Abstract
Dynamics of repolarization, quantified as restitution and electrical memory, impact conduction stability. Relatively less is known about role of slow delayed rectifying potassium current, I Ks, in dynamics of repolarization and memory compared to the rapidly activating current I Kr. Trans-membrane potentials were recorded from right ventricular tissues from pigs during reduction (chromanol 293B) and increases in I Ks (mefenamic acid). A novel pacing protocol was used to explicitly control diastolic intervals to quantify memory. Restitution hysteresis, a consequence of memory, increased after chromanol 293B (loop thickness and area increased 27 and 38 %) and decreased after mefenamic acid (52 and 53 %). Standard and dynamic restitutions showed an increase in average slope after chromanol 293B and a decrease after mefenamic acid. Increase in slope and memory are hypothesized to have opposite effects on electrical stability; therefore, these results suggest that reduction and enhancement of I Ks likely also have offsetting components that affect stability.
Keywords: Slow delayed rectifier potassium current, Restitution, Action potential duration, Cardiac memory, Hysteresis, Ventricular arrhythmia
Introduction
The slow delayed rectifier potassium current, I Ks, plays a critical role in the late repolarization phase of an action potential (AP) by serving as a repolarization reserve when other repolarization currents, such as the rapid delayed rectifier potassium current (I Kr), are reduced during diseased conditions or by the use of drugs such as class III antiarrhythmic drugs [1]. Although prolongation of action potential duration (APD) is proposed to be proarrhythmic in situations such as long QT syndrome [2], there are contrasting results showing that lengthening of APD at short cycle lengths (CLs), i.e. higher heart rate, is an effective way to suppress arrhythmia, and block of I Ks is hypothesized to be more effective in increasing APDs in a frequency-independent way compared to I Kr blockers [3–7]. During inherited or acquired long QT as a result of application of I Kr blocker, activation of I Ks can compensate for the loss of repolarization current, prevent excessive APD prolongation, and reduce proarrhythmic risk [3, 8]. Thus, depending on different pathological conditions, both blockers and activators of I Ks can potentially prove to be beneficial. Although the potential role of modification of I Ks has been considered by several studies, compared to I Kr, less is known about how I Ks affects repolarization dynamics in terms of restitution and electrical memory.
Initiation of ventricular arrhythmias has been reported to be affected by two main factors: restitution of APD, which refers to the relationship between the current APD and its preceding diastolic interval (DI), and cardiac memory [9–11], which, in this context, means the dependence of APD on its previous history in the past few seconds. Although the concept of memory is widely hypothesized to impact stability, very few studies have quantified memory. We have previously developed a novel pacing protocol that allows sequential and precise changes in DI, independent of APDs [12, 13], that can be used to quantify memory. Oscillatory changes in DIs result in the trajectories of APD to diverge into the resulting hysteresis in restitution which permits quantification of memory.
Chromanol 293B has been reported to selectively block I Ks [4, 14, 15]. Block of I Ks has been shown to have a frequency-independent effect on APD in guinea pig and in humans [13], but have minimal effect in rabbit and canines [1, 7, 16–19]. L364, 373, a drug which selectively activates I Ks in guinea pigs [20] and rabbits [21], has been recently reported to have no effect in canines while mefenamic acid is a non-selective activator of I Ks which has been shown to shorten APD in canines [8]. Given these previously reported interspecies differences in the effects of this important repolarization current, the main objective of our study was to characterize the effects of manipulation of I Ks on dynamics of repolarization of ventricular AP in the pig, a widely used animal model to study arrhythmia [22–27]. Our results show that reduction (increase) of I Ks increased (decreased) both memory and slope of restitution curves, which suggest a mixed effect on electrical stability. While occurrence of alternans of APD after increase and reduction of I Ks showed results consistent with slope of restitution, there were several trials in the control group where alternans was not seen, yet the slopes of restitution were greater than one. However, at least in terms of generation of alternans, these results suggest a weak pro-arrhythmic effect of I Ks reduction and, likewise, a weak anti-arrhythmic effect of I Ks enhancement.
Methods
Data were collected from 7 farm pigs, weighing 18–21 kg. All animal related procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky. Animals were sedated/tranquilized using a combination of telazol (4–8 mg/kg), ketamine (2–4 mg/kg), and xylazine (2–4 mg/kg), and then anesthetized by sodium pentobarbital (30–50 mg/kg, IV). After anesthesia, hearts were quickly excised and placed in cold Tyrode’s solution. Two endocardial tissue slices of approximate size 20 mm × 10 mm × 5 mm, one for antagonist and the other for agonist, were collected adjacent to each other from the mid- to apical antero-lateral region of the right ventricle. The tissues were pinned in plastic tissue chambers, and superfused with warmed (36 ± 1 °C), gassed (95 % O2 and 5 % CO2) Tyrode’s solution. Composition of the Tyrode’s solution was (in mmol/L): 0.5 MgCl2, 0.9 NaH2PO4, 2.0 CaCl2, 137.0 NaCl, 4.0 KCl and 5.5 Dextrose. NaHCO3 was added to this solution to obtain a pH of 7.3 ± 0.05. Tissues were paced at a CL of 500 ms for approximately an hour to allow equilibration before recording was started.
When testing the effect of reduction of I Ks, the superfusate was switched to a buffer of the same composition as given above but containing antagonist chromanol 293B (Sigma-Aldrich, St. Louis, MO, USA). A concentration of 10–50 μM has been used in previous studies to reduce or block I Ks [4, 14, 15]. In the current study, we used a final concentration of 20 μM to substantially reduce I Ks without completely blocking it. To increase I Ks, agonist L364, 373 (dissolved in DMSO; Tocris Bioscience, Bristol, UK) was used at first in one animal with the highest concentration (3 μM) that has been used in a previous study [8]. However, once it became apparent that APDs were not affected at this concentration, mefenamic acid (100 μM, dissolved in 0.1 M NaOH; Sigma-Aldrich) was used as the agonist in all subsequent experiments. The concentration of mefenamic acid was consistent with that used by Magyar et al. [8]. After each drug was added, tissues were paced at 500 ms CL for approximately 40 min for equilibration before data were collected.
Tissues were paced using 3-ms biphasic stimuli delivered via platinum-iridium electrodes. Transmembrane potentials (TMPs) were recorded using machine-pulled glass microelectrodes filled with 3 M KCl. A commercial data acquisition system was used to digitize the TMPs at 50,000 samples/s. The following protocols were used to explore dynamics of repolarization of APs both before and after the drug application. First, standard S1S2 and dynamic protocols similar to that previously described by Gilmour et al. [28] were used to obtain restitution curves. In the standard protocol, S1S1 interval was 300 ms and S1S2 interval decreased from 600 to 300 ms in steps of 50 ms, and then from 300 to 200 ms in steps of 20 ms. For S1S2 < 200 ms, the decrement was 10 ms until block occurred. In the dynamic protocol, CL decreased from 600 ms progressively with the same decrement as used in the standard protocol until block occurred. Second, two feedback-based DI protocols were used to obtain hysteresis, i.e. memory in restitution. The novel feedback-based pacing permitted explicit control of DIs as described previously [12, 29, 30]. Unlike the widely used CL control protocols, which result in a correlation of APD and DI (DI = CL-APD), the feedback based protocols allowed precise control of DI, independent of APD. The DIs oscillated in a sinusoidal pattern with a period of 100 beats. Each protocol had two cycles, preceded by 20 beats at its mean DI value. In one protocol, the mean DI was 400 ms with a variation of ±300 ms; in the other, the mean value was 150 ms with a variation of ±140 ms. Figure 1a shows an example of the sinusoidal DI protocol with a mean DI of 400 ms. And, third, to test effects of I Ks changes on arrhythmia, we recorded TMPs when the tissue was paced using constant CL protocols where pacing CLs ≤ 300 ms and constant DI protocols where the DIs ≤ 40 ms were kept constant for all beats in one trial. CLs and DIs were progressively reduced until block occurred. Data were analyzed for the occurrence of alternans of APD, i.e. beat to beat variation in APDs, which is thought to presage and be conducive to ventricular arrhythmia. Each protocol was repeated 2 to 3 times. Between trials, the tissue was paced at 500 ms CL.
Fig. 1.
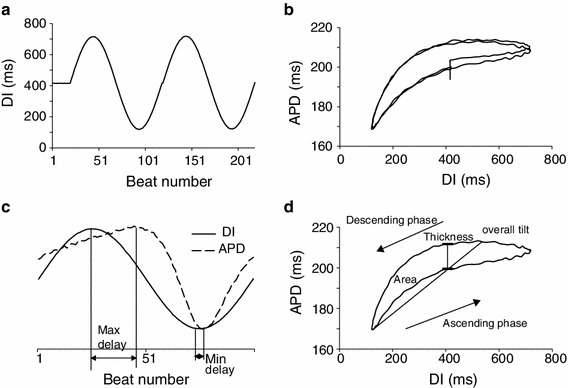
a An example of sinusoidal DI protocol, with 20 beats of DIs at center value of 400 ms followed by 2 cycles of sinusoidal change ranging from 100 to 700 ms. b Restitution relationship between APD and DI obtained from the DI change shown in (a), which shows two complete hysteresis loops after 20 beats adaptation at center DI. c The second cycle of the DI sequence shown in (a) (solid line) and the resulting APD trace (dashed line). The APD trace is scaled and offset to clearly illustrate maximum delay and minimum delay, which are measured in beats. d Hysteresis loop generated by the second cycle of the DI sequence shown in (a), with illustration of loop thickness, overall tilt and loop area
A custom developed program written in MATLAB (Mathworks, Natick, MA, USA) was used for offline data analysis. The TMPs were low-pass filtered at 1,000 Hz cutoff frequency, and APD was calculated for each AP as the duration between the start of an AP, i.e. the point where the derivative of TMP becomes positive, and the instance when TMP repolarized to 90 % of its amplitude. Alternans of APD was considered to occur when beat to beat variations in APDs were ≥4 ms for 5 successive beats. The threshold of 4 ms is the same as that reported previously by Pruvot et al. [31]. From the hysteresis in restitution between APD and DI resulting from the sinusoidal DI protocols (Fig. 1b), five parameters were computed to quantify memory: (1) loop thickness, defined as difference in APDs between the ascending and descending phases when DI was at its mean value; (2) loop area, i.e. area contained within the hysteresis loop; (3) overall tilt, defined as the slope of the line composed by connecting the two points where the APD was at its maximum and minimum; (4) maximum delay, which is the number of beats between the peaks of DI and APD; and (5) minimum delay, which is the delay (in number of beats) between the nadirs of DI and APD. In some cases, APD adaptation caused a baseline shift in the first cycle of the sinusoidal DI sequence, which was induced by the transition from 500 ms CL to constant DI pacing; therefore, we only computed the measures from the second cycle. In rare cases when DI control was transiently lost during real-time control for 1 or 2 beats, the missed DI and corresponding APD values were replaced using linear interpolation from their adjacent 2 values. Those trials where DI control was lost for more than 5 beats from the 200 beats sequence were not used in analysis. If there was more than one trial for any protocol that met the conditions stated above, then results from those trials were averaged and the measures were calculated from the averaged loop/restitution curve and used for further analysis. Paired Student’s t test was conducted to test for statistical significance. Significant difference was accepted at p ≤ 0.05.
Results
Effect of IKs change on baseline APDs
Because L364, 373 had been reported as a selective I Ks agonist in previous studies of guinea pigs and rabbits, we started with L364, 373 in the first animal. However, APDs at 500 ms CL showed no difference between control and post-drug (227 vs. 222 ms). This observation is consistent with a previous study in dogs [8]. As the main objective of the current study was to explore the effect of changes in I Ks on dynamics of repolarization of AP, and not to explore whether L364, 373 works as an agonist in this species, for the rest of the experiments we used mefenamic acid to activate I Ks.
Figure 2 shows an example of TMPs recorded at 500 ms CL before (solid line) and after application of chromanol 293B (dashed line). and mefenamic acid (dotted line). All the traces were collected from one animal and the upstroke of APs were aligned to better show the difference among APDs. The average APD (n = 6) was lengthened by 14 % after chromanol 293B (205 ± 6 vs. 234 ± 7 ms) and shortened by 20 % after mefenamic acid (218 ± 4 to 173 ± 6 ms), both of which were significant.
Fig. 2.
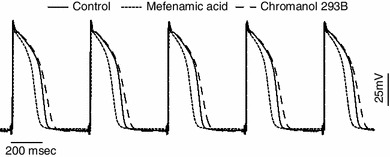
An example of transmembrane potentials recorded in one pig at CL of 500 ms which shows the APD differences among control (solid line), chromanol 293B (dashed line), and mefenamic acid (dotted line). APD increased after chromanol 293B and decreased after mefenamic acid
Effect of IKs change on restitution
To quantify changes in restitution, standard and dynamic restitution were obtained from all animals. Figure 3 shows examples of standard and dynamic restitution during control and post-drug in one animal. For each restitution curve, overall slope was obtained by computing the average value of all slopes over the entire range of DIs. Average overall slopes (n = 6) are summarized in Table 1. Compared to control, both standard (Fig. 3a) and dynamic restitution (Fig. 3b) had steeper slopes after chromanol 293B, especially at short CL. However, only the slopes of dynamic restitution were significantly different (0.75 control vs. 0.97 chromanol 293B). In contrast, slopes of both standard and dynamic restitution flattened after mefenamic acid (Fig. 3c, d); both were significantly smaller than the control (0.26 vs. 0.45, and 0.59 vs. 0.91). In both drugs, changes in slope of dynamic restitution were more prominent than those of standard restitution. Average slope for each restitution curve was also computed for all points where CL < 300 ms, since alternans is mostly seen at higher activation rates. These results are shown in Table 1: all slopes were larger at shorter CL compared to overall slopes, and all dynamic slopes were close to or greater than 1, a condition believed to be requisite for occurrence of alternans and decrease in electrical stability.
Fig. 3.
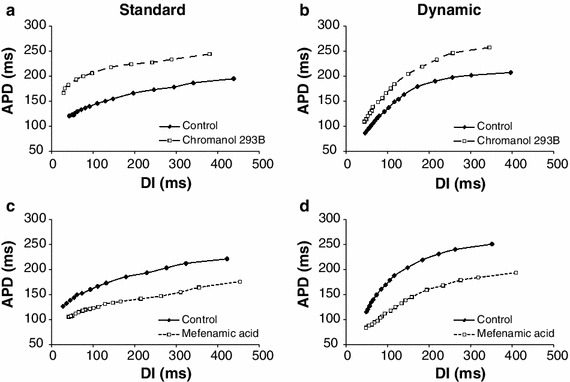
Standard (a, c) and dynamic (b, d) restitution curves recorded from one trial show the change in slope of restitution after chromanol 293B (a, b) and mefenamic acid (c, d). Slopes in this trial are comparable to the average slopes (n = 6). Slopes of standard and dynamic restitution both increased after chromanol 293B and decreased after mefenamic acid, with more prominent changes in dynamic restitution
Table 1.
Average overall slopes for standard and dynamic restitution (n = 6)
| Control | Chromanol 293B | Control | Mefenamicid acid | |
|---|---|---|---|---|
| Standard | ||||
| Overall | 0.30 ± 0.05 | 0.44 ± 0.09 | 0.45 ± 0.08 | 0.26 ± 0.03* |
| CL ≤ 300 ms | 0.45 ± 0.06 | 0.67 ± 0.15 | 0.63 ± 0.13 | 0.34 ± 0.04* |
| Dynamic | ||||
| Overall | 0.75 ± 0.12 | 0.97 ± 0.15* | 0.91 ± 0.11 | 0.59 ± 0.08* |
| CL ≤ 300 ms | 1.08 ± 0.14 | 1.29 ± 0.23 | 1.19 ± 0.15 | 0.81 ± 0.09* |
Slopes at all values of DIs in each restitution curve were averaged to obtain an overall slope
* p < 0.05
Effect of IKs change on memory
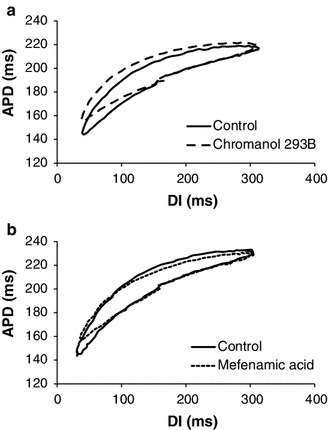
Hysteresis in restitution, i.e. memory, was observed in both sinusoidal DI protocols. Figure 4 shows the averaged hysteresis loops for the sinusoidal protocol with a center DI of 400 ms (n = 5; due to technical difficulties with DI control, we could not obtain data in one animal). In the 5 measures of hysteresis, the most prominent changes were observed in loop thickness and area: after chromanol 293B (Fig. 4a), loop thickness increased from 13 ± 2 to 17 ± 2 ms and area enlarged from 8,084 ± 1,303 to 11,191 ± 1,875 ms2 with a percentage change of 27 and 28 %, respectively; oppositely, after mefenamic acid (Fig. 4b), loop thickness decreased from 16 ± 2 to 8 ± 1 ms, and area shrunk from 8,960 ± 664 to 4,215 ± 810 ms2, with a percentage change of 52 and 53 %, respectively (all changes significant). Overall tilt showed inconsistent changes during both drugs across animals, where both increases and decreases were observed, resulting in minimal change between control and post-drug. Changes in maximum and minimum delay were also minimal and no significant differences were present. Table 2 includes a summary of these measures (mean ± SEM) for the 400 ms DI protocol.
Fig. 4.
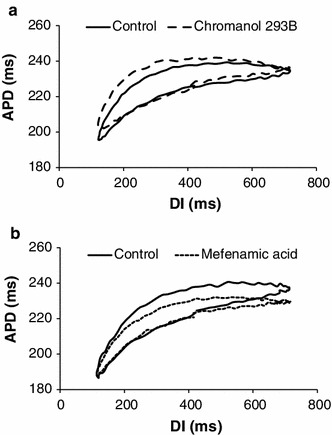
Averaged hysteresis loops (n = 5) during sinusoidal DI protocol with mean DI = 400 ms for control and post-drug. a Control (solid line) versus chromanol 293B (dashed line). b Control (solid line) versus mefenamic acid (dotted line). Curves were shifted vertically (to adjust for differences in APD) in both panels to facilitate comparison between the hysteresis loops
Table 2.
A summary of mean (±SEM) values of measures of hysteresis for 400 ms DI protocol for both drugs (control and post-drug), n = 5
| Control | Chromanol 293B | Control | Mefenamic acid | |
|---|---|---|---|---|
| Thickness (ms) | 13 ± 2 | 17 ± 2* | 16 ± 2 | 8 ± 1* |
| Overall tilt | 0.065 ± 0.017 | 0.070 ± 0.023 | 0.080 ± 0.017 | 0.063 ± 0.015 |
| Area (ms2) | 8,084 ± 1,303 | 11,191 ± 1,875* | 8,960 ± 664 | 4,215 ± 810* |
| Max delay (beats) | 20 ± 3 | 23 ± 2 | 18 ± 3 | 17 ± 1 |
| Min delay (beats) | 1 ± 0 | 3 ± 1 | 2 ± 1 | 3 ± 1 |
* p < 0.05
Figure 5 shows the averaged hysteresis loops (n = 6) of control and post-drug data obtained from the sinusoidal DI protocol with a150 ms center DI. The results are similar to that during the 400 ms DI protocol; loop thickness and area were significantly different while the changes in overall tilt were mixed. Loop thickness increased 52 % after chromanol 293B, decreased 29 % after mefenamic acid, and accordingly loop area increased 31 % and decreased 35 %, respectively. A summary of these measures is in Table 3.
Fig. 5.
Averaged hysteresis loops (n = 6) during sinusoidal DI protocol with mean DI of 150 ms for control and post-drug data. As in Fig. 4, the curves were vertically shifted to facilitate comparison between the hysteresis loops
Table 3.
A summary of mean (±SEM) values of measures of hysteresis for 150 ms DI protocol, n = 6
| Control | Chromanol 293B | Control | Mefenamic acid | |
|---|---|---|---|---|
| Thickness (ms) | 15 ± 2 | 23 ± 2* | 19 ± 1 | 14 ± 2* |
| Overall tilt | 0.257 ± 0.035 | 0.234 ± 0.028 | 0.311 ± 0.039 | 0.259 ± 0.045 |
| Area (ms2) | 4,283 ± 636 | 5,629 ± 954* | 5,267 ± 745 | 3,409 ± 535* |
| Max delay (beats) | 10 ± 2 | 10 ± 1 | 8 ± 1 | 6 ± 1 |
| Min delay (beats) | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 |
* p < 0.05
Effects of IKs change on APD alternans
During reduction of I Ks, alternans occurred only in 2 animals during control, with an average amplitude of 8 ms, and was observed after chromanol 293B in 2 animals (not the same animals) with an amplitude of 5 ms. The average CL when alternans started to occur was longer after chromanol 293B compared with control (200 vs. 130 ms). In all trials, activation blocked at longer CL after chromanol 293B was added, (average CL 118 vs. 150 ms, p < 0.05).
During enhancement of I Ks, alternans was present during the control study in 4 animals, with an average amplitude of 12 ms. No alternans was seen after adding mefenamic acid. This result is consistent with what would be predicted by changes in the average slope of dynamic restitution, i.e. 1.19 in control vs. 0.81 post-drug. The average CLs where activation block occurred were 115 and 106 ms for control and post-drug, respectively, with no significant difference between the two.
Discussion
The focus of the current study was to characterize the effects of changes in I Ks on restitution and memory in swine ventricular tissue. The main observations of our study are: first, chromanol 293B-induced reduction of I Ks in swine ventricles results in APD prolongation, increased measures of hysteresis in restitution, i.e. in memory, but also produces steeper restitution curves. Alternans of APD was present in limited samples during both control and post-drug but occurred at longer CL post-drug; and, second, enhancement of I Ks, which was achieved by mefenamic acid, shortened APD, decreased memory as well as restitution slopes, and minimized occurrence of alternans.
Effect of IKs manipulation on APDs
I Kr blocking drugs are most commonly class III antiarrhythmic drugs used to prolong repolarization and prevent arrhythmia; however, due to their reverse frequency response, they have limited therapeutic effect on arrhythmia suppression and, moreover, have increased risk of Torsades de Pointes (TdP) [32–34]. In the current study, our results show that chromanol 293B produced frequency independent changes in APDs during fast CL pacing (Fig. 3), presumably by reducing I Ks at all levels of CL. If prolonging repolarization prevents re-entrant arrhythmia and ventricular fibrillation as suggested by previous studies [5, 6], these results suggest that blockade of I Ks could provide antiarrhythmic benefit. Heterogeneity in I Ks expression in different species and in its kinetic properties has been widely reported. Presence of I Ks channels in pigs and its sensitivity to chromanol 293B has also been shown in previous studies [31, 35]. Our results show that I Ks is more functional in repolarization in pigs compared with rabbits and dogs [7, 16, 19, 36], another two species widely used in arrhythmia research. Considering the consistent observations in our study with those reported in humans [14], in regards to effects on APD, one would predict similar changes in restitution properties with manipulation of I Ks in human ventricles to those observed in pigs in the current study.
Electrical stability indicated by changes in dynamics of repolarization
Restitution of APD has been believed to be the dominant mechanism underlying initiation of APD alternans, which is hypothesized to presage and be conducive to re-entry and ventricular arrhythmias [28, 37, 38]. The restitution hypothesis states that an increase in restitution slope indicates pro-arrhythmic effect and electrical instability. In our results, we observed an increase in average restitution slope after chromanol 293B, i.e. after decrease of I Ks, and a decrease in slope after mefenamic acid, i.e. after an increase of I Ks (Fig. 3; Table 1). These observations are consistent with results from our previous simulation study using the Luo Rudy model, a model of guinea pig ventricular cell [13]. Therefore, based on the restitution theory, our results would suggest that electrical stability in tissues with decreased I Ks is compromised and it would be more susceptible to arrhythmia induction, while enhancement of I Ks could stabilize activation and provide antiarrhythmic protection. Our results of APD alternans are partly consistent with this conclusion, as alternans was only present when the dynamic curves were steeper than 1, during both controls and after chromanol 293B, but was not present after mefenamic acid. However, studies [9–11] related to cardiac memory have shown that restitution alone is not adequate to predict initiation of arrhythmia, rather, memory should be taken into account to provide a more comprehensive prediction. The fact that, in the current study, although the slopes were consistently >1 for all chromanol 293B trials, yet alternans was only observed in 2 out of 6 animals supports this conclusion. Increased memory is proposed to be indicative of increase in electrical stability [9, 39]. In the current study, 2 out of 5 measures of hysteresis were significantly larger (smaller) after chromanol 293B (mefenamic acid) suggesting an increase (decrease) in memory. Therefore, in the context of the hypothesized effects of memory, our results suggest that reduction of I Ks I Ks would decrease electrical stability while enhancement of I Ks would have a stabilizing effect. This conclusion is opposite to that predicted by results of restitution slopes. Therefore, the effect of I Ks manipulation, i.e. reduction and enhancement, using the contemporary hypothesized mechanisms affecting stability of activation, is mixed. With the presence of two offsetting components that affect stability, the ultimate effect of I Ks manipulation on stability would depend on which of the two mechanisms plays a dominant role in generation of certain type of arrhythmias. Unfortunately, at this stage, without further investigations and experimental or clinical evidence, it is not clear which of the two, i.e. restitution or memory, is the predominant contributor.
Clinical indications of arrhythmogenic effect of IKs blocker/activator
The divergent effects on restitution and memory are consistent with divergent effects of changes in I Ks on electrical stability that have been reported previously. A previous study [40] has shown that I Ks blockade reduced dispersion of repolarization, which is a critical mechanism underlying discordant alternans and ventricular fibrillation. However, other studies reported that suppression of I Ks decreased the repolarization reserve and increased the risk of TdP generation [41, 42] as a result of excessive prolongation. In the current study, we did not observe early after-depolarization after I Ks reduction. However, observations that reduction of I Ks leading to alternans and conduction block at longer CL suggest, albeit weakly, a proarrhythmic effect of I Ks blocker. The experiments were conducted in tissue samples that were small in size; therefore, induction of ventricular tachycardia or fibrillation was not possible. Future studies using optical mapping of isolated heart would be needed to investigate the effect of chromanol on initiation of arrhythmia.
The agonist that we used, mefenamic acid, is somewhat non-selective and is known as a blocker of Cl− current. However, it has also been shown to increase I Ks in several studies (which is also shown by Magyar et al. in their fig. 1) [8, 43, 44]. Results show that increase in I Ks by mefenamic acid decreases slope of restitution and measures of memory (Figs. 3, 4), suggesting the existence of both stabilizing and destabilizing effects. Further, our results of alternans suggest that this drug might have a suppressive effect on alternans generation. As stated above, induction of ventricular tachycardia is not possible in small-sized tissues; therefore, the exact role of mefenamic acid on arrhythmia generation remains to be determined. Therapeutic usefulness of enhancement of I Ks is uncertain, but it is hypothesized to be able to prevent excessive APD prolongation by increasing the repolarization reserve, which could compensate the adverse effect caused by application of I Kr blocking drugs [18, 33, 42]. Nevertheless, our results suggest that the effect of I Ks activator on restitution and memory should be taken into account when considering the therapeutic benefit of I Kr blocking drugs.
Limitations
As stated above, we used a non-selective I Ks I Ks agonist to test the effect of increase in I Ks. Currently, L364, 373 is the only drug that selectively activates I Ks, but it has been proven only in rodents and rabbits. Consistent with a previous study on canines, our results also showed that it did not have an effect on APD at 500 ms CL in pigs. Previously, several studies have used mefenamic acid to activate I Ks [8, 43, 44]. However, these previous studies also showed that, in addition to I Ks enhancement, mefenamic acid could inhibit Cl− current and Ca2+ current. Block of Cl− would prolong APD, while block of Ca2+ would shorten APD, i.e. the effect on these two currents on APD would be opposite and potentially offsetting each other. Therefore, the net shortening of APD observed in this study after mefenamic acid was likely due to I Ks activation.
We chose to use one concentration of each drug without testing the dose effect on I Ks, as the focus of the current study was to investigate the effect of changing I Ks on dynamics of repolarization, but not on the way these change I Ks per se. Therefore, we chose one concentration from what has been reported in the literature to meet our objective of manipulating the current by both attenuating and accentuating it.
All our recordings were made from the endocardial side of the right ventricle. Whether similar results would be obtained from other cell types in other areas of the heart is unknown. Heterogeneity of I Ks expression has been reported by a number of studies in different species. For example, Bryant et al. reported a smaller I Ks density in endocardium compared to epicardium [45]; in rabbits, I Ks expression was more abundant in the base than in the apex [46]; I Ks density was reported to be similar between endo- and epicardial myocytes in canine ventricles [47]; and, in minipigs, the expression of mRNA for I Ks was similar among endo-, mid- and epicardial myocytes in the left ventricle as well as the right ventricle [35]. However, the regional differences in I Ks expression in pig ventricles are unknown. Considering the heterogeneity of I Ks expression in other species, it is likely that heterogeneity exists in pig ventricles, and changes in this current may also alter electrical substrate heterogeneously. Future studies will be required to further investigate the role of heterogeneity in dynamics of repolarization.
Acknowledgement
Supported by grants from the National Science Foundation (0730450, 0814194) and the Commonwealth of Kentucky.
Abbreviations
- IKs
Slow delayed rectifier potassium current
- AP
Action potential
- IKr
Rapid delayed rectifier potassium current
- APD
Action potential duration
- CL
Cycle length
- DI
Diastolic interval
- TMP
Transmembrane potential
- TdP
Torsades de Pointes
References
- 1.Abi-Gerges N, Small BG, Lawrence CL, Hammond TG, Valentin JP, Pollard CE. Gender differences in the slow delayed (IKs) but not in inward (IK1) rectifier K+ currents of canine Purkinje fibre cardiac action potential: key roles for IKs, beta-adrenoceptor stimulation, pacing rate and gender. Br J Pharmacol. 2006;147(6):653–660. doi: 10.1038/sj.bjp.0706491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayakawa EH, Furutani M, Matsuoka R, Takakuwa Y. Comparison of protein behavior between wild-type and G601S hERG in living cells by fluorescence correlation spectroscopy. J Physiol Sci. 2011;61(4):313–319. doi: 10.1007/s12576-011-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng JH, Kodama I. Two components of delayed rectifier K+ current in heart: molecular basis, functional diversity, and contribution to repolarization. Acta Pharmacol Sin. 2004;25(2):137–145. [PubMed] [Google Scholar]
- 4.Lu Z, Kamiya K, Opthof T, Yasui K, Kodama I. Density and kinetics of I(Kr) and I(Ks) in guinea pig and rabbit ventricular myocytes explain different efficacy of I(Ks) blockade at high heart rate in guinea pig and rabbit: implications for arrhythmogenesis in humans. Circulation. 2001;104(8):951–956. doi: 10.1161/hc3401.093151. [DOI] [PubMed] [Google Scholar]
- 5.Singh BN. Control of cardiac arrhythmias by lengthening repolarization. Mount Kisco: Futura; 1988. [Google Scholar]
- 6.Singh BN, Vaughan Williams EM. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ, 1999 and AH 3474. Br J Pharmacol. 1970;39(4):675–687. doi: 10.1111/j.1476-5381.1970.tb09893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stengl M, Volders PG, Thomsen MB, Spatjens RL, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol. 2003;551(Pt 3):777–786. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magyar J, Horvath B, Banyasz T, Szentandrassy N, Birinyi P, Varro A, Szakonyi Z, Fulop F, Nanasi PP. L-364,373 fails to activate the slow delayed rectifier K+ current in canine ventricular cardiomyocytes. Naunyn-Schmiedeberg’s Arch Pharmacol. 2006;373(1):85–89. doi: 10.1007/s00210-006-0047-4. [DOI] [PubMed] [Google Scholar]
- 9.Cherry EM, Fenton FH. Suppression of alternans and conduction blocks despite steep APD restitution: electrotonic, memory, and conduction velocity restitution effects. Am J Physiol Heart Circ Physiol. 2004;286(6):H2332–H2341. doi: 10.1152/ajpheart.00747.2003. [DOI] [PubMed] [Google Scholar]
- 10.Choi BR, Liu T, Salama G. Adaptation of cardiac action potential durations to stimulation history with random diastolic intervals. J Cardiovasc Electrophysiol. 2004;15(10):1188–1197. doi: 10.1046/j.1540-8167.2004.04070.x. [DOI] [PubMed] [Google Scholar]
- 11.Jordan PN, Christini DJ. Determining the effects of memory and action potential duration alternans on cardiac restitution using a constant-memory restitution protocol. Physiol Meas. 2004;25(4):1013–1024. doi: 10.1088/0967-3334/25/4/018. [DOI] [PubMed] [Google Scholar]
- 12.Wu R, Patwardhan A. Restitution of action potential duration during sequential changes in diastolic intervals shows multimodal behavior. Circ Res. 2004;94(5):634–641. doi: 10.1161/01.RES.0000119322.87051.A9. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Patwardhan A. Effects of rapid and slow potassium repolarization currents and calcium dynamics on hysteresis in restitution of action potential duration. J Electrocardiol. 2007;40(2):188–199. doi: 10.1016/j.jelectrocard.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res. 1998;38(2):441–450. doi: 10.1016/S0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 15.Sun ZQ, Thomas GP, Antzelevitch C. Chromanol 293B inhibits slowly activating delayed rectifier and transient outward currents in canine left ventricular myocytes. J Cardiovasc Electrophysiol. 2001;12(4):472–478. doi: 10.1046/j.1540-8167.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- 16.Guerard NC, Traebert M, Suter W, Dumotier BM. Selective block of IKs plays a significant role in MAP triangulation induced by IKr block in isolated rabbit heart. J Pharmacol Toxicol Methods. 2008;58(1):32–40. doi: 10.1016/j.vascn.2008.05.129. [DOI] [PubMed] [Google Scholar]
- 17.Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG, Varro A. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112(10):1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Du L, Li M. Update on the slow delayed rectifier potassium current (I(Ks)): role in modulating cardiac function. Curr Med Chem. 2012;19(9):1405–1420. doi: 10.2174/092986712799462595. [DOI] [PubMed] [Google Scholar]
- 19.Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation. 2003;107(21):2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- 20.Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54(1):220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Salata JJ, Wang J, Wu Y, Yan GX, Liu T, Marinchak RA, Kowey PR. Increasing I(Ks) corrects abnormal repolarization in rabbit models of acquired LQT2 and ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2002;283(2):H664–H670. doi: 10.1152/ajpheart.00076.2002. [DOI] [PubMed] [Google Scholar]
- 22.Banville I, Chattipakorn N, Gray RA. Restitution dynamics during pacing and arrhythmias in isolated pig hearts. J Cardiovasc Electrophysiol. 2004;15(4):455–463. doi: 10.1046/j.1540-8167.2004.03330.x. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell BJ, Legrice IJ, Hooks DA, Tai DC, Pullan AJ, Smaill BH. Intramural measurement of transmembrane potential in the isolated pig heart: validation of a novel technique. J Cardiovasc Electrophysiol. 2005;16(9):1001–1010. doi: 10.1111/j.1540-8167.2005.40558.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Zhao D, Cui B, Lu Z, Lu J, Chen F, Bao M. Electrical restitution determined by epicardial contact mapping and surface electrocardiogram: its role in ventricular fibrillation inducibility in swine. J Electrocardiol. 2008;41(2):152–159. doi: 10.1016/j.jelectrocard.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Ristagno G, Yu T, Quan WL, Freeman G, Li YQ. Current is better than energy as predictor of success for biphasic defibrillatory shocks in a porcine model of ventricular fibrillation. Resuscitation. 2013;84(5):678–683. doi: 10.1016/j.resuscitation.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Voroshilovsky O, Qu Z, Lee MH, Ohara T, Fishbein GA, Huang HL, Swerdlow CD, Lin SF, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS. Mechanisms of ventricular fibrillation induction by 60-Hz alternating current in isolated swine right ventricle. Circulation. 2000;102(13):1569–1574. doi: 10.1161/01.CIR.102.13.1569. [DOI] [PubMed] [Google Scholar]
- 27.Walcott GP, Kroll MW, Ideker RE. Ventricular fibrillation threshold of rapid short pulses. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:255–258. doi: 10.1109/IEMBS.2011.6090049. [DOI] [PubMed] [Google Scholar]
- 28.Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275(5 Pt 2):H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 29.Guzman KM, Jing L, Patwardhan A. Effects of changes in the L-type calcium current on hysteresis in restitution of action potential duration. Pacing Clin Electrophysiol. 2010;33(4):451–459. doi: 10.1111/j.1540-8159.2009.02637.x. [DOI] [PubMed] [Google Scholar]
- 30.Jing L, Chourasia S, Patwardhan A. Heterogeneous memory in restitution of action potential duration in pig ventricles. J Electrocardiol. 2010;43(5):425–432. doi: 10.1016/j.jelectrocard.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94(8):1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 32.Nattel S, Zeng FD. Frequency-dependent effects of antiarrhythmic drugs on action potential duration and refractoriness of canine cardiac Purkinje fibers. J Pharmacol Exp Ther. 1984;229(1):283–291. [PubMed] [Google Scholar]
- 33.Barhanin J, Attali B, Lazdunski M. IKs, a slow and intriguing cardiac K+ channel and its associated long QT diseases. Trends Cardiovasc Med. 1998;8(5):207–214. doi: 10.1016/S1050-1738(98)00013-9. [DOI] [PubMed] [Google Scholar]
- 34.Inanobe A, Kamiya N, Murakami S, Fukunishi Y, Nakamura H, Kurachi Y. In silico prediction of the chemical block of human ether-a-go–go-related gene (hERG) K+ current. J Physiol Sci. 2008;58(7):459–470. doi: 10.2170/physiolsci.RV011408. [DOI] [PubMed] [Google Scholar]
- 35.Laursen M, Olesen SP, Grunnet M, Mow T, Jespersen T. Characterization of cardiac repolarization in the Gottingen minipig. J Pharmacol Toxicol Methods. 2011;63(2):186–195. doi: 10.1016/j.vascn.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (I(Ks)) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br J Pharmacol. 2001;132(1):101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karma A. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 1994;4(3):461–472. doi: 10.1063/1.166024. [DOI] [PubMed] [Google Scholar]
- 38.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102(14):1664–1670. doi: 10.1161/01.CIR.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 39.Chialvo DR, Michaels DC, Jalife J. Supernormal excitability as a mechanism of chaotic dynamics of activation in cardiac Purkinje-Fibers. Circ Res. 1990;66(2):525–545. doi: 10.1161/01.RES.66.2.525. [DOI] [PubMed] [Google Scholar]
- 40.Pajouh M, Wilson LD, Poelzing S, Johnson NJ, Rosenbaum DS. IKs blockade reduces dispersion of repolarization in heart failure. Heart Rhythm. 2005;2(7):731–738. doi: 10.1016/j.hrthm.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Cheng HC, Incardona J. Models of torsades de pointes: effects of FPL64176, DPI201106, dofetilide, and chromanol 293B in isolated rabbit and guinea pig hearts. J Pharmacol Toxicol Methods. 2009;60(2):174–184. doi: 10.1016/j.vascn.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Jost N, Papp JG, Varro A. Slow delayed rectifier potassium current (IKs) and the repolarization reserve. Ann Noninvasive Electrocardiol. 2007;12(1):64–78. doi: 10.1111/j.1542-474X.2007.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busch AE, Busch GL, Ford E, Suessbrich H, Lang HJ, Greger R, Kunzelmann K, Attali B, Stuhmer W. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. Br J Pharmacol. 1997;122(2):187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busch AE, Herzer T, Wagner CA, Schmidt F, Raber G, Waldegger S, Lang F. Positive regulation by chloride channel blockers of I-Sk channels expressed in Xenopus oocytes. Mol Pharmacol. 1994;46(4):750–753. [PubMed] [Google Scholar]
- 45.Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovasc Res. 1998;40(2):322–331. doi: 10.1016/S0008-6363(98)00133-3. [DOI] [PubMed] [Google Scholar]
- 46.Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama I. Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilideclass III agents. Cardiovasc Res. 1999;43(1):135–147. doi: 10.1016/S0008-6363(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 47.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res. 1995;76(3):351–365. doi: 10.1161/01.RES.76.3.351. [DOI] [PubMed] [Google Scholar]


