Abstract
Background
Carotid plaque (CP) formation is an important consequence of atherosclerosis and leads to significant complications. Levels of neuropeptide Y (NPY), which is a sympathetic neurotransmitter, are elevated in cardiovascular diseases. It also has important roles in inflammatory conditions. This study aimed to explore the relationship between serum NPY and CP and to study further the influence of NPY and inflammatory factors on CP.
Methods
This cross-sectional study was conducted among 300 adults who underwent a health examination at the Second Affiliated Hospital of Fujian Medical University in Fujian Province, of whom 177 were finally enrolled. The participants were divided into the CP (n = 120) and non-CP (NCP) or control (n = 57) groups according to the results of carotid artery color Doppler ultrasound. The CP group was further classified into stable plaque (SP, n = 80) and vulnerable plaque (VP, n = 40) groups based on plaque characteristics. Serum NPY and pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) levels were examined. Univariate and correlation analyses were used to evaluate the correlation between serum NPY levels, pro-inflammatory cytokines, and the CP phenotype.
Results
The serum NPY and TNF-α levels of patients in the CP group were significantly higher than those in individuals from the NCP group [ (177.30 ± 43.29) pg.mL− 1 vs. (121.53 ± 40.16)pg.mL− 1, P < 0.001; (41.94 ± 14.19) pg.mL− 1 vs.(33.54 ± 13.37)pg.mL− 1, P = 0.003]. The serum NPY levels of the patients in the VP group were significantly higher than those in patients from the SP group [(191.67 ± 39.87)ng.L− 1 vs.(170.12 ± 43.37)ng.L− 1, P = 0.01, P < 0.05]. Serum TNF-α and NPY levels were positively correlated among patients from the CP group (r = 0.184, P = 0.044). The binary logistic regression analysis showed that serum NPY and TNF-α were independent influencing factors of CP [(OR = 1.029, P < 0.001);(OR = 1.030, P = 0.023)]. The area under the ROC curve of NPY predicting the CP showed statistical significance at a value of 0.819.
Conclusion
Together, elevated serum NPY levels seem to be associated with the occurrence of coronary atherosclerosis in Chinese adults.
Keywords: Carotid plaque, Atherosclerosis, Neuropeptide Y, TNF-α
Background
Atherosclerosis (AS) is the primary pathological basis of most cardiovascular diseases and is characterized by chronic inflammatory damage caused by endothelial dysfunction, which usually affects multiple arteries [1]. Atherosclerotic cardiovascular disease is a major public health concern and a primary cause of death worldwide [2]. Inflammation-related cells are essential components of AS plaques. Inflammatory reactions cause dysfunction of the carotid artery endothelial cells and increase the production of intercellular adhesion molecules by promoting macrophage phagocytosis and transforming them into foam cells. When AS develops, unstable AS plaques rupture, possibly causing platelet aggregation and thrombosis and inducing acute cardiovascular events, such as vascular stenosis or occlusion [3]. The carotid artery is more prone to AS owing to its unique “Y-shaped” bifurcation and impact from high pressure due to blood flow. Carotid AS (CAS) induces systemic AS [4]. Therefore, early assessment of the occurrence of CAS and carotid plaque (CP) stability could significantly aid in preventing adverse cardiovascular events.
Neuropeptide Y (NPY) is an abundant sympathetic neurotransmitter distributed throughout the central and peripheral regions of the body [5]. Serum NPY is released from the sympathetic postganglionic fibers, adrenal medulla, and platelets [6]. However, elevated plasma NPY levels have been documented in diseases, such as hypertension [7], chronic heart failure [8], and renal failure [9], wherein sympathetic outflow is increased. NPY can regulate inflammatory responses, vascular endothelial function, and oxidative stress and promote platelet activation by binding to G protein-coupled receptors [10]. Local injections of NPY into the carotid artery of rats also promote the occurrence of AS lesions and vascular occlusion after carotid angioplasty [11].
Therefore, as an active neuroendocrine regulatory product, NPY may be significantly associated with AS. However, there is little real-world data on the relationship between NPY and CP, which is necessary to determine effective interventions and strategies in this regard. This study aimed to investigate whether serum NPY levels are related to the progression of CP lesions and to further explore the correlation between serum NPY levels and inflammatory responses.
Materials and methods
Study type
This was a cross-sectional study.
Research participants
Between June 2020 and January 2022, 300 participants who underwent color Doppler examination of the carotid artery at the Second Affiliated Hospital of Fujian Medical University, China were screened. All participants signed an informed consent form. This study was approved by the Ethics Committee of our hospital.
The major inclusion criteria were as follows: (1) age, 18–75 years, (2) patients who underwent color Doppler examination of the carotid artery at a designated time in this hospital, and (3) patients who were conscious and could co-operate until study completion.
The exclusion criteria were as follows: (1) cancer, hematological disease, organic heart disease, autoimmune disease, hyperthyroidism, Cushing syndrome, hyperparathyroidism, infection, history of myocardial infarction and stroke, history of tuberculosis, chronic alcohol abuse, pregnancy, lactation, diabetic foot, diabetic ketoacidosis, or hyperglycemic hyperosmolar state; (2) diseases causing abnormal NPY levels, such as psychiatric disorders and epilepsy; (3) participants with unqualified specimens (e.g., hemolysis); and (4) missing information on anthropometric data and metabolic components.
Finally, 120 participants were included in the CP group (n = 120). Based on the plaque characteristics, the CP group was further divided into stable plaque (SP, n = 40) and vulnerable plaque (VP, n = 80) groups. Patients without CP were included in the control group (NCP group, n = 57).
Data collection
The anthropometric data collected primarily included sex, age, smoking history, height, and weight. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured with the participants in seated and resting positions.
All blood samples were collected at 8 am after fasting for 10–12 h and centrifuged at 3,000 × g for 5 min to separate the serum. One part of the supernatant was immediately assessed for routine clinical biochemical indicators using a fully automated biochemical analyzer (Beckman LX20, USA) in the Laboratory Medicine Department of our hospital, and the other part of the supernatant was stored at -80 °C for enzyme-linked immunosorbent assay (ELISA). Serum NPY and tumor necrosis factor-α (TNF-α) levels were measured using specific ELISA with an intra-assay error of < 10% and an inter-assay error of < 12%.
Carotid artery ultrasonography
Carotid artery ultrasound was performed on all participants using a color Doppler ultrasound diagnostic instrument (LOGIQ S7 Expert) at a transducer frequency of 4–9 MHz by two experienced physicians blinded to the patients’ conditions. Using the Mannheim criteria, CP was described as a focal zone invading the arterial lumen by at least 0.5 mm, > 50% of the surrounding intima-media thickness values, or a thickness of 1.5 mm above the distance between the lumen-intima and the media-adventitia interfaces [12]. If plaques were found, further evaluation of plaque stability was carried out. Plaques with smooth surfaces, intact intima, and high internal echogenicity, were defined as stable plaques while those with fewer smooth surfaces, incomplete inner membranes, and internal low echo, isoechoic, and mixed echo plaques, were defined as unstable plaques. If the ultrasound results were inconclusive, two senior physicians, expert at color ultrasound, reached a consensus after discussion.
Statistical analyses
All data were statistically analyzed using SPSS v26.0. We reported patient characteristics using means and standard deviations for normally distributed variables, medians and interquartile ranges (IQR) for non-normally distributed variables, and proportions for categorical variables. Independent samples t-test, Mann–Whitney U test, and χ2 test were used for comparison of normally distributed continuous, non-normally distributed continuous, and categorical variables, respectively. Spearman’s correlation coefficient was performed to evaluate the relationship of NPY with TNF-α. A binary logistic regression analysis was performed to determine the association between serum NPY levels and CP. ROC curves were drawn to evaluate the effect of NPY in predicting CP. A two-tailed p < 0.05 indicated statistical significance.
Results
Comparison of baseline data
The age showed a significant difference between the CP and normal groups (P = 0.001). No significant differences were observed between the CP and normal groups with respect to sex, smoking, hypertension, diabetes, body mass index (BMI), and levels of UA, SBP, DBP, MAP, total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), or FPG (P > 0.05) (Table 1).
Table 1.
Baseline characteristics of the study participants (N = 177)
| Characteristic | NCP group | CP group | P |
|---|---|---|---|
| n | 57 | 120 | |
| Sex | 0.458 | ||
| Male | 27 | 64 | |
| Female | 30 | 56 | |
| Smoking | 8(14.04%) | 26(21.67%) | 0.228 |
| Hypertension | 21(36.84%) | 54(45%) | 0.305 |
| Diabetes | 19(33.33%) | 36(30%) | 0.723 |
| Age(y, x ± s) | 53.14 ± 9.33 | 58.07 ± 8.77 | 0.001 |
| BMI(kg.m− 2, x ± s) | 26.06 ± 4.19 | 25.55 ± 4.00 | 0.436 |
| UA(umol.L− 1, x ± s) | 338.40 ± 116.62 | 369.69 ± 121.93 | 0.108 |
| SBP/mmHg | 126.0(115.00-139.00) | 132.5(124.00-151.75) | 0.146 |
| DBP/mmHg | 78.00(71.00-89.50) | 83.00(75.25–89.75) | 0.095 |
| MAP/mmHg | 96.33(86.83-105.67) | 99.1(92.42-110.58) | 0.258 |
| TC/mmol.L− 1 | 4.53(3.79–5.27) | 4.64(3.75–5.66) | 0.787 |
| TG/mmol.L− 1 | 1.35(0.88–2.01) | 1.46(1.16–1.98) | 0.554 |
| LDLC/mmol.L− 1 | 2.64(2.10–3.51) | 2.91(2.06–3.66) | 0.361 |
| HDLC/mmol.L− 1 | 1.12(0.96–1.30) | 1.08(0.84–1.38) | 0.424 |
| FPG/mmol.L− 1 | 5.64(4.63–7.20) | 5.63(5.00-7.23) | 0.959 |
Data were reported as the mean (SD) or median (IQR: Q1-Q3). The t-test or Mann-Whitney U test or χ2 test was used for comparisons between NCP group and CP group
BMI, body mass index; UC, uric acid; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; TC, total cholesterol; TG, triglyceride; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholestero; FPG, fasting plasma glucose
Comparison of NPY and TNF-α levels in CP conditions
Serum NPY and TNF-α levels were higher in the CP group than in the NCP group(P < 0.05) (Table 2). Specifically, however, the NPY levels in the SP group were significantly lower than those in the VP group (P < 0.05) (Table 3).
Table 2.
Comparison of serum NPY and TNF-α levels between the NCP group and the CP group
| Item | NCP group | CP group | P |
|---|---|---|---|
| n | 57 | 120 | |
| NPY/ng.L-1 | 121.53 ± 40.16 | 177.30 ± 43.29 | < 0.001 |
| TNF-α/pg.mL-1 | 33.54 ± 13.37 | 41.94 ± 14.19 | < 0.001 |
Table 3.
Comparison of serum NPY and TNF-α levels between the SP group and the VP group
| Item | SP group | VP group | P |
|---|---|---|---|
| n | 80 | 40 | |
| NPY/ng.L− 1 | 170.12 ± 43.37 | 191.67 ± 39.87 | P = 0.010 |
| TNF-α/pg.mL-1 | 41.13 ± 14.65 | 43.57 ± 13.26 | P = 0.376 |
Correlation between NPY and TNF-α in the CP group
Serum NPY levels were positively correlated to TNF-α levels in the CP group (r = 0.184, P = 0.044) (Fig. 1).
Fig. 1.
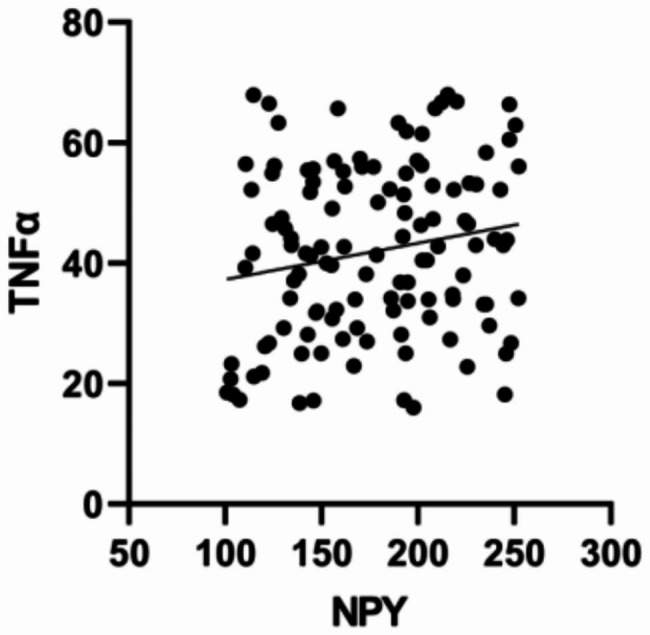
The correlation analysis between NPY and TNF-α in the CP group
Correlation between NPY and TNF-α levels and CP
We performed bivariate regression analysis to analyze the specific correlation between serum NPY and TNF-α levels and CP. Correlations were measured using odds ratios. After adjusting for age and TNF-α, higher levels of NPY remained independently associated with CP (OR = 1.029, P < 0.001). After adjusting for age and NPY, TNF-α was an independent influencing factor for CP (OR = 1.030, P = 0.023)(Table 4).
Table 4.
Binary logistic regression for CP
| Variable | Binary logistic regression | |||||
|---|---|---|---|---|---|---|
| B | Sex | Wald | OR | 95%CI | P | |
| Age | 0.027 | 0.025 | 1.717 | 1.027 | 0.984–1.086 | 0.263 |
| NPY | 0.029 | 0.006 | 19.564 | 1.029 | 1.016–1.041 | < 0.001 |
| TNF-α | 0.030 | 0.016 | 3.097 | 1.030 | 0.997–1.062 | 0.023 |
NPY predicts CP
To evaluate the predictive value of NPY for the occurrence of CP, the ROC curve was mapped, where NPY was used as a test variable and CP was assigned as a state variable (0 for NCP, 1 for CP). NPY had a good predictive value for the occurrence of CP (AUC = 0.819; 95% CI, 0.756–0.882; σ = 0.032; P < 0.001; Fig. 2). The optimal cutoff value for NPY is 189.60 ng.L− 1.
Fig. 2.
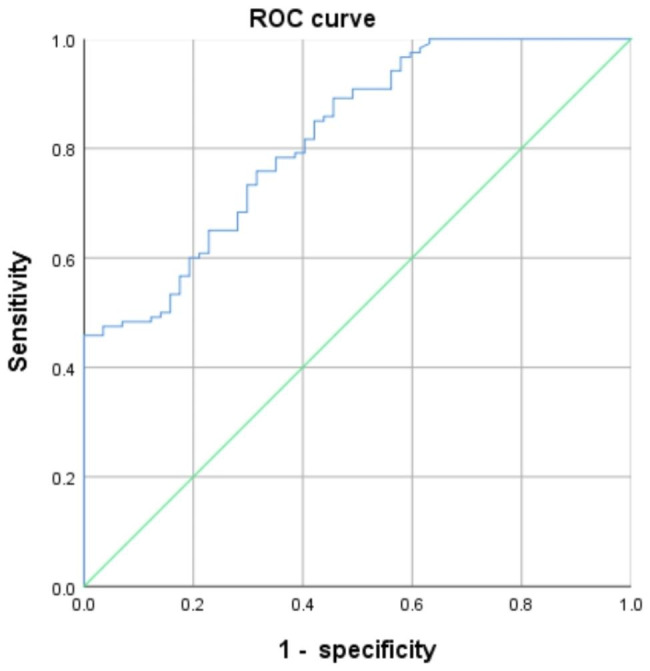
The ROC curve
Discussion
CP is a well-known imaging biomarker for predicting recurrent vascular events. Carotid artery ultrasound is increasingly being used to assess the activity, quality, and morphology of plaques. Herr et al. suggested that increased carotid intima-media thickness was associated with an increased risk of cardiovascular events [13]. Moreover, the echogenicity and fragility of the CP have been considered independent predictors of coronary artery events [14]. Exploring the relationship between serum NPY levels and CP may provide a potential biomarker for assessing the risk of cardiovascular events and a perspective on the onset of AS.
Our results showed that serum NPY levels were higher in the CP group than in the NCP group. After adjusting for factors, such as age, blood lipids, and TNF-α, serum NPY levels independently correlated with the occurrence of CP. Previous clinical studies have suggested that NPY concentration is an independent predictor of coronary heart disease and ischemic stroke [15]. Wu et al. showed that downregulation of NPY and its receptor expression in animals had an anti-atherosclerotic effect [16]. This is consistent with our findings, which suggest that NPY may be associated with the occurrence of CP.
In addition, the serum NPY levels of participants in the VP group were higher than those of individuals in the SP group. Previous studies have shown that NPY activates the extracellular signal-regulated protein kinase (ERK1/2) pathway in mice through the Y1 receptor, thereby inducing the expression of matrix metalloproteinase 8 in macrophages in a dose-dependent manner [17], hydrolyzing the extracellular matrix, and disrupting plaque stability. NPY binds to the Y2 receptor on endothelial cells, thereby promoting the secretion of nitric oxide and vascular endothelial growth factor by these cells within these plaques and inducing angiogenesis [18]. New blood vessels in plaques exacerbate plaque growth and increase the risk of bleeding and rupture, causing thrombosis. The areas with myocardial infarction, myocardial cell apoptosis, and expression and activity of caspase-3 were significantly reduced in NPY-knockout rats, and cardiac systolic function was significantly improved [19]. These findings suggest that, in the presence of CAS, NPY could promote unstable lesions in patients with CP.
Similarly, our results showed that the peripheral blood TNF-α levels of patients in the CP group were higher than those in the patients in the NCP group. This suggests that the inflammatory levels in blood vessels in patients with CP may significantly increase. TNF-α is a mononuclear factor primarily produced by monocytes and macrophages. It upregulates the expression of adhesion molecules and inflammatory cytokines and induces an AS inflammatory response [20]. Inhibiting TNF-α can reduce the progression of AS in ApoE−/− mice [21]. We found a positive correlation between NPY and TNF-α levels, thereby implying a possible correlation between NPY and a significantly elevated inflammatory response in the CP group. Inflammatory reactions can gradually increase lipid core volume, decrease extracellular matrix components, and cause thinning of plaque fiber caps, thereby increasing plaque fragility [22], which may explain the high levels of NPY in the VP group.
However, no significant differences were found in the serum TNF-α levels between the SP and VP groups (P = 0.376). This may be related to the time of procuring the serum TNF-α samples from patients with VP. The degree of inflammation may vary at different stages of AS development [23]. During the process of plaque formation and rupture, we could not accurately determine the timing of the most severe inflammation. Therefore, predicting the optimal time point for the peak release of serum TNF-α levels in CAS participants was challenging. Whether the serum TNF-α levels differ between the SP and VP groups requires further studies.
This study had some limitations. First, the participants in this study were primarily from the Fujian province; therefore, the conclusion is primarily applicable to the southern China population and partially to those in its other parts, such as northern and western China. Second, this study did not identify a concentration range within which serum NPY exerts its biological functions and did not investigate the number of and functional changes in NPY receptors. Thirdly, participants with chronic diseases regularly administer medications under the guidance of specialized physicians, but there are significant differences in the duration of administration, type, dosage, and manufacturer of these medications. Therefore, we did not include specific medication information in the analysis of the results. Finally, this was a cross-sectional study, and we could not conclude that elevated serum NPY levels can promote the development of CAS in Chinese adults.
NPY can regulate inflammatory responses, vascular endothelial function, and oxidative stress; promote platelet activation by binding to G protein-coupled receptors; activate the ERK1/2 pathway in mice through the Y1 receptor, inducing the expression of matrix metalloproteinase 8 in macrophages in a dose-dependent manner [17]; hydrolyze the extracellular matrix and disrupt the stability of plaques; and bind to the Y2 receptor on endothelial cells, promoting the secretion of NO and vascular endothelial growth factor by endothelial cells in plaques, and promoting angiogenesis [24]. New blood vessels in AS not only exacerbate the growth of plaques, but also increase the risk of bleeding and rupture, leading to thrombosis. The study by Pankajakshan et al. revealed that NPY-Y2 mRNA expression was 10 times greater in the smooth muscle cells of symptomatic plaque than that in healthy carotid artery, and the TNF-α-stimulated smooth muscle cells of healthy carotid artery increased the transcription of NPY-Y2 mRNA [25]. Accordingly, these mechanisms suggest that in the presence of CAS, NPY is likely to promote unstable plaques in patients with CAS.
NPY exerts its biological effects by binding to receptors, specifically the inflammation related NPY-Y1, NPY-Y2, and NPY-Y5 receptors [26]. The high distribution of these receptors in the hypothalamic tissue suggests that exploring the correlation between NPY levels in the brain, peripheral inflammatory response, and CAS may be a valuable approach. In addition, NPY regulates immune responses in diverse, complex, and contradicting manners [27]. Exploring the regulatory mechanisms of NPY on innate/adaptive immunity may provide novel insights and effective interventions for the management of AS.
Conclusions
This study suggests that higher serum NPY levels are independently associated with CP and vulnerability to CP, possibly owing to the promotion of inflammation by NPY.
Acknowledgements
Not applicable.
Abbreviations
- NPY
Neuropeptide Y
- CP
carotid plaque
- NCP
non-carotid plaque
- CAS
Carotid atherosclerosis
- AS
atherosclerosis
- ELISA
enzyme-linked immunosorbent assay
Author contributions
W.D. and S.N. wrote the original draft manuscript. H.L.and Y.L. contributed to acquisition references and revision. Y.G.reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fujian Provincial Natural Science Foundation (2021J01249, 2022J01789), the Fujian Medical University Start-up Fund Project(2022QH1111), and also funded by the Second Affiliated Hospital of Fujian Medical University PHD Project Foundation(2021GCC08, 2022DB0801, 2022DB0802, 2022DB0803, 2022DB0804).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The ethics committee of the Second Affiliated Hospital of Fujian Medical University provided the approval of this study. All experimental protocols were approved by the institutional ethical board, all methods were carried out in accordance with relevant guidelines and regulations,and written informed consents were obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hui-li Lin, Email: 1627974150@qq.com.
Yan-li Zheng, Email: 1336133575@qq.com.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K. Heart Disease and Stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2010;117(4):25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Zhu YH, Xian XM, Wang ZZ, Bi YC, Chen QG, Han XF, Tang DQ, Chen RJ. Research Progress on the relationship between Atherosclerosis and inflammation. Biomolecules. 2018;8(3):80. doi: 10.3390/biom8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. The changing landscape of Atherosclerosis. Nature. 2021;592(7855):524–33. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 4.Lombardo A, Rizzello V, Natale L, Lombardi M, Coli S, Snider F, Bonomo L, Crea F. Magnetic resonance imaging of carotid plaque inflammation in acute coronary syndromes: a sign of multisite plaque activation. Int J Cardiol. 2009;136(1):103–5. doi: 10.1016/j.ijcard.2008.03.077. [DOI] [PubMed] [Google Scholar]
- 5.Pain S, Brot S, Gaillard A. Neuroprotective effects of Neuropeptide Y against Neurodegenerative Disease. Curr Neuropharmacol. 2022;20(9):1717–25. doi: 10.2174/1570159x19666210906120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zukowska-Grojec Z, Neuropeptide Y. A novel sympathetic stress hormone and more. Ann N Y Acad Sci 1995; 771219–33. [DOI] [PubMed]
- 7.Wocial B, Ignatowska-Switalska H, Pruszczyk P, Jedrusik P, Januszewicz A, Lapinski M, Januszewicz W, Zukowska-Grojec Z. Plasma neuropeptide Y and catecholamines in women and men with Essential Hypertension. Blood Press. 1995;4(3):143–7. doi: 10.3109/08037059509077586. [DOI] [PubMed] [Google Scholar]
- 8.Callanan EY, Lee EW, Tilan JU, Winaver J, Haramati A, Mulroney SE, Zukowska Z. Renal and cardiac neuropeptide Y and NPY receptors in a rat model of Congestive Heart Failure. Am J Physiology-Renal Physiol. 2007;293(6):F1811–7. doi: 10.1152/ajprenal.00191.2007. [DOI] [PubMed] [Google Scholar]
- 9.Bald M, Gerigk M, Rascher W. Elevated plasma concentrations of neuropeptide Y in children and adults with chronic and terminal Renal Failure. Am J Kidney Dis. 1997;30(1):23–7. doi: 10.1016/S0272-6386(97)90560-6. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32(5):645–59. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li LJ, Lee EW, Ji H, Zukowska Z, Neuropeptide, Arteriosclerosis Thromb Vascular Biology. 2003;23(7):1204–10. doi: 10.1161/01.ATV.0000071349.30914.25. [DOI] [PubMed] [Google Scholar]
- 12.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez R. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004?2006?2011) Cerebrovasc Dis. 2012;34(4):290–6. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herr JE, Hétu MF, Li TY, Ewart P, Johri AM. Presence of Calcium-Like Tissue Composition in Carotid Plaque is indicative of significant coronary artery Disease in high-risk patients. J Am Soc Echocardiogr. 2019;32(5):633–42. doi: 10.1016/j.echo.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, Kavousi M, van der Lugt A. Atherosclerotic carotid plaque composition and incident Stroke and coronary events. J Am Coll Cardiol. 2021;77(11):1426–35. doi: 10.1016/j.jacc.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 15.Zhu P, Sun WW, Zhang CL, Song ZY, Lin S. The role of neuropeptide Y in the pathophysiology of atherosclerotic Cardiovascular Disease. Int J Cardiol. 2016;220:235–41. doi: 10.1016/j.ijcard.2016.06.138. [DOI] [PubMed] [Google Scholar]
- 16.Wu WQ, Peng S, Wan XQ, Lin S, Li LY, Song ZY. Physical exercise inhibits Atherosclerosis development by regulating the expression of neuropeptide Y in apolipoprotein E-deficient mice. Life Sci. 2019;237. 10.1016/j.lfs.2019.116896. [DOI] [PubMed]
- 17.Wu WQ, Peng S, Shi YC, Li LY, Song ZY, Lin S. NPY promotes macrophage migration by upregulating matrix metalloproteinase-8 expression. J Cell Physiol. 2021;236(3):1903–12. doi: 10.1002/jcp.29973. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Jönsson-Rylander AC, Abe K, Zukowska Z. Chronic stress induces rapid occlusion of angioplasty-injured rat carotid artery by activating neuropeptide Y and its Y1 receptors. Arterioscler Thromb Vasc Biol. 2005;25(10):2075–80. doi: 10.1161/01.ATV.0000179601.19888.19. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Zhang QH, Qi HP, Shi PL, Song C, Liu YS, Sun HL. Deletion of Neuropeptide Y attenuates Cardiac Dysfunction and apoptosis during Acute Myocardial Infarction. Front Pharmacol. 2019;10. 10.3389/fphar.2019.01268. [DOI] [PMC free article] [PubMed]
- 20.Ha SJ, Lee J, Song KM, Kim YH, Lee NH, Kim YE, Jung SK. Ultrasonicated Lespedeza cuneata extract prevents TNF-α-induced early Atherosclerosis in vitro and in vivo. Food Funct. 2018;9(4):2090–101. doi: 10.1039/c7fo01666b. [DOI] [PubMed] [Google Scholar]
- 21.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of Tumor necrosis factor-alpha reduces Atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 22.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circul Res. 2014;114(12):1852–66. doi: 10.1161/circresaha.114.302721. [DOI] [PubMed] [Google Scholar]
- 23.Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132(12):1243–52. doi: 10.1042/cs20180306. [DOI] [PubMed] [Google Scholar]
- 24.Li LJ, Jönsson-Rylander AC, Abe K, Zukowska Z. Chronic stress induces Rapid occlusion of angioplasty-injured rat carotid artery by activating Neuropeptide Y and its Y1 receptors. Arteriosclerosis, Thrombosis, and Vascular Biology 2005, 25(10): 2075–80. 10.1161/01.atv.0000179601.19888.19. [DOI] [PubMed]
- 25.Pankajakshan D, Jia GH, Pipinos I, Tyndall SH, Agrawal DK. Neuropeptide Y receptors in carotid plaques of symptomatic and asymptomatic patients: Effect of inflammatory cytokines. Exp Mol Pathol. 2011;90(3):280–6. doi: 10.1016/j.yexmp.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang WD, Zheng YL, Li MM, Lin S, Lin HL. Recent advances in studies on the role of neuroendocrine disorders in Obstructive Sleep Apnea-Hypopnea syndrome-related Atherosclerosis. Nat Sci Sleep. 2021;13:1331–45. doi: 10.2147/nss.S315375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WC, Liu YB, Liu WF, Zhou YY, He HF, Lin S. Neuropeptide Y is an Immunomodulatory factor: direct and indirect. Front Immunol. 2020;11. 10.3389/fimmu.2020.580378. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


