease in MDA concentration, as a lipid peroxidation marker. Therefore, the therapeutic approach is aimed at attenuation of oxidative stress. In addition, free radical scavengers may also be helpful in prolonging the survival time of dopaminergic neurons [29].
In this respect, pretreatment with thimbleweed extract (200 mg/kg/day, rich in isoflavones) and orange epicarp (35 mg/kg/day, rich in polymethoxylated flavones) could improve rats in which the substantia nigra had been destroyed with 6-OHDA [30]. Polyphenolic compounds have protective effects against oxidative stress destruction. In a previous report, phenolic compounds in green tea, grape juice and curcumin demonstrated a protection effect on neurodegenerative disorders [31]. In the present study, AO extract may have attenuated neuronal damage and loss through counteracting oxidative stress. It has been known that Althaea officinalis contains several antioxidants and polyphenols which possess antioxidant and anti-inflammation properties [32]. Many flavonoids (hypolaetin-8-glucoside, isoquercitrin, kaempferol, caffeic acid, p-coumaric acid), coumarins, scopoletin, phytosterols, tannins, asparagines and many amino acids were extracted from leaves of Althaea officinalis [33]. Therefore, flavenoids and tannins are able to chelate free radicals such as hydroxyl radicals and subsequently dismutate ROS. In this regard, AO extract is reported to be rich in phenolics with a strong neuroprotective and antineurodegenerative activity which protects neuronal cells (PC12) from oxidative stress in a dose-dependent manner [34, 35].
Conclusions
The results of the present study suggest, for the first time, a potential basis for the protective effect of AO extract administration against experimental Parkinsonism and this may be put forward as a novel adjuvant treatment strategy for PD.
Acknowledgments
The cooperation and support of the Herbal Medicine Research Center, Lorestan University of Medical Sciences and all the participants who helped us in conducting this research are highly appreciated.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 2.Henze C, Earl C, Sautter J, Schmidt N, Themann C, Hartmann A, Oertel WH. Reactive oxidative and nitrogen species in the nigrostriatal system following striatal 6-hydroxydopamine lesion in rats. Brain Res. 2005;1052:97–104. doi: 10.1016/j.brainres.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:643–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Liao PC, Kuo YM, Chang YC, Lin C, Cherng CF, Yu L. Striatal formation of 6-hydroxydopamine in mice treated with pargyline, pyrogallol and methamphetamine. J Neural Transm. 2003;110:487–494. doi: 10.1007/s00702-002-0829-x. [DOI] [PubMed] [Google Scholar]
- 6.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 7.Agid Y, Chase T, Marsden D. Adverse reactions to levodopa: drug toxicity or progression of disease? Lancet. 1998;351:851–852. doi: 10.1016/S0140-6736(05)70285-3. [DOI] [PubMed] [Google Scholar]
- 8.Ali Shah SM, Akhtar N, Akram M, Akhtar Shah P, Saeed T, Ahmed K, Asif HM. Pharmacological activity of Althaea officinalis L. J Med Plants Res. 2011;5(24):5662–5666. [Google Scholar]
- 9.Elmastaş M, Öztürk L, Gökçe I, Erenler R, Aboul-Enein HY. Determination of antioxidant activity of marshmallow flower (Althaea officinalis L.) Anal Lett. 2004;37(9):1859–1869. doi: 10.1081/AL-120039431. [DOI] [Google Scholar]
- 10.Gudej J. Polyphenolic compounds in Althaea officinalis leaves. Acta Pol Pharm. 1981;38:385. [Google Scholar]
- 11.Gudej J. Polyphenolic compounds in Althaea officinalis flowers. Acta Pol Pharm. 1988;45(4):340–345. [Google Scholar]
- 12.Sutovska M, Capek P, Franova S, Joskova M, Sutovsky J, Marcinek J, Kalman M. Antitussive activity of Althaea officinalis L. polysaccharide rhamnogalacturonan and its changes in guinea pigs with ovalbumine-induced airways inflammation. Bratisl Lek Listy. 2011;112(12):670–675. [PubMed] [Google Scholar]
- 13.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Nishino H, Kumazaki M, Shimada S, Tohyama M, Nishimura T. Expression of dopamine transporter mRNA and its binding site in fetal nigral cells transplanted into the striatum of 6-OHDA lesioned rat. Brain Res Mol Brain Res. 1996;39:127–136. doi: 10.1016/0169-328X(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 15.Pedrosa R, Soares-Da-Silva P. Oxidative and non-oxidative mechanisms of neuronal cell death and apoptosis by L-dihydroxy phenylalanine (L-DOPA) and dopamine. Br Pharmacol. 2002;137(8):1305–1313. doi: 10.1038/sj.bjp.0704982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alirezaei M, Jelodar G, Niknam P, Ghayemi Z, Nazifi S. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J Physiol Biochem. 2011;67(4):605–612. doi: 10.1007/s13105-011-0107-1. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 18.Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem. 1990;55(1):342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 19.Soto-Otero R, Me’ndez-A’lvarez E, Hermida-Ameijeiras A, Lo’pez-Real AM, Labandeira-Garcia JL. Effects of (–)-nicotine and (–)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol. 2002;64:125–135. doi: 10.1016/S0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- 20.Borah A, Mohanakumar KP. Melatonin inhibits 6-hydroxydopamine production in the brain to protect against experimental Parkinsonism in rodents. J Pineal Res. 2009;47:293–300. doi: 10.1111/j.1600-079X.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 21.Cossette M, Lecomte F, Partent A. Morphology and distribution of dopaminergic neurons intrinsic to human striatum. J Chem Neuroanat. 2005;29:1–11. doi: 10.1016/j.jchemneu.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Muller T, Jugel C, Ehret R, Ebersbach G, Bengel G, Muhlack S, Klostermann F. Elevation of total homocysteine levels in patients with Parkinson’s disease treated with duodenal levodopa/carbidopa gel. J Neural Transm. 2011;118(9):1329–1333. doi: 10.1007/s00702-011-0614-9. [DOI] [PubMed] [Google Scholar]
- 23.Nissinen E, Nissinen H, Larjonmaa H, Vaananen A, Helkamaa T, Reenila I, Rauhala P. The COMT inhibitor, entacapone, reduces levodopa-induced elevations in plasma homocysteine in healthy adult rats. J Neural Transm. 2005;112(9):1213–1221. doi: 10.1007/s00702-004-0262-4. [DOI] [PubMed] [Google Scholar]
- 24.Schapira AHV. The clinical relevance of levodopa toxicity in the treatment of Parkinson’s disease. Mov Disord. 2008;23(S3):S515–S520. doi: 10.1002/mds.22146. [DOI] [PubMed] [Google Scholar]
- 25.Ravati A, Junker V, Kouklei M, Ahlemeyer B, Culmsee C, Kriegstein AR. Enalapril and moexipril protect from free radical-induced neuronal damage in vitro and reduce ischemic brain injury in mice and rats. Eur J Pharmacol. 1999;373:21–33. doi: 10.1016/S0014-2999(99)00211-3. [DOI] [PubMed] [Google Scholar]
- 26.Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson′s disease? J Neurol. 2005;252(4):iv37–iv42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 27.Shulman LM. Levodopa toxicity in Parkinson disease: reality or myth? Reality—practice patterns should change. Arch Neurol. 2000;57(3):406–407. doi: 10.1001/archneur.57.3.406. [DOI] [PubMed] [Google Scholar]
- 28.Weiner WJ. Is levodopa toxic? Arch Neurol. 2000;57(3):408. doi: 10.1001/archneur.57.3.408. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub D, Comella CL, Horn S. Parkinson’s disease—Part 1: pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. 2008;14(2 Suppl):S40–S48. [PubMed] [Google Scholar]
- 30.Astarloa R, Mena MA, Sanchez V, de la Vega L, de Yebenes JG. Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin Neuropharmacol. 1992;15(5):375–380. doi: 10.1097/00002826-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Charles R. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Didry N, Torck M, Pinkas M. Polyphenolic compounds from the flowers of Althaea officinalis . Fitoterapia. 1990;61(3):280. [Google Scholar]
- 33.Al-Snafi AE. The pharmaceutical importance of Althaea officinalis and Althaea rosea: a review. Int J PharmTech Res. 2013;5(3):1378–1385. [Google Scholar]
- 34.Gudej J. Flavonoids, phenolic acids and coumarins from the roots of Althaea officinalis . Planta Med. 1991;57(3):284–285. doi: 10.1055/s-2006-960092. [DOI] [PubMed] [Google Scholar]
- 35.Akhtardzhiev KH, Koleva M, Kitanov G, Ninov S. Pharmacognistic study of representatives of Arum, Althaea and Hypericum species. Farmatsiya (Sofia) 1984;34(3):1–6. [Google Scholar]


