Abstract
Muscle atrophy with aging is closely associated with chronic systemic inflammation and lifestyle-related diseases. Here, we assessed whether dried tofu intake during 5-month interval walking training (IWT) enhanced increases in thigh muscle mass and strength and ameliorated susceptibility to inflammation in older women. Subjects (n = 32, ~ 65 years) who performed IWT for > 6 months participated in this study. They were randomly divided into 2 groups: IWT + placebo intake (PLG, n = 16; 108 kcal, 0.2 g protein, 5.5 g fat, and 14.4 g carbohydrate) and IWT + dried tofu intake (DTG, n = 16; 111 kcal, 9.6 g protein, 6.0 g fat, and 4.6 g carbohydrate). They were instructed to repeat ≥ 5 sets of fast and slow walking for 3 min each at ≥ 70 and 40% peak aerobic capacity for walking, respectively, per day for ≥ 4 days/week. Immediately after daily exercise, subjects were instructed to consume the supplements assigned to each group. In the DTG, after IWT, the methylation increased at 4/6 sites in the promoter region of the NFKB2 gene in the whole blood (all, P < 0.04), with an 18% increase in the average methylation of the 6 sites (P = 0.035). On the other hand, in the PLG, the increase occurred at only 2/6 sites, with no significant increase in the average methylation of the 6 sites. No significant differences were observed in increases in thigh muscle strength or cross-sectional area between the groups (all, P > 0.2). Altogether, dried tofu supplementation during IWT likely enhanced the methylation of the NFKB2 gene more than IWT alone, without detectably enhanced increases in thigh muscle strength or cross-sectional area.
Keywords: Soy protein intake, Anti-inflammatory response, Thigh muscle strength, Interval walking training
Introduction
Physical fitness deterioration mainly due to muscle atrophy with advanced aging (sarcopenia) is suggested to cause chronic systemic inflammation and age- and lifestyle-related diseases (LSDs) [1–5]. To prevent this, nutritional supplementation during exercise has been recommended for middle-aged and older people [6, 7], but no system has been established to monitor exercise training achievement, including exercise intensity and frequency, in a large population of people for a long period of several months, which is required to examine the effects of nutritional supplementation during exercise [6].
To solve this problem, we have developed an exercise training system that is broadly applicable to middle-aged and older people in the field with minimal personnel and financial requirements. The system includes interval walking training (IWT) and the use of an information technology network system that tracks exercise intensity and energy expenditure during training [8–10]. Using this system, we reported that in a large population of middle-aged and older subjects, 5-month IWT increased thigh muscle strength and the peak aerobic capacity for walking () by ~ 10% [8, 11], which was accompanied by improved LSD symptoms by ~ 20% [8, 11] and increased DNA methylation (inactivation) of the NFKB2 gene, one of the chief pro-inflammatory response genes [12]. These results suggest that IWT ameliorates the susceptibility to inflammation by increasing physical fitness in middle-aged and older people.
Moreover, this system enabled us to examine the effects of nutritional supplementation during aerobic exercise training. We recently found that milk protein supplementation during a 5-month IWT enhanced methylation of pro-inflammatory cytokine genes with increases in thigh muscle mass and strength more than IWT alone in middle-aged and older women [13, 14]. Accordingly, the prescription has been broadly accepted by middle-aged and older people in Japan; however, some people show physical and psychological rejection against milk products and desire replacements. One candidate for replacement is soy protein; however, the composition of amino acids is different from that of milk protein [15], and the increasing rate of blood concentrations of the essential amino acids after ingestion, including leucine, which is reported to accelerate the muscle protein synthesis rate [16], is lower in soy protein than in milk protein [17]. These results suggest that the effects of soy protein intake would be attenuated compared with those of milk protein intake. Thus, it is unknown whether dried tofu supplementation during 5-month IWT would enhance the methylation of the NFKB2 gene with increases in thigh muscle mass and strength. Therefore, the present study was conducted to examine this theory. The reason for the use of dried tofu, also called “kori-tofu”, as a supplement was that it is a familiar food for Japanese people, and is rich in soy protein.
Materials and methods
Subjects
This study was approved by the Review Board on Human Experiments, Shinshu University School of Medicine (UMIN000014957), and conformed to the standards set by the Declaration of Helsinki. As shown in Fig. 1, subjects were recruited from among the 97 participants in the “Jukunen Taiikudaigaku Project”, a health promotion program for middle-aged and older people in Matsumoto City, Japan.
Fig. 1.
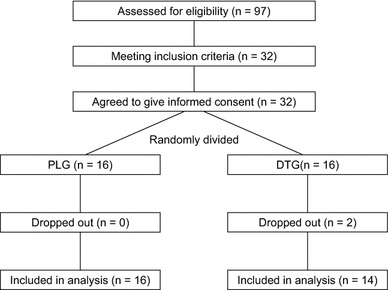
Flow diagram outlining the experimental protocol. PLG placebo group, DTG dried tofu group
We recruited female subjects to minimize any confounding effects of gender. We also recruited subjects who had participated in the IWT program for more than 6 months prior to this study because they were familiar with the exercise testing procedures used in the present study and also because their increases in had likely reached a steady state. Therefore, we surmised that we could detect any effects elicited by dried tofu intake in addition to those of IWT alone.
After the experimental protocol was fully explained, 32 healthy female volunteers (51–70 years of age) provided written informed consent before participating in this study. Each subject provided a complete medical history and underwent a physical examination. All subjects were nonsmokers, had no overt history of hepatic, thyroid, renal, metabolic, cardiovascular, or pulmonary disease, and had no orthopedic limitations that could affect exercise testing or training. We also confirmed that all participants were postmenopausal.
Protocol
The experiments were conducted from October 1, 2014 to April 24, 2015. The subjects were instructed to arrive at a gym at 09:00 on their assigned day in October. We measured their physical characteristics, thigh muscle strength, and and sampled their blood for the determination of blood lipid and glucose levels and also DNA methylation levels. All measurements except thigh muscle strength and were performed after overnight fasting. On a separate day assigned to each subject, the subjects were instructed to visit the Shinshu University Hospital at 17:00–18:00 with a normal hydration status, but without having eaten any food for at least 2 h for the measurement of the thigh muscle cross-sectional area with computer tomography [13].
The subjects were randomly divided into two groups, IWT + placebo intake (PLG, n = 16) and IWT + dried tofu intake (DTG, n = 16) (see below for details), to evaluate the effects of dried tofu intake. No significant differences were observed in the physical characteristics or fitness between the groups (Tables 1, 2).
Table 1.
Physical characteristics and blood pressures
| PLG (n = 16) | DTG (n = 14) | P value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | G × T | |
| Age (years) | 65 ± 4 | – | 64 ± 5 | – | NS | – | – |
| Height (cm) | 155 ± 4 | – | 156 ± 3 | – | NS | – | – |
| Body weight (kg) | 51.5 ± 1.6 | 52.0 ± 1.8** | 53.3 ± 1.9 | 54.2 ± 1.9** | NS | 0.005 | NS |
| BMI (kg/m2) | 21.4 ± 0.7 | 21.6 ± 0.7** | 21.9 ± 0.7 | 22.2 ± 0.7** | NS | 0.006 | NS |
| % Fat | 28.3 ± 1.5 | 29.1 ± 1.7* | 29.1 ± 1.5 | 30.0 ± 1.4* | NS | 0.020 | NS |
| Abdominal circumstance (cm) | 79.5 ± 2.1 | 78.9 ± 2.4 | 77.8 ± 2.2 | 77.9 ± 2.4 | NS | NS | NS |
| SBP (mmHg) | 131 ± 4 | 141 ± 5** | 130 ± 3 | 139 ± 4.4** | NS | 0.002 | NS |
| DBP (mmHg) | 77 ± 2 | 78 ± 3 | 75 ± 3 | 77 ± 3 | NS | NS | NS |
The values are the mean ± SD for age and height and mean ± SE for other variables
Significant differences from the pre-training values, *P < 0.05 and **P < 0.01
PLG placebo group, DTG dried tofu group, pre and post before and after 5-month interval walking training with placebo or dried tofu supplementation, respectively, SBP systolic blood pressure, DBP diastolic blood pressure, G × T interactive effects of group (G; PLG and DTG) and time (T; pre and post) on the variables by two-way ANOVA (analysis of variance), NS not significantly different
Table 2.
Thigh muscle strength and cross-sectional areas and the
| PLG (n = 16) | DTG (n = 14) | P value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | G × T | |
| FEXT (Nm) | 111 ± 6 | 113 ± 5 | 107 ± 9 | 112 ± 10 | NS | NS | NS |
| FFLX (Nm) | 54 ± 2 | 56 ± 2 | 61 ± 3 | 59 ± 3 | NS | NS | NS |
| M. rectus femoris (cm2) | 4.1 ± 0.3 | 4.5 ± 0.2** | 4.6 ± 0.3 | 4.9 ± 0.2** | NS | 0.004 | NS |
| M. hamstrings (cm2) | 20.8 ± 0.6 | 21.2 ± 0.7 | 21.1 ± 1.0 | 22.0 ± 0.8* | NS | 0.037 | NS |
| (ml/min) | 1,345 ± 74 | 1,353 ± 61 | 1,367 ± 45 | 1,382 ± 66 | NS | NS | NS |
| PR peak (beats/min) | 144 ± 6 | 153 ± 7 | 156 ± 4 | 155 ± 4 | NS | NS | NS |
The values are the mean ± SE
Significant differences from the pre-training values, *P < 0.05 and **P < 0.01
FEXT isometric knee extension force, FFLX isometric knee flexion force, M. rectus femoris rectus femoris muscle tissue area, M. hamstrings hamstrings muscle tissue area, peak aerobic capacity for walking, PR peak pulse rate at , G × T interactive effects of group (G; PLG and DTG) and time (T; pre and post) on the variables by two-way ANOVA, NS not significantly different
During the 5-month training period, the average atmospheric temperature ranged from − 1 to 14 °C, and the average relative humidity ranged from 59 to 74%. All measurements performed before training were repeated after training using the same procedures. All subjects in the PLG completed 5 months of training and returned for post-training assessments. Two of 16 subjects in the DTG dropped out; one left because of a family issue, while the other left because of a health issue. Therefore, we used the data from 16 subjects in the PLG and 14 subjects in the DTG for the following analyses.
IWT regimen
As reported previously [8], all subjects were instructed to repeat ≥ 5 sets of 3 min of low-intensity walking at ~ 40% of their pre-training for walking (see below for details), followed by 3 min of high-intensity walking at ≥ 70% of their for ≥ 4 days/week. Energy expenditure during daily walking at their favorite time and place was monitored with a convenient calorimeter (Jukudai Mate; Kissei Comtec, Matsumoto, Japan) on the right or the left side of the waist at the midclavicular line. A beeping signal alerted the participants when a change in intensity was scheduled, and another signal alerted them when their walking intensity reached 70% of their .
Every 2 weeks, the subjects visited a local community office near their homes to upload their walking records from the calorimeter to a central server at the administrative center through the Internet for automatic analysis and reporting. The trainers used these reports to track daily walking intensity, energy expenditure, and other parameters, which are shown in Table 3, and to instruct the participants on how best to achieve their target levels. Using these records, adherence to the exercise training program was calculated as the number of walking days completed divided by the total number of walking days prescribed as the target (4 days/week).
Table 3 .
Training achievements
| PLG (n = 16) | DTG (n = 14) | P values | |
|---|---|---|---|
| Walking (days/week) | 4.1 ± 0.4 | 3.6 ± 0.4 | NS |
| Fast walking time/week (min) | 74 ± 13 | 63 ± 8 | NS |
| Fast walking | |||
| Time (min/walking day) | 17 ± 2 | 18 ± 1 | NS |
| Energy expenditure (mlO2/walking daya) | 15,430 ± 2053 | 17,785 ± 1605 | NS |
| Intensity (mlO2/mina) | 902 ± 49 | 999 ± 29 | NS |
| Slow walking | |||
| Time (min/walking day) | 32 ± 4 | 31 ± 5 | NS |
| Energy expenditure (mlO2/walking daya) | 15,506 ± 1947 | 13,942 ± 1512 | NS |
| Intensity (mlO2/mina) | 490 ± 29 | 503 ± 36 | NS |
The values are the mean ± SE
NS not significantly different
aResting oxygen consumption is not included
Supplement intake during IWT
Subjects in the PLG and the DTG were instructed to ingest the supplements within 30 min after each IWT session. If no training was performed, they were instructed to take the supplements the next day before breakfast. The nutritional components of the supplements per dose were 108 kcal, 0.2 g of protein, 5.5 g of fat, and 14.4 g of carbohydrate for the PLG (20.5 g of blocked sugar with powdered soybean oil and fat) and 111 kcal, 9.6 g of protein, 6.0 g of fat, and 4.6 g of carbohydrate for the DTG (21.9 g of freeze–dried cooked kori-tofu). The two supplements were similar in appearance. The subjects in both groups were instructed to refrain from eating and drinking any foods or fluids other than tap water during and for at least 60 min before and after each IWT session. To examine adherence to the intervention, all subjects were required to keep daily logs describing whether they completed IWT and whether they ingested the supplements as instructed. Using this information, adherence to the supplement intake regimen was determined by dividing the number of times that the subjects ingested the supplements as instructed, with a supplement intake period of 140 days.
Dietary intake
All subjects were instructed to maintain their dietary habits during the study period. In addition, the subjects were instructed to report the foods that they consumed for 7 consecutive days during the training period in October and March by completing a questionnaire that was prepared by a dietician (FFQg Ver 3.5; Kenpakusya, Tokyo, Japan).
Measurements
Thigh muscle strength
The isometric knee extension (FEXT) and flexion (FFLX) forces were measured twice on the dominant side of the leg with an isometric force meter (GT330, OG Giken, Okayama, Japan), while the knee angle was fixed at 60°; the highest values were used in the present analysis. The measurements were performed by staff members who were blinded to the groups assigned to the evaluated subjects.
Thigh muscle tissue area
Subjects were instructed to refrain from vigorous exercise for 2 days and from beverages containing caffeine and alcohol for 24 h before the day of the measurements. After emptying their bladders, the subjects rested in a supine position for at least 10 min before the measurements were performed. The thigh sectional images were obtained using computer tomography (LightSpeed VCT, GE Healthcare, Tokyo, Japan) with the subjects in the supine position with their thigh muscles relaxed and their arms crossed in front of their chests. An anterior–posterior scout scan of the entire femur was conducted in the dominant leg, which was used for the thigh muscle strength test. Cross-sectional slices (1.25mm thick) were obtained from the distal edge of the medical condyle to the head of the femur and were then reconstructed as 10-mm thick images from the data. The scanning parameters for images were 12 kVp, 170–180 mA, 48 cm2 of FOV, and 512 × 512 matrix to attain a pixel resolution of 0.94 mm. The mid-thigh image was used to determine the cross-sectional area of both the muscle and the fat tissue (cm2), with the Hounsfield units for fat tissue defined as -190–0 and the muscle tissue as 0–100 [13], using image processing software (Dicom Works 1.3.5 software, Lyon, France).
O2peak
On the same day as the muscle strength measurements, was determined by measuring energy expenditure with the calorimeter (Jukudai Mate; Kissei Comtec, Matsumoto, Japan) during graded-intensity walking on a flat surface at subjectively slow, moderate, and the fastest speeds for 3 min each, as reported previously [9].
Blood samples
Blood samples were collected from the antecubital vein to measure blood lipid and glucose levels and to extract DNA before and after training in all subjects. Serum cholesterol and triglyceride concentrations and plasma glucose concentrations were determined using standard enzymatic methods. Genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
DNA methylation by pyrosequencing
Because the NFKB2 gene plays a key role in inflammation [18] and is suggested to be inactivated through DNA methylation after ~ 5 months of IWT [12], we examined the effects of dried tofu intake during IWT on the DNA methylation of the NFKB2 gene.
We analyzed samples from all subjects in the PLG (n = 16) and the DTG (n = 14) before and after training by pyrosequencing (PyroMark Q24ID; Qiagen, Hilden, Germany). PCR and sequencing primers were designed using PyroMark Assay Design 2.0 software (Qiagen), and all procedures were performed according to recommended protocols. The promoter region of NFKB2 (−1331 to −1153 upstream of the transcription start site) was amplified by PCR. The primers are shown in Table 4. DNA methylation predominantly occurs on cytosines at sites of CpG dinucleotides in mammals. Therefore, the target region of NFKB2 was 5′-AAAGGGCGCGAGGCGTGACGCACGGAAACGTCATGGGA-3′ (−1238 to −1201 upstream of the transcription start site). Briefly, bisulfite conversion of 500 ng of genomic DNA was performed with an EpiTect Kit (Qiagen). Bisulfite-converted DNA was amplified by PCR with a reverse primer biotinylated at its 5′ end using a PyroMark PCR Master Mix Kit (Qiagen). Biotinylated PCR products were immobilized onto streptavidin-coated beads (GE Healthcare, Uppsala, Sweden), and the DNA strands were separated using a denaturation buffer. After washing and neutralization at a PyroMark Q24 Vacuum Workstation, the sequencing primer was annealed to the immobilized strand. DNA methylation was analyzed via highly quantitative bisulfite pyrosequencing with a PyroMark Q24 system (Qiagen). The data were analyzed using PyroMark Q24 software (Qiagen), and the results are presented in Table 5.
Table 4.
Primers used for the pyrosequencing assay
| Gene | Amplification site | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | *Sequencing primer (5′ to 3′) |
|---|---|---|---|---|
| NFKB2 | −1331 to −1153 | GGGTTTAGGGTAGGGAAAGTTTTT | Biotin-CCCCCCCTAAATAAACCTATCCCCTCT | −1185-TTTAGGAGGGGGGAAT |
The sequencing primer was designed to analyze antisense DNA
Table 5.
Methylation of the NFKB2 promoter region
| PLG (n = 16) | DTG (n = 14) | P value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | G × T | |
| NFKB2 methylation, % cytosine methylated | |||||||
| Site 1 | 21.4 ± 1.6 | 23.2 ± 1.1 | 19.1 ± 2.2 | 22.9 ± 1.9 | NS | NS | NS |
| Site 2 | 29.4 ± 2.1 | 32.8 ± 1.6 | 27.9 ± 2.9 | 32.5 ± 2.8* | NS | 0.030 | NS |
| Site 3 | 28.3 ± 2.0 | 31.4 ± 1.4 | 25.7 ± 2.6 | 31.0 ± 2.7* | NS | 0.030 | NS |
| Site 4 | 26.3 ± 2.1 | 30.2 ± 1.4* | 24.4 ± 2.8 | 28.7 ± 2.3* | NS | 0.031 | NS |
| Site 5 | 27.8 ± 2.2 | 32.6 ± 1.5** | 24.8 ± 2.8 | 31.4 ± 2.4** | NS | 0.008 | NS |
| Site 6 | 32.2 ± 2.2 | 34.1 ± 1.5 | 29.9 ± 3.0 | 33.2 ± 2.7 | NS | NS | NS |
| Average of sites 1–6 | 27.6 ± 2.0 | 30.7 ± 1.4 | 25.3 ± 2.7 | 29.9 ± 2.4* | NS | 0.035 | NS |
The values are the mean ± SE
Significant differences from the pre-training values, *P < 0.05 and **P < 0.01
G × T interactive effects of group (G; PLG and DTG) and time (T; pre and post) on the variables by two-way ANOVA, NS not significantly different
Statistics
One-way ANOVA was used to examine any significant differences in physical characteristics, thigh muscle strength and cross-sectional area, , and NFKB2 methylation before training between the groups (Tables 1, 2, 5). This model was also used to examine any significant differences in training achievements and dietary intake during the training period between the groups (Table 3). Two-way ANOVA for repeated measures was used to examine any significant effects of supplementation (group) and training (time) on the variables with [group × time] interaction analysis (Tables 1, 2, 5). The Tukey–Kramer test was used as a subsequent post hoc test to perform any pairwise comparisons between the groups. P values < 0.05 were considered significant. The values are expressed as the mean ± SE unless otherwise indicated.
Results
Adherence to the exercise training program
As shown in Table 3, the training days per week and the fast walking time per week in both groups were almost 4 days per week and 60 min per week, respectively, which were the targets instructed prior to starting training; however, both values were significantly higher than the 3.0 ± 0.4 and 2.3 ± 0.4 days per week and 59 ± 14 and 42 ± 8 min per week for the 2 months observed prior to initiating the intervention in the PLG and DTG groups, respectively (both, P < 0.03).
Adherence to supplement intake
Before the training began, we instructed the subjects in the PLG and the DTG to consume the supplements immediately after daily IWT. If they did not perform IWT, we instructed them to consume the supplements the next day before breakfast. Adherence to the supplement intake regimen during IWT was 94.2 ± 2.5 and 90.9 ± 3.0% in the PLG and the DTG, respectively, with no significant difference between the groups (P > 0.39).
Dietary intake
Without the supplements, all subjects in both groups (n = 30) consumed 1763 ± 310 kcal with diet, including 247 ± 46 g of carbohydrate, 66 ± 14 g of protein (animal protein:textured vegetable protein = 55:45), 52 ± 12 g of fat, and 3609 ± 780 mg of sodium per day at the measurement in October. No significant differences were observed between the groups (P > 0.10), and the measurements remained unchanged in March in both groups, which met the recommended dietary allowances (RDA) for active, older Japanese women, except for the relatively high sodium intake: total calories, 1500–1750 kcal; carbohydrate, 231–358 g; protein, > 50 g; fat, 41–73 g; and sodium, < 2800 mg per day [19].
Physical characteristics and fitness
The BMI, FEXT, and FFLX values prior to initiating training in the present study (Tables 1, 2) were similar between the groups. These values were also similar to those previously reported in age-matched female Japanese populations [11, 20, 21], whereas was slightly higher in this study population than previously published populations, likely because the subjects had performed IWT for more than 6 months prior to participating in the present study. Thus, the physical characteristics in the present study reflected those of this age group in the Japanese population.
After training, body weight, BMI, %fat, and SBP (systolic blood pressure) significantly increased from the baseline in both groups (P < 0.02), but no significant differences were observed in the increases between the groups (P > 0.3). Additionally, blood glucose, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and hemoglobin A1c levels before training and the changes after training were similar between the groups (P = 0.10–0.90).
As shown in Table 2, before training, thigh muscle cross-sectional areas were similar between the groups. After training, these areas significantly increased from the baseline in both groups (P < 0.04) except for M. hamstrings in the PLG; however, no significant differences were observed in the increases between the groups (P > 0.1). On the other hand, no significant increases were observed in FEXT, FFLX, or after training in either group, with no significant differences in the increases between the groups.
DNA methylation by pyrosequencing
As shown in Table 5, before training, methylation of the NFKB2 promoter region at each CpG site was similar between the groups. After training, the %methylation in NFKB2 significantly increased at sites 2, 3, 4, and 5 in the DTG, while it increased at only sites 4 and 5 in the PLG, with a significant increase in the average across CpG sites 1–6 in the DTG but not in the PLG (Table 5). Because the methylation of promoter regions is associated with the transcriptional suppression of the corresponding gene, enhanced methylation in NFKB2 in the DTG suggests reduced NFKB2 gene expression [22, 23].
Discussion
The major finding of the present study was that dried tofu supplementation during 5 months of home-based IWT in older women who had performed habitual training prior to this study marginally enhanced the increases in NFKB2 gene methylation compared with placebo supplementation, without detectably enhanced increases in thigh muscle strength or cross-sectional area.
As shown in Tables 2 and 5, the methylation of the NFKB2 gene increased as the thigh muscle area increased after intervention in DTG and PLG. A close relationship between muscle atrophy with aging and chronic systemic inflammation has been suggested in large-scale population-based studies [2–5]. Also, a previous cross-sectional study reported that older men who exercise exhibited increased muscle strength and decreased NF-κB activity compared with older inactive men [24]. In the present study, we confirmed the relationship between NFKB2 gene methylation and thigh muscle mass.
Regarding the mechanisms underlying the hypo-methylation of pro-inflammatory genes with muscle atrophy in aging, previous studies have suggested that deteriorated mitochondrial function due to muscle atrophy generates reactive oxygen species (ROS), injures cells and tissue, and induces chronic inflammatory responses, resulting in lifestyle-related diseases [1, 25]. NF-κB2 protein, which we focused on in the present study, is a member of the NF-κB family and is a well-known transcriptional regulator that plays a central role in inflammation through its ability to induce pro-inflammatory cytokine gene transcription [18]. For example, NF-κB mediates the synthesis of cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8 [18]. Because the NF-κB2-dependent pathway is one of the key NF-κB signaling pathways, hyper-methylation of the NFKB2 gene promoter region may elicit decreases in its protein expression, suppressing this pathway and resulting in decreased NF-κB activity and pro-inflammatory cytokine inhibition [26]. Therefore, the hyper-methylation of the NFKB2 gene after intervention in PLG suggests that IWT alone suppressed inflammatory responses to prevent lifestyle-related diseases, and soy protein supplementation during the training in DTG enhanced this suppressive effect.
However, more importantly, increases in the methylation of the NFK2 gene, thigh muscle mass and thigh muscle strength were not significantly higher in DTG than in PLG, in contrast to the results of previous studies examining the effects of milk protein intake by a similar protocol as used in the present study [13, 14]. Okazaki et al. [13] suggested that the hamstring muscle tissue mass significantly increased by ~ 3% and isometric knee flexion force increased by ~ 16% in the milk protein intake group while neither significantly increased in the control group, and Masuki et al. [14] suggested that the methylation of the NFKB2 gene increased by ~ 44% with an increase in thigh muscle strength by ~ 8% in the milk product intake group while neither increased in the control group.
The precise reasons for this discrepancy remain unknown; however, Tang et al. [17] compared the effects of whey (10 g), casein (10 g), and soy (10 g) proteins intake immediately after resistance training on the profiles of essential amino acids, insulin, and leucine concentrations in the blood for the following 180 min. The reason for focusing on the profile of the leucine concentration in the blood was that this amino acid was suggested to enhance protein synthesis in the muscle [17]. As a result, they suggested that the concentrations were higher in the order of whey, soy, and casein proteins. In addition, the mixed muscle protein fractional synthesis rate, which was determined by a primed constant infusion of stable isotope (L-[ring-13C6] phenylalanine) uptake by active muscle tissue, was higher in the order of whey, soy, and casein proteins. These results suggest that the smaller increasing rate of essential amino acids in the blood, including leucine, after the supplement intake causes smaller increases in thigh muscle strength and the cross-sectional area and thereby decreased enhancement of NFKB2 gene methylation.
Alternatively, the increases in thigh muscle mass and strength by IWT might have not reached steady state before starting the present study. Indeed, the thigh muscle mass increased, even in PLG, with 37% more walking days/week and 25% more fast walking time/week than those before starting the present intervention. Thus, the effects of increased IWT achievement might be too strong to detect the mere effects of soy protein intake on thigh muscle mass and NFKB2 gene methylation in DTG. Nevertheless, after the intervention, we observed significant increases in hamstring muscle mass as well as rectus femoris muscle mass and hyper-methylation at more sites of NFKB2 in DTG than in PLG. These results suggest that soy protein intake enhanced the effects of IWT alone.
There are three experimental considerations that deserve additional discussion. First, as seen in Table 1, body weight, BMI, %fat, and SBP increased after the intervention in PLG and DTG. These effects were likely caused by the seasonal change from October 2014 to April 2015 during which time the mechanisms for adaptation to lower atmospheric temperature, including increased food intake, were likely contributing factors [27]. Although we did not observe any significant changes in dietary intake during the period, this might be due to the limited time resolution of the dietary intake measurements. Second, in the present study, we did not measure inflammatory cytokine levels because in a pilot study we observed that the cytokine levels were below the lowest levels guaranteed by the commercially available assays. Third, we did not observe any increases in FEXT and FFLX, despite the increase in rectus femoris muscle mass in PLG and DTG and the increase in hamstring muscle mass in DTG. Although the detailed reasons for this finding remain unknown, the technical limitation of detecting the expected difference by the increase in the muscle mass might be a contributing factor.
In conclusion, dried tofu supplementation during IWT might enhance the methylation of the NFKB2 gene, without detectably enhanced increases in thigh muscle strength or the cross-sectional area of thigh muscles in older women.
Author contributions
MM, NM, HM, SM, and HN conceived and designed the research; MM and SN performed experiments; MM, SN, SM, and HN analyzed data; MM, SN, SM, and HN interpreted the results of the experiments; MM, SM, and HN prepared the figure; MM, SM, and HN drafted the manuscript; MM, SM, and HN edited and revised the manuscript; MM, SN, NM, HM, SM, and HN approved the final version of the manuscript.
Funding
This study was supported by a grant from the Society of Nagano Kori-tofu Companies in 2014.
Compliance with ethical standards
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Handschin C, Spiegelman BM. The role of exercise and pgc1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526 e529-517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 3.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and c-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.M709. [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health abc study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.M326. [DOI] [PubMed] [Google Scholar]
- 6.American College of Sports Medicine . Exercise prescription. In: Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s guidelines for exercise testing and prescription. 8. Baltimore: Lippincott Williams & Wilkins; 2010. pp. 151–271. [Google Scholar]
- 7.McArdle WD, Katch FI, Katch VL. Sports and exercise nutrition. Baltimore: Lippincott Williams & Wilkins; 1999. Nutrient bioenergetics in exercise and training; pp. 106–180. [Google Scholar]
- 8.Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82:803–811. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki T, Gen-No H, Kamijo Y, Okazaki K, Masuki S, Nose H. A new device to estimate VO2 during incline walking by accelerometry and barometry. Med Sci Sports Exerc. 2009;41:2213–2219. doi: 10.1249/MSS.0b013e3181a9c452. [DOI] [PubMed] [Google Scholar]
- 10.Nose H, Morikawa M, Yamazaki T, Nemoto K, Okazaki K, Masuki S, Kamijo Y, Gen-no H. Beyond epidemiology: field studies and the physiology laboratory as the whole world. J Physiol. 2009;587:5569–5575. doi: 10.1113/jphysiol.2009.179499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morikawa M, Okazaki K, Masuki S, Kamijo Y, Yamazaki T, Gen-no H, Nose H. Physical fitness and indices of lifestyle-related diseases before and after interval walking training in middle-aged and older males and females. Br J Sports Med. 2011;45:216–224. doi: 10.1136/bjsm.2009.064816. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Hashimoto S, Fujii C, Hida S, Ito K, Matsumura T, Sakaizawa T, Morikawa M, Masuki S, Nose H, Higuchi K, Nakajima K, Taniguchi S. NFκB2 gene as a novel candidate that epigenetically responds to interval walking training. Int J Sports Med. 2015;36:769–775. doi: 10.1055/s-0035-1547221. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki K, Yazawa D, Goto M, Kamijo YI, Furihata M, Gen-no H, Hamada K, Nose H. Effects of macronutrient intake on thigh muscle mass during home-based walking training in middle-aged and older women. Scand J Med Sci Sports. 2013;23:e286–e292. doi: 10.1111/sms.12076. [DOI] [PubMed] [Google Scholar]
- 14.Masuki S, Nishida K, Hashimoto S, Morikawa M, Takasugi S, Nagata M, Taniguchi S, Rokutan K, Nose H. Effects of milk product intake on thigh muscle strength and NFKB gene methylation during home-based interval walking training in older women: a randomized, controlled pilot study. PLoS One. 2017;12:e0176757. doi: 10.1371/journal.pone.0176757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Education, Culture, Sports, Science and Technology of Japan . Standard tables of food composition in Japan 2015 [in Japanese] Tokyo: Official Gazette co-operation of Japan; 2015. Amino acid tables; pp. 1–341. [Google Scholar]
- 16.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mtor signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 1985. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 18.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health, Labor and Welfare of Japan (2010) Recommended dietary allowances. In: Dietary reference intakes for Japanese 2010 (1st ed) [in Japanese]. Tokyo, Japan: Daiichi Shuppan, pp 1–306
- 20.Laboratory of Physical Fitness Standards, Tokyo Metropolitan University (2007) In: New physical fitness standards of Japanese people (2nd ed) [in Japanese]. Fumaido Shuppan, Tokyo, pp 1–421
- 21.Niizeki T, Takeishi Y, Takabatake N, Shibata Y, Konta T, Kato T, Kawata S, Kubota I. Circulating levels of heart-type fatty acid-binding protein in a general Japanese population: effects of age, gender, and physiologic characteristics. Circ J. 2007;71:1452–1457. doi: 10.1253/circj.71.1452. [DOI] [PubMed] [Google Scholar]
- 22.Ching T, Song MA, Tiirikainen M, Molnar J, Berry M, Towner D, Garmire LX. Genome-wide hypermethylation coupled with promoter hypomethylation in the chorioamniotic membranes of early onset pre-eclampsia. Mol Hum Reprod. 2014;20:885–904. doi: 10.1093/molehr/gau046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unterberg M, Kreuzer MJ, Schafer ST, Bazzi Z, Adamzik M, Rump K. NFKB1 promoter DNA from nt + 402 to nt + 99 is hypomethylated in different human immune cells. PLoS One. 2016;11:e0156702. doi: 10.1371/journal.pone.0156702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci. 2010;65:532–537. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimshony T, Zhang J, Keshet I, Bustin M, Cedar H. The role of DNA methylation in setting up chromatin structure during development. Nat Genet. 2003;34:187–192. doi: 10.1038/ng1158. [DOI] [PubMed] [Google Scholar]
- 27.Shahar DR, Froom P, Harari G, Yerushalmi N, Lubin F, Kristal-Boneh E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur J Clin Nutr. 1999;53:395–400. doi: 10.1038/sj.ejcn.1600761. [DOI] [PubMed] [Google Scholar]


