Abstract
Maternal behavior has a substantial impact on the behavioral, endocrine, and neural development of the pups. This study investigated the effect of altering the neonatal nutritional environment by modifying the litter size on maternal care and anxiety- and fear-like behaviors in rats during adulthood. On postnatal day (PND) 2, litters were adjusted to a small litter (SL) size of three pups per dam or normal litter (NL) size of 12 pups per dam. Maternal behaviors were scored daily during lactation (PND2-21). The weight gain, food intake, adiposity, and biochemical landmarks of offspring rats were evaluated. On PND60, performances in the open field, elevated plus-maze (EPM), and fear conditioning test were measured. The reduction of the litter size enhanced maternal care in lactating rats, increasing the arched-back posture and licking pups. SL offspring exhibited accelerated weight gain, hyperphagia, increased visceral fat mass, dyslipidemia, and hyperleptinemia in adulthood. The SL offspring of both sexes showed an increase in the anti-thigmotactic effect in the open field, an intact anxious-phenotype in the EPM, and a decrease in the time spent freezing during the fear-conditioning test, compared to NL. The neonatal environment as determined by litter size plays a crucial role in programming the adult metabolic phenotype as well as behavioral responses to stressful stimuli, with an impact on anxiety-like and fear behaviors. These behavioral changes in offspring may be, at least in part, a result of increased maternal care.
Keywords: Anxiety, Lactation, Maternal behavior, Overnutrition
Introduction
Overnutrition during early postnatal life represents a risk factor for persistent obesity and associated metabolic and cardiovascular disturbances [1]. Artificially adjusted litter size and its consequences on the offspring during development and adulthood have been well studied, including the timing of the appearance of developmental landmarks, and the neurobehavioral development of pups [1–3]. It has been shown that animals raised in small litters (SL) show an accelerated body weight gain before weaning, which is associated with permanent modulation of adiposity and hypothalamic circuits that control food intake and energy balance in adulthood [2, 4–6]. Raising rats in SL reduces competition for milk during the suckling period and, therefore, leads to overnourishment because the total calorie intake for each pup is increased [7]. Increased milk intake in each individual pup in SL contributes, at least in part, to the alteration in metabolic phenotype [8–10]. However, the impact of changes in maternal care cannot be excluded [7].
Disturbances in the nutritional environment that alter the supply of nutrients from the mother to pups can induce structural and functional adaptations during postnatal development, with consequences for offspring growth and metabolism throughout life [1]. In fact, the perinatal environment and maternal care can have long-lasting effects on behavior and physiology [11]. Together with nutritional environment, maternal behavior has a substantial impact on the behavioral, endocrine, and neural development of pups [12].
Animal models provide strong evidence that perinatal nutrition has an enduring impact on numerous aspects of offspring physiology and behavior, including impairments in social behaviors [13, 14], decreased cognitive abilities [15], enhanced response to stress [1], and altered reward-based behaviors [16].
In the present investigation, we hypothesized that alterations in neonatal nutritional environment, induced by raising the rats in litters of different sizes, would alter maternal care and the behavioral features of the offspring in adulthood.
Materials and methods
Animals
Adult Wistar nulliparous female rats, 9 weeks of age, were obtained from the Central Animal Facility of the Federal University of Alfenas and were housed in a temperature-controlled room (22 °C), on a 12 h light–12 h dark cycle (lights on at 7:00 A.M.), with access to water and food ad libitum. In all experiments, the females were timed-mated by housing them with sexually experienced males. The presence of spermatozoa in the vaginal lavage on the following morning designated day 0 of pregnancy. Pregnant females were individually housed in transparent cages (42 cm × 34 cm × 16 cm). On the second day postpartum (PND2), certain litters were adjusted to a size of three pups (one female and two males; small litter — SL) to induce early postnatal overnutrition by reducing the competition for milk [1, 7, 8]. In the control group, the litter size was maintained at 12 pups per litter (male to female ratio 1:1; normal litter — NL) for normal postnatal nutrition as described previously [5, 6]. When necessary, there was a small adjustment using puppies born on the same days for the litters to be correctly culled in SL or NL groups. The animals were weaned on PND21, and the pups of each litter size were housed in groups of four rats per cage (separated by sex), with free access to pelleted food and tap water. To avoid the litter effect, one male pup and one female pup from each litter were marked with ink and used for body weight tracking, hormonal and biochemical analyses, and behavioral tests [14, 17].
Maternal studies
Maternal behavior
Maternal behavior in lactating females was scored daily in four 72-min observation sessions during 20 days of lactation (LD). The observations were performed at regular times, with three sessions taking place during the light phase (08:00 A.M., 12:00 P.M., and 04:00 P.M.) and one session during the dark phase (08:00 P.M.). Within each session, the behavior of each mother was scored every 3 min (25 observations per 4 periods per day for a total of 100 observations per mother per day). We identified five maternal and four non-maternal parameters, as follows: (1) licking pups (either its body surface or its anogenital region), (2) nursing pups in an arched-back posture, (3) “blanket” posture in which the mother lays over the pups, (4) passive posture in which the mother is lying either on her back or side while the pups nurse, (5) nest building, (6) eating, (7) exploring the cage, (8) non-explorative movement away from the pups, and (9) self-grooming [18, 19]. Data were reported as the percentage of observations in which the pups were subjected to a target behavior (number of observations in which the target behavior was recorded divided by the total number of observations × 100).
Performance of lactating rats in the elevated plus-maze and open field tests
On lactation day 6 (LD6), lactating rats with normal and small litters (n = 8 per group) and non-lactating virgin female rats in their diestrus phase (n = 8 animals) were subjected to behavioral assessment in the elevated plus-maze and open field tests.
The elevated plus-maze had two opposite open arms (50 cm in length and 10 cm in width) and two opposite closed arms (50 cm in length, 10 cm in width, and 40 cm in height), extending out from a central platform (10 × 10 cm). The whole apparatus was elevated 50 cm above the floor. Each rat was placed in the center of the maze facing one of the closed arms, and then allowed to explore the open or closed arms of the maze for 5 min. The number of entries and the time spent in the different arms was recorded. The presence of four paws inside the entrance line to the arm was used as a signal to start measuring the time interval spent in the specific arm, and the end time point was recorded when all four paws were outside the line again. The maze was cleaned with 5% ethanol after each test to prevent the influence of previously tested rats. For each day of the experiment, a different group of lactating rats was used.
Immediately after being tested in the EPM, the rats were placed in the open field arena for evaluation of their locomotor activity. In this test, each female rat was placed in the center of the open field that was novel to the animal. The open field apparatus consists of a circular arena with a diameter of 60 cm with walls of 45 cm in height, and a floor that is divided into 12 areas. A circle of 30 cm in diameter in the center was divided into four areas defined as the central areas, and the eight areas along the walls were considered as the peripheral area. The number of peripheral (adjacent to the walls) and central (away from the walls) squares that the rat entered with all four paws during a 5-min interval was recorded [20]. The anti-thigmotactic effect was defined as the proportion of entries into the central part of the open-field arena relative to the total number of entries [20]. The arena was carefully cleaned with 5% ethanol solution after every test. The behavioral sessions were video-recorded and analyzed by an experimenter blinded to the experimental condition.
Offspring studies
Biometrical analysis, food intake, adipose tissue, and biochemical analysis
The body weight of offspring was monitored every 3 days (starting on PND2) until weaning (PND21) and weekly thereafter, until the eighth week. The average 24-h food intake (g) was evaluated weekly between the fourth and the eighth week by carefully collecting and weighing the food remaining in the metabolic cage, and then subtracting this amount from the amount given to the rats. To verify whether overfeeding early in life could induce abnormal weight gain, body weight and nasoanal length were measured in rats on PND60, and the Lee index was calculated [21]. Another set of animals belonging to NL and SL groups was sacrificed at PND60, after 12 h of fasting, and trunk blood samples were collected and centrifuged for 15 min at 3000 rpm and 4 °C in order to separate the plasma. The samples were stored at − 20 °C until use. Plasma leptin and insulin levels were assayed using ELISA kits (Linco Research, St. Charles, MO, USA). Plasma glucose and lipids [total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides] were analyzed using enzymatic colorimetric assays (In vitro, SP, Brazil). The visceral fat mass (epididymal, ovarian, and retroperitoneal white adipose tissue) was excised and immediately weighed for evaluation of adiposity.
Offspring behavioral tests
All behavioral tests were performed at PND60. The open field and elevated plus-maze test were performed as described for lactating rats. For the evaluation of fear conditioning in adult offspring, 16 male and 16 female rats were used. These were randomly selected from 16 different litters (2 pups per litter, 1 pup to non-conditioned group and 1 pup to conditioned group) of SL and NL dams. Habituation, conditioning, and testing were performed in 25 cm × 22 cm × 22 cm foot shock chambers. The chambers had a grid floor composed of 18 stainless steel rods (2 mm in diameter), spaced 1.5 cm apart, and wired to a shock generator. The chambers were cleaned with 5% ethanol after each animal. In the conditioning shock session performed 24 h after the habituation session, the animals were separated into two experimental groups: non-conditioned and conditioned. The non-conditioned group (n = 8 animals) was exposed to the foot shock chamber for 10 min but no shock was delivered. The conditioned group (n = 8 animals) was submitted to a shock session consisting of six electric 1.5 mA/3 s foot shocks delivered at 20-s to 1-min intervals. The behavioral response (freezing) evoked by conditioned emotional responses to the context was evaluated 1 day after the conditioning session. The test session consisted of a 10-min re-exposure to the foot shock chamber without shock delivery [18].
Data analysis
Data were analyzed using the GraphPad software program version 6.0 and are expressed as the mean ± SEM. A two-way analysis of variance (ANOVA) with repeated measures and Tukey’s post hoc test were used where appropriate. Performances of lactating rats with normal or small litters and non-lactating rats on elevated plus-maze and open field apparatus were analyzed by one-way ANOVA, followed by Tukey’s post hoc test. To assess the effects of litter size on offspring behavior tests, data were analyzed with Student t-tests. A p value of less than 0.05 (p < 0.05) was used to establish significance.
Results
Maternal behavior
Two-way ANOVA showed that the lactating rats with SL exhibit an increase in total maternal behavior (Fig. 1a), including an increase in arched nursing (Fig. 1b) and licking pups (Fig. 1c) during lactation. The parameters blanket-nursing (Fig. 1d), passive-nursing (Fig. 1e), and nest building (Fig. 1f), changed during lactation, but were not influenced by litter size (statistical data: Table 1). Regarding non-maternal behavioral parameters, the reduction in litter size and lactating rats with SL were associated with a reduction in total non-maternal behavior (Fig. 1g), including a decrease in eating (Fig. 1h), non-explorative (Fig. 1i), and explorative behavior (Fig. 1j) during lactation. The parameter self-grooming (Fig. 1k) changed during lactation, but was not influenced by litter size (statistical data: Table 1).
Fig. 1.
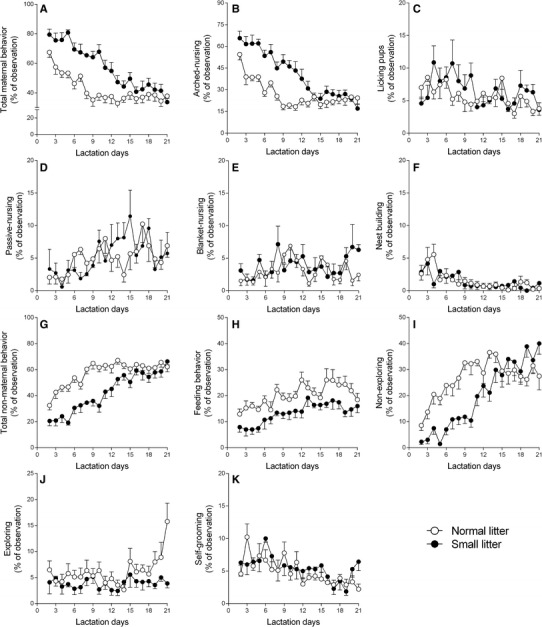
Composite maternal and non-maternal behavior of lactating rats after litter adjustment to 12 pups per nest (normal litters, n = 8) or 3 pups per nest (small litters, n = 8) on PND2. Data are plotted as the mean ± SEM percentage of episodes across 100 observations per day. a Percentage of total maternal behavior; b arched-nursing; c licking pups; d passive-nursing; e blanket nursing; f nest building; g percentage of total non-maternal behavior; h feeding behavior; i non-exploring; j exploring; k self-grooming
Table 1.
Two-way ANOVA results for all analyses relevant to maternal and non-maternal behavior with litter size (normal or small) and lactation day as main factors
| Parameters | Litter size (LS) factor | Lactation day (LD) factor | LS × LD interaction |
|---|---|---|---|
| Total maternal behavior | F1,280 = 133.6; p < 0.001 | F19,280 = 15.9; p < 0.001 | F19,280 = 2.80; p < 0.001 |
| Arched nursing | F1,280 = 129.1; p < 0.001 | F19,280 = 19.5; p < 0.001 | F19,280 = 4.47; p < 0.001 |
| Licking pups | F1,280 = 213.7; p < 0.001 | F19,280 = 2.07; p < 0.001 | F19,280 = 1.69; p < 0.001 |
| Blanket-nursing | F1,280 = 3.31; p = 0.07 | F19,280 = 1.76; p < 0.05 | F19,280 = 1.17; p = 0.28 |
| Passive-nursing | F1,280 = 0.05; p = 0.81 | F19,280 = 2.21; p < 0.001 | F19,280 = 0.99; p = 0.47 |
| Nest building | F1,280 = 0.12; p = 0.73 | F19,280 = 3.55; p < 0.001 | F19,280 = 1.23; p = 0.23 |
| Total non-maternal behavior | F1,280 = 137.9; p < 0.001 | F19,280 = 16.4; p < 0.001 | F19,280 = 2.88; p < 0.001 |
| Feeding | F1,280 = 67.4; p < 0.001 | F19,280 = 4.21; p < 0.001 | F19,280 = 0.52; p = 0.94 |
| Non-exploring | F1,280 = 38.0; p < 0.001 | F19,280 = 12.7; p < 0.001 | F19,280 = 4.39; p < 0.001 |
| Exploring | F1,280 = 14.1, p < 0.001 | F19,280 = 0.94; p = 0.51 | F19,280 = 0.48; p = 0.97 |
| Self-grooming | F1,280 = 2.85; p = 0.09 | F19,280 = 2.69; p < 0.001 | F19,280 = 0.85; p = 0.65 |
F F value, p p value
Performance of lactating rats in elevated plus-maze and open field tests
The evaluation of animals in the elevated plus-maze test showed an increase in % time [Fig. 2a; F(2,21) = 4.36; p < 0.05] and entries [Fig. 2b; F(2,21) = 5.78; p < 0.01] into the open arms in lactating rats compared to non-lactating rats, but a similar total number of entries into the arms [Fig. 2c; F(2,21) = 1.41; p > 0.05]. There was no significant difference between lactating rats with normal litters and those with small litters. We observed a significant increase in the number of central entries in the open field test [Fig. 2d; F(2,21) = 5.78; p < 0.01] in lactating rats with small litters compared with non-lactating or lactating rats with normal litters, as well as an increase in the ratio of central/total entries [Fig. 2d; F(2,21) = 16.33; p < 0.01] in lactating rats compared with non-lactating rats.
Fig. 2.
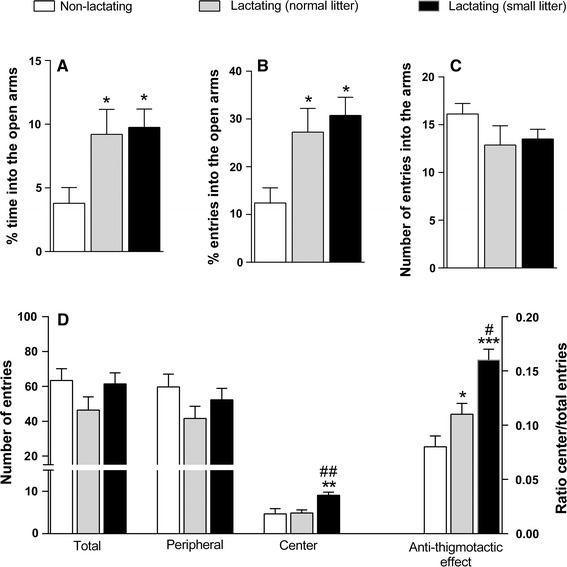
Performances of lactating rats with normal or small litters and non-lactating rats on elevated plus-maze (a % time into the open arms; b % entries into the open arms, and c number of entries into the arms) and open field apparatus (d number of entries into the center and periphery, total entries, and ratio center/total entries). Data are presented as the mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001, compared to non-lactating rats. #p < 0.05, p < 0.01 compared to lactating (normal litter) rats
Biometric analysis, food intake, adipose tissue and biochemical analysis of offspring
The pup body weights were measured during lactation (Fig. 3a), and a two-way repeated-measures ANOVA indicated that there were significant main effects for the litter size [F(1,98) = 304.9; p < 0.001] and time [F(6,98) = 23.4; p < 0.001] and a significant interaction between the litter size and time [F(6,98) = 443.5; p < 0.001]. After weaning (Fig. 3b), both male and female offspring from SL had a higher weight compared to control animals (p > 0.001). These results indicated that the weight gain during lactation was increased in SL offspring compared to NL offspring, and this difference remained until adulthood. The patterns of food intake post weaning in male and female offspring are shown in Fig. 3c. A significant increase in food intake was observed in both male and female SL offspring compared to NL offspring.
Fig. 3.
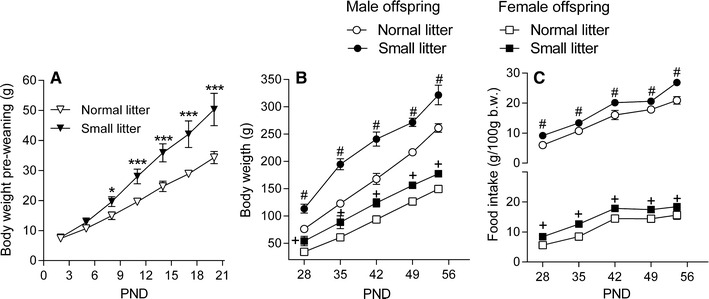
Effect of the litter size on body weight prior to the weaning of pups at PND2–21 (a), body weight post-weaning at PND28–56 (b); and food intake at PND28–56 (c). Data are presented as the mean ± SEM. *p < 0.05; ***p < 0.001 compared to normal litter; #p < 0.05 compared to male offspring from normal litter; +p < 0.05 compared to female offspring from normal litter
Table 2 shows that litter size manipulation altered the Lee index, adiposity, and blood biomarkers in offspring at PND60. SL offspring presented an increase in Lee index (male: p < 0.001; female: p < 0.01), higher retroperitoneal (male: p < 0.001; female: p < 0.001), epididymal (male: p < 0.001), and ovarian fat mass (female: p < 0.001) compared to NL offspring. Female and male offspring of the SL group presented higher fasting glycemia (male: p < 0.01; female: p < 0.01), plasma triglycerides (male: p < 0.05; female: p < 0.05), total cholesterol (male: p < 0.05; female: p < 0.05), and leptin levels (male: p < 0.01; female: p < 0.001), and reduction in HDL level (male: p < 0.001; female: p < 0.01). Fasting plasma levels of insulin were higher in overfed female SL offspring (p > 0.05) compared to NL, but no differences were observed in overfed male offspring.
Table 2.
Biometrical analysis, adipose tissue, adipose depot mass, and biochemical and hormonal determinations in adult male and female rats raised in small and normal litters (PND60, n = 8/group)
| Litter size | ||||
|---|---|---|---|---|
| Normal litter | Small litter | |||
| Male | Female | Male | Female | |
| Biometrical analysis | ||||
| Body weight | 261.3 ± 2.81 | 149.5 ± 2.29 | 321.6 ± 6.36*** | 177.5 ± 1.77*** |
| Lee index | 3.06 ± 0.01 | 2.84 ± 0.04 | 3.27 ± 0.01*** | 3.00 ± 0.02** |
| Adipose tissue (g/100 g b.w.) | ||||
| Retroperitoneal fat | 0.61 ± 0.02 | 0.90 ± 0.06 | 1.07 ± 0.05*** | 1.58 ± 0.17*** |
| Epididymal fat | 0.72 ± 0.01 | 0.97 ± 0.03*** | ||
| Ovarian fat | 1.04 ± 0.07 | 2.06 ± 0.19*** | ||
| Biochemical and hormonal determinations | ||||
| Glucose (mg/dl) | 105.00 ± 0.93 | 97.87 ± 2.40 | 118.00 ± 2.73** | 113.20 ± 4.07** |
| Triglycerides (mg/dl) | 135.70 ± 11.70 | 73.21 ± 11.55 | 169.80 ± 10.61* | 109.30 ± 10.24* |
| Total cholesterol (mg/dl) | 70.93 ± 3.71 | 70.88 ± 3.40 | 85.37 ± 4.12* | 89.48 ± 7.84* |
| HDL-C (mg/dl) | 4.38 ± 0.14 | 4.67 ± 0.29 | 3.32 ± 0.10*** | 3.69 ± 0.15** |
| Insulin (ng/ml) | 0.52 ± 0.04 | 0.29 ± 0.01 | 0.56 ± 0.07 | 0.41 ± 0.04* |
| Leptin (ng/dl) | 0.69 ± 0.10 | 0.70 ± 0.06 | 1.92 ± 0.25** | 1.67 ± 0.05*** |
Values are the mean ± SEM (Student t-test)
*p < 0.05, **p < 0.01; ***p < 0.001 compared with the normal litter
Performance of offspring in the behavioral tests
The analysis of the percentage of the time spent in the open arms, in percentage of open arm entries, and in the total number of arm entries by male (Fig. 4a–c) and female (Fig. 4e–g) offspring revealed no differences between NL and SL groups. However, in the open field test, both male (Fig. 4d) and female (Fig. 4h) SL offspring showed an increase in central entries (male: p < 0.01; female: p < 0.05) and in the ratio of central/total entries (male: p < 0.01; female: p < 0.01) compared to NL offspring. The litter reduction did not affect the total number of crosses in the open field test, indicating that there was no change in locomotion in these animals, either female or male.
Fig. 4.
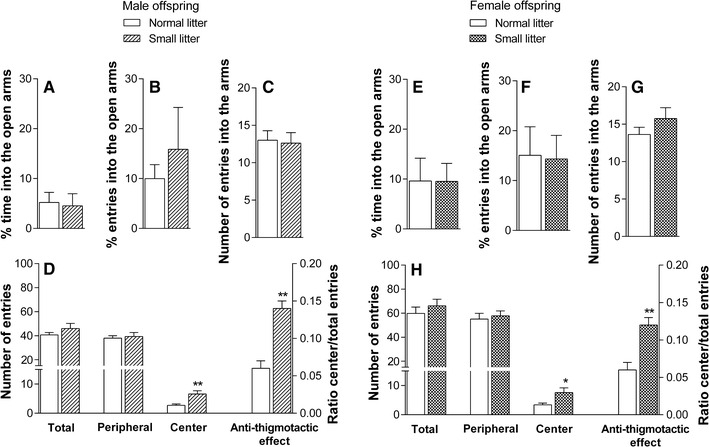
Performances of male (a–d) and female (e–h) offspring from normal versus small litters on elevated plus-maze (% time into the open arms, % entries into the open arms, and number of entries into the arms) and open field apparatus (number of entries into the center and periphery, total entries, and ratio center/total entries). Data are presented as the mean ± SEM. *p < 0.05; **p < 0.01 compared to normal litter
As expected, both male and female offspring that received foot shocks (conditioned group) spent more time freezing during re-exposure to the aversive context than the animals that did not receive a shock (non-conditioned group). Conditioned male rats from the SL group showed a shorter freezing time compared to male rats from the NL group [Fig. 5a: F(conditioning factor)1,28 = 35.3, p < 0.001; F(litter factor)1,28 = 1.63, p = 0.23; F(interaction conditioning × litter)1,28 = 6.97, p < 0.05]. Similarly, conditioned female rats from the SL group showed a shorter freezing time when compared with female rats from the NL group [Fig. 5b: F(conditioning factor)1,28 = 59.1, p < 0.001; F(litter factor)1,28 = 12.2, p < 0.001; F(interaction conditioning × litter)1,28 = 19.6, p < 0.001].
Fig. 5.
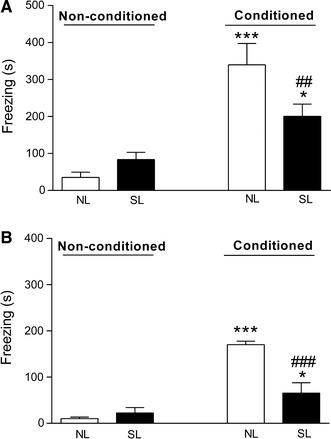
Duration of freezing response in the fear conditioning test by male (a) and female (b) offspring from normal litters (NL) and small litters (SL). Data are presented as the mean ± SEM. *p < 0.05; ***p < 0.001 compared to NL/non-conditioned group. ##p < 0.01; ###p < 0.001 compared to NL/conditioned group
Discussion
The alteration of the neonatal nutritional environment induced by modifications of litter size has important implications for long-term physiology and behavior. In the present study, we demonstrated that the changes in litter size not only alter maternal care, weight gain, and biochemical and metabolic landmarks in offspring, but also influence anxiety-like and fear behavior in adult rats.
In this study, the reduction of the litter size enhanced maternal care in lactating rats, increasing the time spent in arched-back posture and pup licking by mothers, and decreasing the time the mother spent away from the offspring. A potential explanation for increased maternal behavior by SL mothers compared to NL mothers was given by Priestnall [22] in a study using mice, in which mothers spent less time in the nest and licking the animals and more time eating, drinking, and exploring while taking care of larger litters. Larger litters require more nutrients from their progenitor [23], favoring behaviors such as feeding to assure enough milk to sustain them. In addition, the reduction in litter size promotes a reduction in anxiety-like parameters (increase in anti-thigmotactic effect in the open field test) in SL rats when compared to NL rats and non-lactating rats. Lactating dams not only display direct care-giving behaviors toward pups but also show anxiolytic-like responses in the conflict tests [24], open field paradigms [20], and elevated plus-maze test [25], and exhibit less fear following a sudden auditory stimulus [26]. These behavioral adaptations complement direct pup-caring to ensure the survival not only of the offspring but also of the mother herself. The reduced emotional responsiveness observed during lactation can be explained, at least in part, by enhanced activity by the oxytocin and prolactin systems in the brain, as these are associated with anxiolytic properties in male and female rats, especially during peripartum [20, 27]. Such events may be based on increased motivation of these animals to spend the energy and overcome the adversity to guarantee their own survival and that of their offspring [27], particularly in dams with SL.
The neonatal overnutrition resulting from the reduction in litter size leads to weight excess, hyperphagia, increased total visceral fat mass, dyslipidemia, and hyperleptinemia in adulthood. According to Šefčiková and colleagues [10] and Shankar and colleagues [28], the reduction in the number of pups alters the quantity and quality of milk, leading to a higher production of lipids, especially triglycerides; which increases the availability of nutrients to the pups during breast-feeding, and improves care for offspring. The postnatal overfeeding induced by litter size manipulation leads to early malprogramming of the hypothalamic system, inducing persistent central leptin and insulin resistance and an increase in orexigenic signals [3, 29–32]. The malprogramming and resistance of orexigenic as well as anorexigenic neurons in the hypothalamus might contribute to the occurrence of hyperphagia, overweight, and hyperinsulinemia throughout later life [1, 29]. Our study has shown a change in the lipid profile (a rise in total cholesterol and triglycerides plasma levels), higher accumulation of visceral fat, and an increase in the Lee index (obesity predictor index) in adult offspring from the SL group. In summary, our results corroborate that metabolic and endocrine dysfunctions in adulthood, such as metabolic syndrome, may have originated from the nutritional environment in early life.
The behavioral differences between the offspring from NL and SL groups were only observed under aversive conditions in behavioral tests but not under standard conditions. In the open field test, both male and female offspring from the SL group showed a reduced anxiety-like behavior compared to the offspring from the NL group, evidenced by increased exploration of central areas and a higher ratio in central/total entries (anti-thigmotactic effect). However, no differences were observed in the elevated plus-maze test, and the exploratory behavior was not altered, as demonstrated by a comparable number of total entries in the open field and in the elevated plus-maze test. In addition, the SL offspring of both sexes showed a decrease in the time spent freezing in the fear conditioning test compared to the NL group.
The findings reveal that gestation and the juvenile developmental periods may be early-life windows of vulnerability for developing anxiety in later life. Diet-induced obesity in animal models is produced by manipulations of macronutrient content, particularly with respect to fat and/or sugar. The timing of high-fat feeding in rats during the perinatal period appears critical for the neurodevelopment of offspring. Although exposure to a high-fat diet during gestation increases offspring anxiety [33], a high-fat diet during lactation is associated with reduced levels of anxiety in male offspring [34]. In addition, rat studies have noted that the offspring of perinatal high-fat-diet-exposed dams have an age-dependent anxiety-like phenotype. High-fat diet fed dams had reduced anxiety behaviors measured by the EPM and open field in adolescence [35] but higher levels of anxiety-like behavior as adults [36]. However, postnatal overfeeding by litter size reduction has been demonstrated to decrease anxiety-like behavior measured on the EPM as adults, but enhanced neuronal activation in PVN in response to acute restraint stress [37]. The reduced anxiety-like and fear behaviors observed in SL offspring may be due to a higher level of maternal attention received during the neonatal period. It has been reported that variations in maternal care affect the development of individual differences in neuroendocrine and behavioral responses to stressful stimuli in rats [38–42]. The magnitude of the hypothalamic–pituitary–adrenal (HPA) response to stress in adult animals was strongly correlated with maternal licking and grooming [38]. Tactile stimulation derived from maternal licking and grooming regulates pup physiology and affects central nervous system development. The variations in this form of maternal behavior among dams appear to be associated with the development of individual differences in neuroendocrine responses to stress [39, 43].
The development of neural circuits that regulate endocrine and behavioral responses to stress in rats is influenced by natural variations in maternal care [11]. Thus, the rats that received relatively high levels of maternal licking/grooming (LG) from their dams in infancy display lower levels of fear reactivity, as evidenced by decreased acoustic startle responses, increased exploration of a novel open field, and a decreased latency to start eating in a novel test chamber, compared with the offspring of the mother showing low levels of LG [38–40].
The concept of programming refers to the process by which the exposure to environmental stimuli or insults during critical periods of development leads to permanent changes in the physiology, including the metabolism of the organism, whose consequences are often observed much later in life [14, 44, 45]. In this investigation, we have therefore demonstrated that the neonatal nutritional environment determined by litter size can play a crucial role in programming the adult metabolic phenotype as well as behavioral responses to stressful stimuli, with an impact on anxiety-like and fear behaviors. We suggest that these behavioral changes in offspring may be due, in part, to alterations in maternal care.
Acknowledgements
We would like to thank José Reis for his technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; #APQ-0041-15), Conselho Nacional de desenvolvimento Científico e Tecnológico (CNPq; #300977/2013-1) and Coordenação de Aperfeiçoamento de pessoal de nível superior (CAPES; PNPD and Pró-equipamentos).
Compliance with ethical standards
Ethical approval
All experiments were conducted according to the Declaration of Helsinki regulations addressing the welfare of experimental animals and were approved by the Ethics Committee of the Federal University of Alfenas #445/2012).
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- 1.Habbout A, Li N, Rochette L, Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J Nutr. 2013;143:553–562. doi: 10.3945/jn.112.172825. [DOI] [PubMed] [Google Scholar]
- 2.Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dorner G. Increased number of galanin-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Brain Res. 1999;818:160–163. doi: 10.1016/S0006-8993(98)01264-5. [DOI] [PubMed] [Google Scholar]
- 3.Davidowa H, Li Y, Plagemann A. Altered responses to orexigenic (AGRP, MCH) and anorexigenic (alpha-MSH, CART) neuropeptides of paraventricular hypothalamic neurons in early postnatally overfed rats. Eur J Neurosci. 2003;18:613–621. doi: 10.1046/j.1460-9568.2003.02789.x. [DOI] [PubMed] [Google Scholar]
- 4.Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146–155. doi: 10.1016/S0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- 5.Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am J Physiol Endocrinol Metab. 2005;288:E1236–E1243. doi: 10.1152/ajpendo.00505.2004. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 7.Fiorotto ML, Burrin DG, Perez M, Reeds PJ. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol. 1991;260:R1104–R1113. doi: 10.1152/ajpcell.1991.260.5.C1104. [DOI] [PubMed] [Google Scholar]
- 8.Cunha AC, Pereira RO, Pereira MJ, Soares VM, Martins MR, Teixeira MT, Souza EP, Moura AS. Long-term effects of overfeeding during lactation on insulin secretion—the role of GLUT-2. J Nutr Biochem. 2009;20:435–442. doi: 10.1016/j.jnutbio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Moreira AS, Teixeira MT, Osso FS, Pereira RO, Silva-Junior GO, de Souza EPG, Lacerda CAM, Moura AS. Left ventricular hypertrophy induced by overnutrition early in life. Nutr Metab Cardiovasc Dis. 2009;19:805–810. doi: 10.1016/j.numecd.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Šefčiková Z, Bujnakova D, Racek L, Kmet V, Mozes S. Developmental changes in gut microbiota and enzyme activity predict obesity risk in rats arising from reduced nests. Physiol Res. 2011;60:337–646. doi: 10.33549/physiolres.931939. [DOI] [PubMed] [Google Scholar]
- 11.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 12.Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 13.Raygada M, Cho E, Hilakivi-Clarke L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings’ aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. J Nutr. 1998;28:2505–2511. doi: 10.1093/jn/128.12.2505. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho AL, Ferri BG, de Sousa FA, Vilela FC, Giusti-Paiva A. Early life overnutrition induced by litter size manipulation decreases social play behavior in adolescent male rats. Int J Dev Neurosci. 2016;53:75–82. doi: 10.1016/j.ijdevneu.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Bi Y, Ma W, He L, Yuan L, Feng J, Xiao R. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. Int J Dev Neurosci. 2010;28:271–276. doi: 10.1016/j.ijdevneu.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology. 2008;97:83–94. doi: 10.1007/s00213-007-1008-4. [DOI] [PubMed] [Google Scholar]
- 17.Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013;22(14):37. doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa HHV, Vilela FC, Giusti-Paiva A. Continuous central infusion of cannabinoid receptor agonist WIN 55,212-2 decreases maternal care in lactating rats: consequences for fear conditioning in adulthood males. Behav Brain Res. 2013;257:31–38. doi: 10.1016/j.bbr.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Uriarte N, Breigeiron MK, Benetti F, Rosa XF, Lucion AB. Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev Psychobiol. 2007;49:451–462. doi: 10.1002/dev.20241. [DOI] [PubMed] [Google Scholar]
- 20.Vilela FC, Melo CM, Giusti-Paiva A. Glucocorticoids impair maternal anxiolysis during lactation. Neurosci Lett. 2012;509:121–124. doi: 10.1016/j.neulet.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 21.Simson EL, Gold RM. The Lee obesity index vindicated? Physiol Behav. 1982;29:371–376. doi: 10.1016/0031-9384(82)90028-2. [DOI] [PubMed] [Google Scholar]
- 22.Priestnall R. Effects of litter size on the behaviour of lactating female mice (Mus musculus) Anim Behav. 1972;20:386–394. doi: 10.1016/S0003-3472(72)80063-0. [DOI] [Google Scholar]
- 23.Smith BW, McManus JJ. The effects of litter size on the bioenergetics and water requirements of lactating Mus musculus. Comp Biochem Physiol A Comp Physiol. 1975;51:111–115. doi: 10.1016/0300-9629(75)90422-3. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira A, Hansen S, Nielsen M, Archer T, Minor BG. Behavior of mother rats in conflict tests sensitive to antianxiety agents. Behav Neurosci. 1989;103:193–203. doi: 10.1037/0735-7044.103.1.193. [DOI] [PubMed] [Google Scholar]
- 25.Lonstein JS, Simmons DA, Stern JM. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav Neurosci. 1998;112:1502–1518. doi: 10.1037/0735-7044.112.6.1502. [DOI] [PubMed] [Google Scholar]
- 26.Hard E, Hansen S. Reduced fearfulness in the lactating rat. Physiol Behav. 1985;35:641–643. doi: 10.1016/0031-9384(85)90155-6. [DOI] [PubMed] [Google Scholar]
- 27.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;6:858–886. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 28.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:28–38. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- 29.Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65(Suppl 3):83–89. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- 30.Conceição EP, Franco JG, Oliveira E, Resende AC, Amaral TA, Peixoto-Silva N, Passos MC, Moura EG, Lisboa PC. Oxidative stress programming in a rat model of postnatal early overnutrition—role of insulin resistance. J Nutr Biochem. 2013;24:81–87. doi: 10.1016/j.jnutbio.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. NeuroReport. 2000;11(12):2795–2798. doi: 10.1097/00001756-200008210-00037. [DOI] [PubMed] [Google Scholar]
- 32.Davidowa H, Plagemann A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. NeuroReport. 2007;18(5):521–524. doi: 10.1097/WNR.0b013e32805dfb93. [DOI] [PubMed] [Google Scholar]
- 33.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24(6):2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 34.Wright T, Langley-Evans SC, Voigt JP. The impact of maternal cafeteria diet on anxiety-related behaviour and exploration in the offspring. Physiol Behav. 2011;103(2):164–172. doi: 10.1016/j.physbeh.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan PO. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience. 2014;272:92–101. doi: 10.1016/j.neuroscience.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience. 2013;240:1–12. doi: 10.1016/j.neuroscience.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Spencer SJ, Tilbrook A. Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology. 2009;34(8):1133–1143. doi: 10.1016/j.psyneuen.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 39.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis DD. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 41.Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 42.Menard JL, Champagne DL, Meaney MJP. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience. 2004;129:297–308. doi: 10.1016/j.neuroscience.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira VP, Cervilha DA, Cabral LD, Oliveira LM, Incerpi EK, Novaes RD, Ionta M, Soncini R. Postnatal overnutrition in mice leads to impaired pulmonary mechanics in response to salbutamol. J Physiol Sci. 2016;66:221–228. doi: 10.1007/s12576-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. J Anat. 2009;215:36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


