Abstract
We investigated the change in myokine expression related to hypertrophy (IL-4, IL-6, IL-10) and atrophy (TNF-α, NFκB, IL-1β) in middle-aged rats after resistance exercise with ladder climbing. 50- and 10-week-old male Wistar rats were randomly assigned to two groups: the sedentary and exercise groups. The exercise groups underwent a ladder-climbing exercise for 8 weeks. While the tibialis anterior muscle mass in the young group significantly increased after the ladder-climbing exercise, the middle-aged group did not show any changes after undergoing the same exercise. To understand the molecular mechanism causing this difference, we analyzed the change in hypertrophy- and atrophy-related myokine levels from the tibialis anterior muscle. After 8 weeks of ladder-climbing exercise, the IL-4 and IL-10 protein levels did not change. However, the IL-6 level significantly increased after exercise training, but the amount of increase in the young training group was higher than in the middle-aged training group. IL-1β and TNF-α as well as NFκB protein levels were significantly higher in the middle-aged group than in the young group. Except for TNF-α, exercise training did not affect IL-1β and NFκB protein levels. The TNF-α level significantly decreased in the middle-aged exercise training group. AMPK and PGC-1α levels also significantly increased after exercise training, but there was no difference between age-related groups. Therefore, 8-week high-intensity exercise training using ladder climbing downregulates the skeletal muscle production of myokine involved in atrophy and upregulates hypertrophic myokine. However, the extent of these responses was lower in the middle-aged than young group.
Keywords: Ladder-climbing exercise, Atrophy, Hypertrophy, Myokine, Aging
Introduction
Human muscle mass decreases by 0.5 to 1 % per year after 30 years of age, and this reduction significantly increases after 65 years of age, which eventually can cause sarcopenia [28]. It is important to prevent the development of sarcopenia in the elderly because it can limit physical activity and be a direct cause of chronic diseases as well [16, 33]. Although it has not been clearly shown how aging causes the loss of muscle mass, recent studies have shown that there might be a link between decreased functionality of the immune system and loss of muscle mass in the elderly. It is well known that the high level of inflammatory cytokines such as TNF-α and IL-6 appearing in middle-aged people is highly relevant to the muscle mass loss and decreased muscle strength [30, 35]. It has been reported that proinflammatory cytokines can increase protein degradation in skeletal muscle in both in vitro and rodent models [9, 12].
The antiinflammatory effects of exercise training have recently attracted attention, and recent studies have shown that exercise training has a decreasing effect on intraabdominal fat mass and Toll-like receptor (TLR) expression of monocytes and macrophages related to antiinflammatory effects; it also increases the production and secretion of antiinflammatory cytokine from muscle contractions during movement [8]. It is reported that myostatin, which is the myokine secreted by contracting muscle, such as LIF, IL-4, IL-6, IL-7, IL-15 and so on, is involved in the control of the hypertrophy and myogenesis of muscle, and BDNF and IL-6 facilitate fat oxidation by AMPK; IL-8 affects angiogenesis by exercise training [2, 14, 29, 36]. Also, IL-4 and IL-10, which are known to have antiinflammatory effects, are reported to increase myogenesis [6, 15]. As a result, the fact that muscles produce and release myokines provides a molecular basis for understanding how physical activity could protect and improves the human body against premature mortality. The finding that exercise has multiple beneficial effects, which may be mediated by myokines, suggests that the improvement of muscle mass through exercise training is due to the interaction of various myokines.
Most recent studies have investigated the effects of extreme or short-term exercise training on secretion patterns of cytokines; the most common exercise type was endurance exercise [10, 11, 20, 26]. To increase the secretion of cytokines, such as IL-6, long-term or high-intensity exercise is required [7]. It is necessary to find an effective exercise training method that can help maintain or improve the skeletal muscle function of middle-aged people for whom it is impossible to exercise for long times because of their busy lives. We still do not have much information about the effect of exercise training on muscle atrophy/hypertrophy with increasing age. Therefore, the purpose of this study was to investigate the effect of ladder-climbing exercises on young versus middle-aged rats considering inflammatory cytokine expression related to atrophy/hypertrophy.
Methods
Experimental animals
50- and 10-week-old male Wistar rats were purchased and randomly divided into the sedentary group and exercise group after a 1-week adaptation period. The diet included carbohydrates (64.5 %), fat (11.8 %) and protein (23.5 %), vitamins (22 g/kg Teklad vitamin mix no. 40,077), minerals (51 g/kg Teklad mineral mix no. 170,915), methionine (5 g/kg, Teklad Premier no. 10,850) and choline chloride (1.3 g/kg) [13]. All of the animals had free access to water and food. During the test period, body weight and food consumption were measured every other day using an electronic balance (Mettler PJ6, Germany). All animals were maintained at temperatures ranging from 20 to 25 °C, with a 12-h light/dark cycle, and the humidity was maintained at approximately 60 %. We obtained the approval of the Animal Experiment Ethics Committee of Keimyung University School of Medicine (KM-2012-30R). The number of rats in each group was as follows:
Young sedentary group (YS, 10 weeks old, n = 10)
Young exercise group (YE, n = 10)
Middle-aged sedentary group (MS, 50 weeks old, n = 10)
Middle-aged exercise group (ME, n = 10)
Ladder-climbing exercise
We modified the ladder-climbing exercise training program presented by Kreamer et al. [19]. The rats in the YE and ME groups were subjected to one training period per day every third day for 8 weeks. Training was accomplished utilizing a 1-m ladder with 2-cm grid steps and inclined at 75°. Initially, rats were familiarized with the ladder by practicing climbing it from the bottom to top for 3 days. A cylinder containing weights was attached to the base of the tail with foam tape (3 M Conan) and a Velcro strap. The initial weight attached to each animal was 30–50 % of its body weight. Rats were positioned at the bottom of the climbing apparatus and motivated to climb the ladder by touching the tail. When the rats reached the top of the ladder, they were allowed to rest in a simulated home cage for 2 min. After the rest period, additional weights were placed in the cylinder, and the rats were returned to the bottom of the ladder for subsequent climbs. Rats climbed the ladder with 50, 75, 90 and 100 % maximal load from the previous exercise session. This procedure was repeated until eight climbs were achieved or until the rat failed to climb the entire length of the ladder. The training session was stopped when the rat refused to climb up the ladder after three successive shocks to the tail (Fig. 1).
Fig. 1.

Ladder-climbing exercise
Sample collection
In order to remove the last-bout exercise effect, rats took a rest for 48 h after 8 weeks of exercise and were fasted for 12 h before they were anesthetized using ketamine (80 mg/kg of body mass) and xylazine (12 mg/kg of body mass). The tibialis anterior muscles were dissected rapidly and free of fat and connective tissue. Muscle tissues were weighed and clamp frozen, then stored at −80 °C until analysis. The quadriceps muscle, on the other hand, was kept in a 10 % formalin solution at fixed room temperature for 2 days for histological analysis before being analyzed according to the protocol.
Sample preparation
Tibialis anterior muscle was homogenized with an ice-cold buffer [w/250 mM sucrose, 10 mM HEPES/1 mM EDTA (pH 7.4), 1 mM Pefabloc (Roche), 1 mM EDTA, 1 mM NaF, 1 g/ml aprotinin, 1 g/ml leupeptin, 1 g/ml pepstatin, 0.1 mM bpV (phen), 2 mg/ml glycerophosphate]. The homogenized samples underwent a repeated freeze/thaw process three times, and the supernatant was separated by centrifuge (700×g, 10 min). Samples were stored at −80 °C until analysis.
H&E staining and measuring
The quadriceps muscle that was fixed in 10 % formalin solution for 2 days was cut in cross section for observation, and the vertical section embedding was conducted with paraffin to produce paraffin blocks. This block was cut at 4 μm thickness and attached on a slide glass. Samples were observed with hematoxylin and eosin (H&E) staining, which was used with a deparaffinization and hydration process using xylene and alcohol. A virtual file was produced from these slides using Scanscope XT (Aperio, CA92081) from Aperio Technologies, while the average value of the cross-sectional area of the muscle fiber was analyzed using the Imagescope program (Aperio Technologies, version 10.2.2.2319).
Western blotting
The method of Lowry et al. [23] was used for protein assay. The samples were dissolved in Laemmli buffer, and electrophoresis was performed in SDS-polyacrylamide gel. In the immunoblotting using an antibody, we measured IL-6 (Santa Cruz Bio, sc-1265), IL-4 (Santa Cruz Bio, sc-53084), IL-1β (Santa Cruz Bio, sc-365858), TNF-α (Santa Cruz Bio, sc-1350), NFκB (Santa Cruz Bio, sc-109), PGC-1α (abcam, ab106814), phospho-AMPKα1/2 (Santa Cruz Bio, sc-33524), AMPKα1/2 (Santa Cruz Bio, sc-25792) and β-actin (Santa Cruz, sc-47778), which were visualized using ECL after an antibody treatment and quantified by densitometry (SigmaPlot 8.0 system).
Statistical analysis
SPSS 10.0 was used to calculate means and standard errors (mean ± SE) and for all of the analysis. Two-way ANOVA was employed to test the difference between treatments by age. Post-hoc analyses were done using the Tukey method. Statistical significance was set at α = 0.05.
Results
Body weight and food consumption changes
While the weight of the young group was gradually increased from 177.7 ± 4.4 to 461.2 ± 12.5 g, the middle-aged group showed a minimal increase in body weight from 619.9 ± 21.4 to 681.4 ± 26.1 g during the 8-week experimental period. However, there was no statistical difference between the exercise and sedentary group in either the young or middle-aged group (Fig. 2). This means that the resistance training for 8 weeks using ladder climbing did not significantly affect changes in body weight. Daily food consumption was not different between the exercise and sedentary group in either the young or middle-aged group (Fig. 3).
Fig. 2.
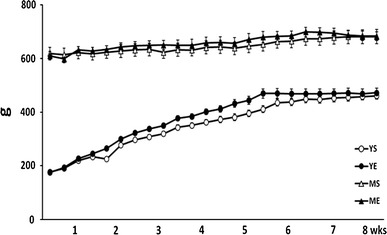
Body weight changes throughout the training period between groups (mean ± SE)
Fig. 3.
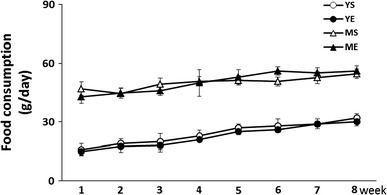
Food consumption changes throughout the training period between groups (mean ± SE)
Tibialis anterior muscle mass changes compared to body weight
To analyze the effect of the ladder-climbing exercise, we quantified the change in tibialis anterior muscle mass. The YE group showed statistically increased muscle mass compared to the YS group (P < 0.05), while there was no statistical difference in muscle mass between the middle-aged groups (Fig. 4). Therefore, this result suggests that 8 weeks of ladder climbing exercise only helped the young group to gain muscle mass but not the middle-aged group.
Fig. 4.
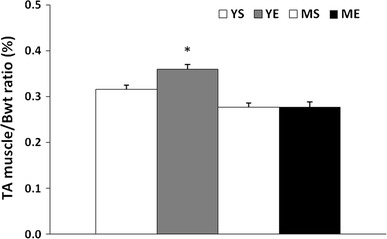
Changes in the tibialis anterior muscle mass to body weight ratio after 8 weeks of ladder-climbing exercise (mean ± SE). *Significantly different from the YS, MS and ME group (P < 0.05)
Diameter changes in quadriceps muscle fibers
We analyzed the change in muscle fibers by H&E staining. The fiber diameter of quadriceps muscle showed an approximately 20 % increase in the YE group compared to the YS group (P < 0.05). As expected, there was no statistically significant change between the middle-aged groups (Figs. 5, 6). Therefore, the ladder-climbing exercise did not help the middle-aged group to gain muscle mass along with increasing fiber diameter.
Fig. 5.
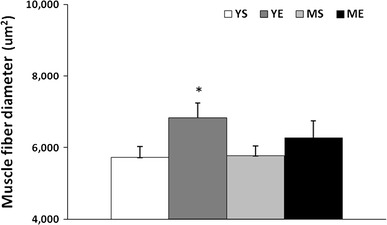
Quadriceps muscle fiber diameter changes after ladder-climbing exercise training for 8 weeks (mean ± SE). *Significantly different from the YS, MS and ME group (P < 0.05)
Fig. 6.
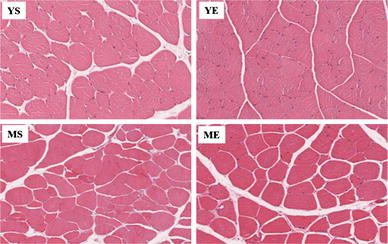
Hematoxylin and eosin-stained slides of representative YS, YE, MS and ME quadriceps muscles. Photographs were taken at ×200 magnification
Expression of myokine related to muscle atrophy
To determine the molecular mechanism behind our observation, we analyzed the molecular level of myokine related to the atrophy effect in tibialis anterior muscle. On the basal level, atrophic myokines such as TNF-α, IL-1β and NFκB protein were expressed two to threefold more in the middle-aged group than in the young group. After 8 weeks of training, there was no change in the level of NFκB and IL-1β in either the middle-aged or young group. Interestingly, the level of TNF-α was greatly decreased in the ME group, but this decreased level was still higher than in any of the young groups (Fig. 7). These results suggest that the basal level of atrophy-related myokine gradually increased throughout aging, and the 8-week exercise training in the middle-aged group did not affect NFκB and IL-1β expression levels except TNF-α, which showed a significant decrease but still a higher level than in the young group.
Fig. 7.
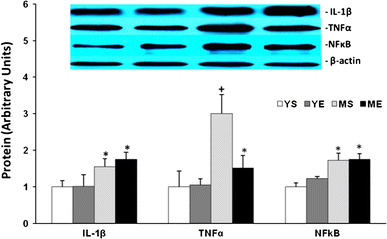
The IL-1β, TNFα and NFκB protein level changes in tibialis anterior muscle after ladder-climbing exercise for 8 weeks (mean ± SE). *Significantly different from YS and YE (P < 0.05). +Significantly different from YS, YE and ME (P < 0.05)
Expression of myokine related to muscle hypertrophy
We investigated the change in myokine (IL-4, IL-6, IL-10) related to hypertrophy effects. Eight weeks of ladder-climbing exercise significantly increased IL-6 protein levels in the muscles of both the young and middle-aged groups. The IL-6 protein level was increased by almost two times after exercise training in the young group, but in the middle-aged group, the IL-6 level was increased approximately 1.5 times. Eight weeks of training did not affect the level of IL-4 or IL-10 in any of the groups. Of note, the basal level of IL-4 was clearly significantly lower in the middle-aged groups (Fig. 8). Thus, these data show the muscle hypertrophy-related myokine response to exercise training was reduced with aging.
Fig. 8.
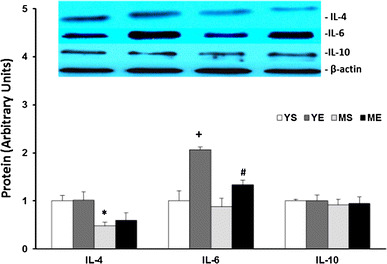
IL-4, IL-6 and IL-10 protein level changes in the tibialis anterior muscle after 8 weeks of ladder-climbing exercise (mean ± SE). *Significantly different from YS and YE (P < 0.05). +Significantly different from YS, MS and ME (P < 0.05). #Significantly different from YS and MS (P < 0.05)
pAMPK/AMPK ratio and PGC-1α expression
When we analyzed the activity by calculating the phospho-AMPK protein ratio to the total AMPK, both exercising groups showed a significant increase compared to the sedentary group. However, the AMPK activity increased by about 100 % in the younger group and only 60 % in the middle-aged group. This showed that AMPK activity gradually decreased with aging (Fig. 9). The protein expression of PGC-1α was significantly increased after 8 weeks of ladder-climbing exercise, but there was no difference between the young and middle-aged groups (Fig. 10).
Fig. 9.
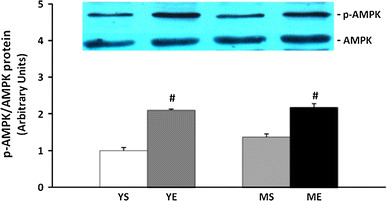
p-AMPK/AMPK protein ratio changes in tibialis anterior muscle after 8 weeks of treatment (mean ± SE). #Significantly different from YS and MS (P < 0.05)
Fig. 10.
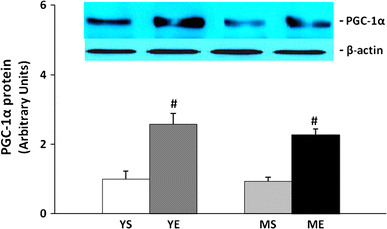
PGC-1α protein level changes in tibialis anterior muscle after 8-week treatment (mean ± SE). #Significantly different from YS and MS (P < 0.05)
Discussion
IL-6 has been considered a proinflammatory cytokine, present in the blood and muscle, secreted in high levels from the immune cells. However, it was recently discovered that both muscle contraction and IL-6 in muscle cells produced by exercise stimulation have an antiinflammatory effect similar to growth factors. The exercise-induced IL-6 in skeletal muscle stimulates the production of IL-1 receptor antagonist (IL-1ra) and IL-10 [31], while also reducing TNF-α synthesis [34]. TNF-α has been reported to promote loss of muscle protein by activation of the oxidative stress signaling pathway via NFκB activation, which is a redox-sensitive transcription factor. It also reduces muscle function without muscle mass change [22]. Comparable to a previous study, 8 weeks of high-intensity interval exercise training significantly increased the IL-6 level in muscle, while the TNF-α level was significantly decreased in the middle-aged group. However, in this study, muscle hypertrophy after 8-week exercise training was only observed in young rats.
Although the mechanism that can explain muscle hypertrophy or atrophy according to aging has not been confirmed yet [3], Della Gatta et al. [5] investigated the effect of exercise training on skeletal muscle cytokine expression in the elderly. After 12 weeks of exercise training, the expression of MCP-1, IL-8 and IL-6 increased significantly, but the expression of IL-4, IL-10 and IL-13 increased only slightly. These responses were not significantly different between young and elderly males. However, in the present investigation, the middle-aged rats did not show a similar hypertrophy pattern as the young rats even though IL-6 in skeletal muscle increased after 8 weeks of ladder-climbing exercise training. It can be supposed that the differences were due to various factors such as the limitation of the 8-week study period, exercise method and type, and so on. However, interestingly, the skeletal muscle of middle-aged rats showed high levels of IL-1β, TNF-α and NFκB before exercise compared to the young rats. Furthermore, the high-intensity interval training using 8 weeks of ladder climbing did not affect the levels of IL-1β and NFκB but of TNF-α. Previous studies reported that the muscle cells of elderly people appear to have more significant protein degradation than those of young people with TNF-α and NFκB treatment [21, 25]. Thus, high levels of TNF-α and NFκB inhibited muscle hypertrophy, even though IL-6 levels were significantly increased. Ludwig et al. [24] found that IL-6 increased through exercise could inhibit NFκB expression by activating HSP70, which can prevent muscle mass loss with aging. In trained young rats, IL-6 increased in response to a bout of downhill running, while TNF-α was unchanged in both sedentary and trained rats. It is possible that IL-6 may downregulate TNF-α secretion. However, the reduction of TNF-α in this study only occurred in the middle-aged group. This may be because the basal level of TNF-α in the young group was significantly lower than in the middle-aged group. Since it is difficult to detect the NFκB level in relation to exercise training in skeletal muscle, the interaction among these factors could not be clearly identified. Further studies should investigate the interaction among TNF-α, NFκB and IL-6.
According to a recent study, IL-6 and AMP-activated protein kinase (AMPK) are closely related, and AMPK, activated by exercise, plays an important role in energy metabolism, which increases both the glucose uptake in skeletal muscle and fat oxidation through increased glucose transporter 4 (GLUT4) translocation [4, 18]. IL-6 has been reported to further enhance AMPK activation in this case [17]. AMPK is the PGC-1α activation signaling factor, so the activated PGC-1α plays a role in the increase of mitochondrial biogenesis and angiogenesis, fiber type switching, fat oxidation in skeletal muscle and activation of muscle in atrophy prevention [1, 27, 32]. However, in this study, AMPK activity and PGC-1α protein expression significantly increased in both young rats and middle-aged rats after 8 weeks of ladder-climbing exercise, but there was no difference in age. In this respect, the difference in the results of this study compared to previous studies may be due to differences in the subjects’ ages and exercise training type. The middle-aged rats we used did not exhibit muscle atrophy in morphology compared to old rats, which may mean the metabolic response to exercise training may be different. Also, we used high-intensity interval training with a ladder, but most previous studies used endurance exercise. This difference may have affected the results. For clarification, an additional study is warranted.
In conclusion, 8-week high-intensity exercise training using ladder climbing downregulates the skeletal muscle production of myokine involved in atrophy and upregulates hypertrophic myokine. However, the extent of these responses was lower in the middle-aged than in the young group. These findings may be partly explained by the specific contribution of total lean body mass or muscle mass to healthy aging.
Compliance with Ethical Standards
Conflict of interest
None.
References
- 1.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bortoluzzi S, Scannapieco P, Cestaro A, Danieli GA, Schiaffino S. Computational reconstruction of the human skeletal muscle secretome. Proteins Mar. 2006;62:776–792. doi: 10.1002/prot.20803. [DOI] [PubMed] [Google Scholar]
- 3.Brooks NE, Schuenke MD, Hikida RS. No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J Physiol Sci. 2009;59(6):465–471. doi: 10.1007/s12576-009-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 5.Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun. 2014;39:80–86. doi: 10.1016/j.bbi.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 8.Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev. 2006;34:176–181. doi: 10.1249/01.jes.0000240027.22749.14. [DOI] [PubMed] [Google Scholar]
- 9.Frost RA, Nystrom GJ, Lang CH. Tumor necrosis factor-alpha decreases insulin-like growth factor-I messenger ribonucleic acid expression in C2C12 myoblasts via a Jun N-terminal kinase pathway. Endocrinology. 2003;144:1770–1779. doi: 10.1210/en.2002-220808. [DOI] [PubMed] [Google Scholar]
- 10.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/S0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 11.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Zaldivar F, Cooper DM, Adams GR. L-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 13.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen I. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 17.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer WJ, Flanagan SD, Volek JS, Nindl BC, Vingren JL, Dunn-Lewis C, Comstock BA, Hooper DR, Szivak TK, Looney DP, Maresh CM, Hymer WC. Resistance exercise induces region-specific adaptations in anterior pituitary gland structure and function in rats. J Appl Physiol. 2013;115:1641–1647. doi: 10.1152/japplphysiol.00687.2013. [DOI] [PubMed] [Google Scholar]
- 20.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lees SJ, Zwetsloot KA, Booth FW. Muscle precursor cells isolated from aged rats exhibit an increased tumor necrosis factor-alpha response. Aging Cell. 2009;8:26–35. doi: 10.1111/j.1474-9726.2008.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol. 2000;279:1165–1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 23.Lowry EC, Blumber JM, Rhea RL, Ranson JP. Serum levels of orally administered penicillin. US Armed Forces Med J. 1951;2:265–270. [PubMed] [Google Scholar]
- 24.Ludwig MS, Minguetti-Câmara VC, Heck TG, Scomazzon SP, Nunes PR, Bazotte RB, Homem de Bittencourt PI., Jr Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: an effect associated with increased IL-6 levels but not with IL-10 or TNF-α. Mol Cell Biochem. 2014;397:97–107. doi: 10.1007/s11010-014-2176-2. [DOI] [PubMed] [Google Scholar]
- 25.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Tuggle SC, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol. 2013;115:937–948. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nader GA, Dastmalchi M, Alexanderson H, Grundtman C, Gernapudi R, Esbjornsson M, Wang Z, Ronnelid J, Hoffman EP, Nagaraju K, Lundberg IE. A longitudinal, integrated, clinical, histological and mRNA profiling study of resistance exercise in myositis. Mol Med. 2010;16:455–464. doi: 10.2119/molmed.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagatomo F, Fujino H, Kondo H, Kouzaki M, Gu N, Takeda I, Tsuda K, Ishihara A. The effects of running exercise on oxidative capacity and PGC-1α mRNA levels in the soleus muscle of rats with metabolic syndrome. J Physiol Sci. 2012;62(2):105–114. doi: 10.1007/s12576-011-0188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N, van Hall G, Plomgaard P, Febbraio MA. Muscle-derived interleukin-6: lipolytic anti-inflammatory and immune regulatory effects. Pflug Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124:495–502. doi: 10.1016/S0047-6374(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 31.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 32.Popov DV, Bachinin AV, Lysenko EA, Miller TF, Vinogradova OL. Exercise-induced expression of peroxisome proliferator-activated receptor γ coactivator-1α isoforms in skeletal muscle of endurance-trained males. J Physiol Sci. 2014;64(5):317–323. doi: 10.1007/s12576-014-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 34.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 35.Visser M, Pluijm SM, Stel VS, Bosscher RJ, Deeg DJ. Physical activity as a determinant of change in mobility performance: the longitudinal aging study Amsterdam. J Am Geriatr Soc. 2002;50:1774–1781. doi: 10.1046/j.1532-5415.2002.50504.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoon JH, Yea K, Kim H, Choi YS, Park S, Lee H, Lee CS, Suh PG, Ryu SH. Comparative proteomic analysis of the insulin-induced L6 myotube secretome. Proteomic. 2009;9:51–60. doi: 10.1002/pmic.200800187. [DOI] [PubMed] [Google Scholar]


