Abstract
Although child abuse has become a serious social problem in most countries, the neural mechanisms by which it induces adulthood mental disorders is not yet fully understood. Mice exposed to early-life stresses, such as maternal deprivation (MD) during lactation, are a good model for studying the effects of neglect of humans in early life. Early-life stress induces structural/functional changes of neurons in the hippocampus, prefrontal cortex, and amygdala, and causes mental disorders in adulthood. In this study, we found motor coordination dysfunction in male MD mice. We also found that the expression levels of the aminomethylphosphonic acid receptor subunits GluA1 and GluA3 were high in the cerebellum of male MD mice. The basal activity of the cerebellum detected by field-potential analysis was higher in male MD mice than in male control mice. Caloric stimulation increased the activity of the cerebellum of control mice, but it did not significantly increase the activity of the cerebellum in male MD mice. We concluded that early-life stress induced a functional change in the cerebellum of MD mice and that this change induced motor coordination dysfunctions.
Keywords: Early-life stress, Cerebellum, In vivo microdialysis, AMPA receptor, Carolic stimulation
Introduction
Early-life stress during the perinatal period, such as maternal deprivation (MD), induces functional and structural disorders of the brain through multiple pathways. Stress-induced alteration of neuronal activity affects several steps of neurological development, such as dendrite arborization, synaptogenesis, and spine formation [1–3]. Early-life stress and its associated changes in several hormonal states induce structural alterations, which in turn induce disorders of brain functions throughout life [4–6]. However, the mechanisms underlying the functional/structural changes induced by early-life stress have not yet been fully clarified [5].
Currently, animal models are frequently used to study the mechanisms underlying early-life stress-induced deficiencies of neuronal development [6, 7]. Models of early-life stress show various neuropsychological disorders in adulthood, such as the worsening of anxiety-related behaviors [6, 8, 9]. The synaptic components in the infralimbic cortex and somatosensory cortex (SSC) are altered in these animals [10–12]. In the SSC of early-life stressed mice, the stability of the mushroom spine is decreased [12] and the amount of glutamate (Glu) released is high [13]. The threshold of somatosensory sensation is low in early-life stressed mice, [12], and this low threshold correlates with the cortical field potential in the SSC [13] (high cortical field potentials are detected in early-life stressed mice, which show a low threshold for nociceptive stimulation). Note that these changes are detected not only in the juvenile stage but also in adulthood [12]. We also found an increase in the motility of microglia in SSC [14]. However, further study is necessary to clarify the mechanisms underlying these neuropsychiatric changes in adulthood induced by exposure to early-life stress.
Recently, it has been reported that the cerebellum is also involved in early-life stress-induced disorders, such as post-traumatic stress disorder (PTSD), and some studies have shown altered functioning of the cerebellum in PTSD patients [15–18]. Early-life stressed animal models also show structural/molecular changes of the cerebellum [19, 20]. However, functional changes of the cerebellum have not been well studied in an early-life stressed model. Because the cerebellum is a good model for studying neuronal circuits, it is useful to study the effects of early-life stress on the neuronal circuits using certain procedures based on the changes induced in the cerebellum.
In this study, we found that the motor coordination function was affected in early-life stressed mice. We also found that the basal activity of the cerebellum increased in MD mice. Because of this increase, caloric stimulation did not induce any significant change in the activity of the cerebellum in MD mice. Based on these results, we propose a new approach to studying the nature of early-life stress and its effect on brain function.
Materials and methods
Animals
Adult male mice derived from wild-type C57BL/6 J mice (age 2–2.5 months; purchased from SLC Japan, Hamamatsu, Japan) were used in this study. All mice were housed with food and water provided ad libitum under controlled temperature (25 ± 5 °C), humidity, and illumination conditions (12:12 light:dark cycle; lights on at 7:00 a.m.). Cages were cleaned once a week.
MD mice were used as an early-life stressed model in this study [12]. MD mice were prepared by separating them for 3 h (usually from 9:00 a.m. to 12:00 a.m.) every day from their mothers from postnatal day (P) 2 to P14. The separated MD mice were individually placed in a locally made incubator under regulated humidity (50–70%) and temperature (37 °C) conditions. There is a possibility that the separation induces a nutritional disorder that affects the anatomical structure of synapses [21]. However, the body weight was not significantly different between the control and MD mice examined at several stages of development [12].
In this study, all experiments were performed using males at 2–2.5 months of age (Control group: n = 35 mice from 13 dams: MD group: n = 37 mice from 15 dams). The number of pups in a litter was kept to between five and eight; if the number of pups in a litter was less than four, the dams were not used in this study; if the number of pups in a litter was more than nine, pups were randomly sacrificed at P2. In experiment 1, 17 control mice and 17 MD mice were used for the rotarod test. In experiment 2, 18 control and 22 MD mice were used for in vivo microdialysis, Western blot analysis, and electrophysiological analysis without the rotarod test. Six control and five MD mice were used in in vivo microdialysis, six control and six MD mice were used in Western blot analysis, and six control and nine MD mice were used in electrophysiological analysis. Among the MD mice, two mice showed irregular spikes during the electrophysiological experiment and died after finishing the experiment; their data were excluded from the analysis.
Rotarod test
The accelerating rotarod test (model LE8500; Panlab, Barcelona, Spain) was performed at 2–2.5 months of age to assess motor coordination [22] (17 control mice, 17 MD mice). Five rotarod test trials were performed, each for a maximum of 3 min. In each test, the speed increased from 4 to 40 rpm in the first 2 min and then remained at 40 rpm for 1 min, for a total of 3 min (if the mouse was still on the rotarod at the 3-min time point, we terminated the test). The duration of remaining on the rotarod in the last three trials was recorded, and average scores of these three tests were used for analysis.
Electrophysiological analysis
The field potential of the vermis of the cerebellum was recorded under anesthesia induced by urethane [intraperitoneal (i.p.) injection, 1.7 g/kg body weight] and atropine (i.p. injection, 0.4 mg/kg body weight), as previously reported [23]. As shown in Fig. 3a, a tungsten electrode (5 MΩ) was inserted into the right side of the vermis. After 300 s of basal recording, caloric stimulation (50 μl of ice-cold serine injected in the left ear canal) was carried out and the recording continues for another 300 s. Caloric stimulation induces electrophysiological changes in Purkinje cells in the cerebellum [24] and is a useful procedure for studying the function of the cochlea–cerebellum neuronal circuits. The epoch sequences were subjected to power spectrum analysis based on fast Fourier transform; then, the power (μV2) was obtained as the output. We divided the spectrum into δ (0.5–4 Hz), θ (4–8 Hz), α (8–14 Hz), and β (14–29.5 Hz) waves.
Fig. 3.
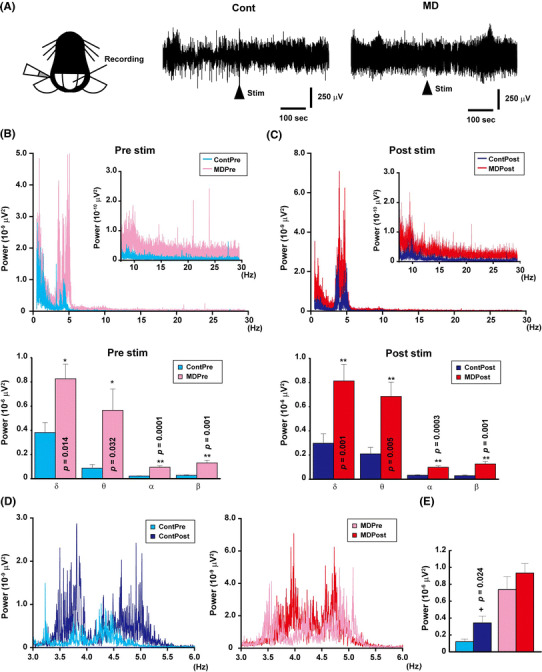
The basal activity of the cerebellum increased in MD mice. a Schematic figure of caloric stimulation and data. Cold buffer was injected after recording the basal activity of the cerebellum for 300 s. b Graph showing the results of fast Fourier transform (FFT) prior (Pre) to the injection (upper panel) and a summary of all waves (lower panel). The basal activity of the cerebellum was higher in MD mice than in control mice. The graph shows the averages of 6 control and 7 MD mice. c Graph showing the results of FFT following (Post) the injection of the buffer (upper panel) and a summary of all waves (lower panel). Injection of cold water into the ear increased the activity of the cerebellum in both control and MD mice, but the activity was still higher in MD mice than in control mice. d Comparison between pre- (left) and post- (right) stimulation in both control and MD mice at 3–6 Hz. e An increase in power was particularly detected in the wave range of 3–6 Hz. In control mice, the power between 3.5 and 5.5 Hz significantly increased after caloric stimulation; in comparison, although an increase in power was also detected in MD mice, this increase was not statistically significant due to the higher basal activity of the cerebellum. Single asterisk indicates a significant difference at p < 0.05 by the paired t test, double asterisks indicate significant different at p < 0.01 by Student’s t test
In vivo microdialysis
A guide cannula was inserted into the cerebellum through the cranial window (diameter 0.5 mm) located 1.5–2 mm posterior to the lambda under uletan-induced anesthesia, as previously reported [23]. After inserting the microdialysis probe, dialysates were collected for 30 min under anesthesia. The concentrations of Glu, glutamine (Gln), glycine (Gly), and gamma-aminobutyric acid (GABA) were immediately measured by high-performance liquid chromatography (Eicon, Kyoto, Japan) [23].
Western blot analysis
The expression levels of glutamate receptors [aminomethylphosphonic acid (AMPA) and N-methyl-d-aspartame (NADA)] in the cerebellar vermis were measured by western blot analysis (per experiment: 6 control mice, 6 MD mice). Total protein was extracted from the brain tissues as described previously [23]. Cerebellar samples were collected before the rotarod test and after the last rotarod test. Immunoblot analysis was performed using specific antibodies [Cell Signaling Technology, Danvers, MA: GluA1 (Cat# 8850S RRID:AB10950223), 1:1000; GluA2 (Cat# 5306S RRID:AB10622024), 1:1000; GluA3 (Cat# 4676S RRID:AB10547136), 1:2000; GluN1 (Cat# 5704S RRID:AB1904067), 1:1000; GluN2A (Cat# 4205S RRID:AB2112295), 1:1000; and GluN2B (Cat# 4212S RRID:AB2112463), 1:1000] and anti-rabbit immunoglobulin G coupled to horseradish peroxidase (GE Healthcare, Little Chalfont, UK). Blots were reprobed with an anti-β-actin antibody (Cell Signaling Technology Cat# 4967L RRID:AB823414) to monitor protein quantity.
Statistical analysis
The treatment effect was compared between the control and MD mice by one- or two-way analysis of variance. Post hoc comparison was performed by Student’s t test, or paired t test using Excel statistics (Esumi, Tokyo, Japan). Differences were considered significant at p < 0.05. All values are presented as the mean ± standard error of the mean.
Results
Motor coordination dysfunction was induced in MD mice
To evaluate the effects of MD on motor coordination, we first performed the rotarod test. As shown in Fig. 1, the average duration of control mice (n = 17) remaining on the rotarod was 80.0 ± 9.7 s compared to 53.4 ± 5.9 s for the MD mice (n = 17). This difference was significantly different (Fig. 1 by Student’s t test (t (32) = 2.33, p = 0.026). This result indicates that the motor coordination function in the mice was affected by early-life stress.
Fig. 1.
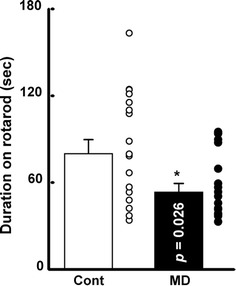
Motor coordination function was affected in early-life stressed mice. The duration of remaining on the rotarod was also shorter in the maternal deprivation (MD) mice. Bars Mean scores of last three rotarod tests with standard deviation of the mean (SEM). Asterisk indicates significant difference between control group (Cont) and MD group at p < 0.05 by Student’s t test
We next observed the expression levels of Glu receptors in the cerebellar vermis because we previously reported a change in the expression level of the Glu receptor subunit in MD mice [13]. As shown in Fig. 2a, in MD mice the expression levels of GluA1 and GluA3 increased, but that of GluA2 did not increase significantly. Similarly to GluA2, the expression levels of GluN1, GluN2A, and GluN2B also did not significantly increased in MD mice.
Fig. 2.
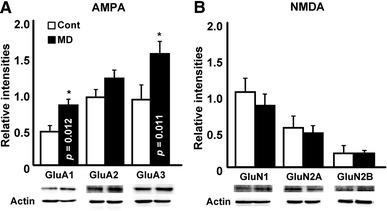
Expression level of the aminomethylphosphonic acid (AMPA) receptor increased in the cerebellum of MD mice. a Graph showing relative intensities of western blot analysis using antibodies to AMPA receptor subunits. The expression levels of glutamate (Glu)A1 and GluA3 significantly increased in MD mice. b Graph showing relative intensities of western blot analysis using antibodies to N-methyl-d-aspartate (NMDA) receptor subunits. The expression levels of NMDA receptor subunits did not significantly change in MD mice. Bars Mean ± SEM
In MD mice, the concentration of Glu increases in the SSC [13] and this increase is detectable even under anesthesia [14]. If the concentration of Glu also increases in the cerebellum of MD mice, this increase could induce the alteration in motor coordination function. Thus, we next determined the concentrations of neurotransmitters in the cerebellum by in vivo microdialysis under anesthesia. In the cerebellum, however, the concentration of Glu did not significantly increase in MD mice as compared with that in the control mice (Table 1). Moreover, the concentrations of Gln, Gly, and GABA did not change in MD mice as compared with those in control mice.
Table 1.
Concentrations of neurotransmitters in cerebellum
| Study group | Glutamate (nM) | Glutaminen (μM) | Glycine (nM) | Gamma-aminobutyric acid (nM) |
|---|---|---|---|---|
| Control (n = 6) | 595.1 ± 90.4 | 55.3 ± 19.9 | 786.7 ± 95.7 | 281.3 ± 82.7 |
| MD (n = 5) | 553.5 ± 53.1 | 48.0 ± 7.6 | 753.7 ± 36.8 | 273.9 ± 36.6 |
Data in table are presented as the mean ± standard error of the mean
MD Maternal deprivation mice
Neuronal function of cerebellum was altered in MD mice
To confirm the neuronal circuits that correlate with the motor coordination function of the cerebellum, we performed caloric stimulation because the increase in expression level of the AMPA receptor subunit could potentially have induced the functional change to the synapses in the cerebellum which affected the fine-tuning of behavior. As shown in Fig. 3a, the frequency of the field potential appears to increase in the control mice (n = 6) following caloric stimulation. In comparison, the frequency of the field potential seems to be high in MD mice even under basal conditions (n = 7), with the powers of the δ, θ, α, and β waves all significantly increasing in this group (Student’s t test) during both pre- and post-stimulation (Figs. 3b, c). Caloric stimulation significantly increased the powers at frequencies from 3.5 to 5.5 Hz in the control group (by paired t-test, t (5) = 3.21, p = 0.024; Figs. 3d, e). However, because of the high basal power in the MD group, a similar increase was not statistically significant (t (6) = 0.85, p = 0.43). These results indicate that the vestibular–cerebellar neuronal circuits were affected by early-life stress and that these effects induced alterations in motor coordination function.
These findings indicate that early-life stress potentially increased both the basal activity of the cerebellum and the expression levels of GluA1 and GluA3. These changes terminated the fine tuning by the cerebellum, resulting in the motor coordination dysfunction in MD mice.
Discussion
In this study, we found a mild change in the motor coordination of MD mice, as detected by the rotarod test (Fig. 1). We also found increases in the basal activity of the cerebellum (Fig. 3) and in the expression levels of AMPA receptor subunits (Fig. 2). These findings indicate that early-life stress may affect fine tuning by the cerebellum.
Motor coordination function and early-life stress
Dysfunction of the motor coordination function induced by the cerebellum is observed in several animal disease models, such as those of hypothyroidism [22, 25, 26], knockout of Glu transporters [27], and autism spectrum dysfunctions [28]. In our study, the motor coordination dysfunction was less severe in our MD mice than has been observed in other previously reported disease models. The MD mice in our study appeared to remain longer on the rotarod seems than did mice with hypothyroidism in previous studies [22, 26].
A decrease in the volume of the cerebellum and in connectivity between the cerebellum and other brain regions has been reported in human PTSD patients [29, 30]. It has also been reported that this reduction in volume of cerebellum is associated with the magnitude of PTSD symptoms [16]. To our knowledge, observation of the function of the cerebellum is not a medical marker for patients who have been exposed to early-life stress. In contrast, it is said that the function which relates to the cerebellum, such as saccade adaptation, is good medical marker for the diagnosis of autism spectrum disorders (ASDs) [31]. Although the dysfunction of the cerebellum in the MD mice in our study is modest compared with the level(s) of dysfunction in other disease models, our results indicate that observation of the function of the cerebellum may be a good medical marker for early-life stress, such as ASDs. Further study is needed to identify a good task related to the function of cerebellum for patients who have been exposed to early-life stress. For this purpose, the caloric test could be good candidate for observe the function of cerebellum (Fig. 3).
As shown in Fig. 1, the values for both the control and MD mice are bimodal, and two distinct clusters seem to exist. There is the possibility that the environmental conditions during lactating and after weaning induced this bimodality in behavior. However, we did not find any significant relationship between the values and the dam, season, number of litters, and breeding space. Further study is necessary to confirm the roles of these phenomena.
Glu concentration in the cerebellum of MD mice
We previously reported that the amount of Glu released by MD mice increases in the somatosensory cortex, which potentially induces synaptic instability and hyper-function of the neuronal circuits [7, 13]. If this Glu release is taken into consideration, the condition could be the same as that of the mice with knockout of the Glu transporter [27]. However, in our study, the concentration of Glu did not increase in the cerebellum in MD mice, as determined by in vivo microdialysis (Table 1). In the cerebellum, synaptically released Glu is rapidly taken up by Glu transporters, which can collect fivefold the Glu released from the presynaptic membrane in the cerebellum [32, 33]. Thus, if the amount of Glu released in MD mice is fivefold smaller than that in control mice, the effect of Glu is potentially masked by the function of Glu transporters. In our study, however, cerebellar function was partially affected, and the expression level of the AMPA receptor changed in MD mice (Figs. 1, 2). To identify the change induced in the MD mouse cerebellum, establishment of in vivo experimental methods is necessary. For example, an in vivo microdialysis probe may be utilized for not only collecting samples but also administering drugs [23].
We found that the expression levels of GluA1 and GluA3 were increased in MD mice cerebellum (Fig. 2). These subunits are widely expressed in both neurons (Purkinje cells, granule cells) and glial cells (Bergmann glia, basket cells, Golgi cells, among others) [34]. Because of the change observed in the electrophysiological experiment, we conclude that at least the condition of the Purkinje cells (and its synapses) was affected by MD and that the change was potentially induced by the increase in expression levels of the AMPA receptor subunit. Further study on the expression level of these subunits in neurons and glia is necessary.
Conclusion
Early-life stress induced mild motor coordination dysfunction via the increase in the basal activity of the cerebellum and the overexpression of the GluA1 and GluA3 subunits, which prevented fine tuning by the cerebellum.
Acknowledgements
We thank Professor Noriyuki Koibuchi for the chance to perform the study.
Author contribution
M.K. and S.T. performed the western blot study and supported behavior study; I.A. supported all experiments and supervised the manuscript; Y.T. designed and performed most of the experiments and analysis, and drafted the manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (17H05919) from Japan Society for the Promotion of Science (JSPS), Gunma University Tenure-Track Program, and Hiroshi and Aya Irisawa Memorial Promotion Award for Young Physiologists to YT.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
This study was performed in accordance with the guidelines and protocols approved by the Animal Care and Experimentation Committee, Gunma University, based on the EU Directive 2010/63/EU on the protection of animals used for scientific purposes. All efforts were made to minimize the suffering and number of animals used.
References
- 1.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 3.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershon A, Sudheimer K, Tirouvanziam R, Williams LM, O’Hara R. The long-term impact of early adversity on late-life psychiatric disorders. Curr Psychiatry Rep. 2013;15:352. doi: 10.1007/s11920-013-0352-9. [DOI] [PubMed] [Google Scholar]
- 5.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann NY Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 6.Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007;182:180–192. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takatsuru Y, Koibuchi N. Alteration of somatosensory response in adulthood by early life stress. Front Mol Neurosci. 2015;8:15. doi: 10.3389/fnmol.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/S0031-9384(98)00300-X. [DOI] [PubMed] [Google Scholar]
- 9.Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL. Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Res. 2004;1016:111–118. doi: 10.1016/j.brainres.2004.04.077. [DOI] [PubMed] [Google Scholar]
- 10.Ovtscharoff W, Jr, Braun K. Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience. 2001;104:33–40. doi: 10.1016/S0306-4522(01)00059-8. [DOI] [PubMed] [Google Scholar]
- 11.Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb Cortex. 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- 12.Takatsuru Y, Yoshitomo M, Nemoto T, Eto K, Nabekura J. Maternal separation decreases the stability of mushroom spines in adult mice somatosensory cortex. Brain Res. 2009;1294:45–51. doi: 10.1016/j.brainres.2009.07.092. [DOI] [PubMed] [Google Scholar]
- 13.Toya S, Takatsuru Y, Kokubo M, Amano I, Shimokawa N, Koibuchi N. Early-life-stress affects the homeostasis of glutamatergic synapses. Eur J Neurosci. 2014;40:3627–3634. doi: 10.1111/ejn.12728. [DOI] [PubMed] [Google Scholar]
- 14.Takatsuru Y, Nabekura J, Ishikawa T, Kohsaka J, Koibuchi N. Early-life stress increases the motility of microglia in adulthood. J Physiol Sci. 2015;65:187–194. doi: 10.1007/s12576-015-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/S0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 16.Baldaçara L, Jackowski AP, Schoedl A, Pupo M, Andreoli SB, Mello MF, Lacerda ALT, Mari JJ, Bressan RA. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J PsychiatryRes. 2011;45:1627–1633. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry. 2001;50:246–253. doi: 10.1016/S0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- 18.Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- 19.Wouters MM, Van Wanrooy S, Casteels C, Nemethova A, de Vries A, Van Oudenhove L, Van den Wijngaard RM, Van Laere K, Boeckxstaens G. Altered brain activation to colorectal distention in visceral hypersensitive maternal-separated rats. Neurogastroenterol Motil. 2012;24(678–685):e297. doi: 10.1111/j.1365-2982.2012.01919.x. [DOI] [PubMed] [Google Scholar]
- 20.Miki T, Yokoyama T, Kusaka T, Suzuki S, Ohta K, Warita K, Wang ZY, Ueki M, Sumitani K, Bellinger FP, Tamai M, Liu JQ, Yakura T, Takeuchi Y. Early postnatal repeated maternal deprivation causes a transient increase in OMpg and BDNF in rat cerebellum suggesting precocious myelination. J Neurol Sci. 2014;336:62–67. doi: 10.1016/j.jns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Crawford MA, Doyle W, Leaf A, Leighfield M, Ghebremeskel K, Phylactos A. Nutrition and neurodevelopmental disorders. Nutr Health. 1993;9:81–97. doi: 10.1177/026010609300900205. [DOI] [PubMed] [Google Scholar]
- 22.Amano I, Takatsuru Y, Toya S, Haijima A, Iwasaki T, Grasberger H, Refetoff S, Koibuchi N. Aberrant cerebellar development in mice lacking dual oxidase maturation factors. Thyroid. 2016;26:741–752. doi: 10.1089/thy.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takatsuru Y, Eto K, Kaneko R, Masuda H, Shimokawa N, Koibuchi N, Nabekura J. Critical role of the astrocyte for functional remodeling in contralateral hemisphere of somatosensory cortex after stroke. J Neurosci. 2013;33:4683–4692. doi: 10.1523/JNEUROSCI.2657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagi N, Chikamori Y, Matsuoka I. Response of single Purkinje neurons in the flocculus of albino rabbits to caloric stimulation. Acta Otolaryngol. 1977;84:98–104. doi: 10.3109/00016487709123947. [DOI] [PubMed] [Google Scholar]
- 25.Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab. 2000;11:123–128. doi: 10.1016/S1043-2760(00)00238-1. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Iwasaki T, Xu M, Lesmana R, Xiong Y, Shimokawa N, Chin WW, Koibuchi N. Aberrant cerebellar development of transgenic mice expressing dominant-negative thyroid hormone receptor in cerebellar Purkinje cells. Endocrinology. 2015;156:1565–1576. doi: 10.1210/en.2014-1079. [DOI] [PubMed] [Google Scholar]
- 27.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins LM, Barba A, Campbell M, Lamar M, Shankman SA, Leow AD, Ajilore O, Langenecker SA. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin. 2016;12:1022–1034. doi: 10.1016/j.nicl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philip NS, Kuras YI, Valentine TR, Sweet LH, Tyrka AR, Price LH, Carpenter LL. Regional homogeneity and resting state functional connectivity: associations with exposure to early life stress. Psychiatry Res. 2013;214:247–253. doi: 10.1016/j.pscychresns.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman EG, Foxe JJ (2017) Eye-movements, sensori-motor adaptation and cerebellar-dependent learning in Autism: Towards potential biomarkers and sub-phenotypes. Eur J Neurosci. doi: 10.1111/ejn.13625 [DOI] [PubMed]
- 32.Takatsuru Y, Takayasu Y, Iino M, Nikkuni O, Ueda Y, Tanaka K, Ozawa S. Roles of glial glutamate transporters in shaping EPSCs at the climbing fiber-Purkinje cell synapses. Neurosci Res. 2006;54:140–148. doi: 10.1016/j.neures.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Takayasu Y, Iino M, Takatsuru Y, Tanaka K, Ozawa S. Functions of glutamate transporters in cerebellar Purkinje cell synapses. Acta Physiol (Oxf) 2009;197:1–12. doi: 10.1111/j.1748-1716.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 34.Bats C, Farrant M, Cull-Candy SG. A role of TARPs in the expression and plasticity of calcium-permeable AMPARs: evidence from cerebellar neurons and glia. Neuropharmacology. 2013;74:76–85. doi: 10.1016/j.neuropharm.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


