Abstract
In mammals, circadian rhythms, such as sleep/wake cycles, are regulated by the central circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN consists of thousands of individual neurons, which exhibit circadian rhythms. They synchronize with each other and produce robust and stable oscillations. Although several neurotransmitters are expressed in the SCN, almost all SCN neurons are γ-amino butyric acid (GABA)-ergic. Several studies have attempted to understand the roles of GABA in the SCN; however, precise mechanisms of the action of GABA in the SCN are still unclear. GABA exhibits excitatory and/or inhibitory characteristics depending on the circadian phase or region in the SCN. It can both synchronize and destabilize cellular circadian rhythms in individual SCN cells. Differing environmental light conditions, such as a long photoperiod, result in the decoupling of circadian oscillators of the dorsal and ventral SCN. This is due to high intracellular chloride concentrations in the dorsal SCN. Because mice with functional GABA deficiency, such as vesicular GABA transporter- and glutamate decarboxylase-deficient mice, are neonatal lethal, research has been limited to pharmacological approaches. Furthermore, different recording methods have been used to understand the roles of GABA in the SCN. The excitability of GABAergic neurons also changes during the postnatal period. Although there are technical difficulties in understanding the functions of GABA in the SCN, technical developments may help uncover new roles of GABA in circadian physiology and behavior.
Keywords: Circadian rhythm, Suprachiasmatic nucleus, Clock gene, Cellular networks, GABA, Photoperiod
The central circadian clock: the suprachiasmatic nucleus (SCN)
Several physiological functions in our body exhibit oscillations on various time scales, including electrical activity in the brain, heart rate, breathing, and the sleep/wake cycle. Among them, circadian rhythms are defined as approximately 24-h oscillations in physiology and behavior. In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus is known as the central circadian pacemaker. Circadian behavioral rhythms were abolished by SCN lesions [71, 105], and restored by implantation of the SCN [56]. Importantly, the restored circadian period was identical to that of the donor, rather than the host [91, 107]. In addition, implantation of the SCN contained in a semipermeable polymeric capsule also restored circadian behavioral rhythms in SCN-lesioned hamsters, indicating that diffusible signals from the SCN control circadian behavior [102].
The SCN contains approximately 20,000 neurons [113] that have heterogeneous circadian properties. In dispersed SCN cell culture, individual SCN neurons exhibit autonomous oscillations. However, the circadian period, phase, and amplitude, differ from cell to cell [34, 37, 120]. In cultured SCN slices, circadian rhythms of individual cells synchronize with each other and they express stable circadian oscillations [33, 36, 79, 84] (Fig. 1). Cellular networks in the SCN are important for synchronization [121] and the stability of circadian oscillation in individual cells [59]. Synchronized circadian rhythms within the SCN are responsible for coordinating peripheral circadian oscillators [123], and exhibiting circadian behavior. In the SCN, there are multiple oscillators. Environmental light/dark conditions change the coupling between these regional oscillators in the SCN, which is critical for the output for the circadian behavior and peripheral clock [35, 41, 78, 82, 114].
Fig. 1.
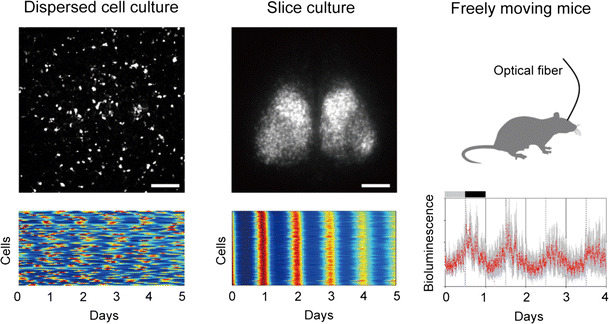
PER2::LUC rhythms in dispersed SCN cells, SCN slices, and the SCN of freely moving mice. Per2 protein fusion luciferase activity was measured using bioluminescence imaging from dispersed (left) and slice (middle) SCN culture using an EM-CCD camera. Circadian rhythms of individual SCN cells in dispersed (lower left) and slice (lower middle) cultures are expressed as pseudo colors, in which red and blue indicate peak and trough circadian rhythm phases. PER2::LUC rhythms in the SCN in freely moving mice were measured using an optical fiber under constant darkness for 4 days. Neuronal networks are important for SCN cellular circadian rhythm synchronization. Scale bars represent 200 μm
Circadian rhythms of individual SCN cells are generated by transcription-translation feedback loops involving several clock genes and protein products [93]. In this feedback loop, the positive elements, BMAL1 and CLOCK form a heterodimer that initiates the transcription of genes that contain E-box enhancer sequences, including Period (Per) and Cryptochrome (Cry) [12, 23, 53]. The protein products of Per and Cry then suppress transactivation by the BMAL1/CLOCK heterodimer [96, 100]. This clock machinery is widely observed on the single cell level.
Anatomical properties of the SCN
The SCN is divided into dorsal (shell) and ventral (core) subdivisions. Retinal projections to the ventral SCN [11, 108] and dorsal SCN [29] are observed. The SCN contains several neurotransmitters located within specific regions (Fig. 2). This general organization has been studied in hamsters, mice, rats, and humans [1, 14, 62, 74]. Arginine vasopressin (AVP) neurons are located in the SCN shell. Conversely, the SCN core expresses several neuropeptides, such as vasoactive intestinal peptides (VIP), gastrin-releasing peptide (GRP), and calbindin [1, 103].
Fig. 2.
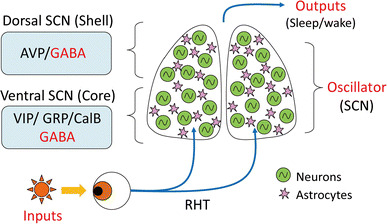
Schematic diagram of circadian organization in the SCN. Light input is transmitted to the ventral SCN through the retinohypothalamic tract (RHT). Several neuropeptides are expressed in the SCN and almost all SCN cells are GABAergic neurons. Circadian rhythms in individual SCN cells synchronize via neurotransmitters, and synchronized circadian rhythms in the SCN regulate behaviors such as sleep and wake cycles
Remarkably, almost all SCN neurons express γ-amino butyric acid (GABA) [1, 73]. GABAergic neurons in the SCN contain GABA vesicular transporters (VGAT), [8] and GABA synthesizing enzymes, such as glutamate decarboxylase (GAD) [73, 83]. Two distinct isoforms of GAD (GAD65 and GAD67) are found within the SCN, and they have different molecular weights and subcellular distributions. A study using immunohistochemistry determined that GAD65 and GAD67 are highly expressed in the ventral SCN of rats, similar to the distribution of VGAT [8]. GABA receptors, GABAA and GABAB, are also found in the SCN [8, 21, 106]. GABAB receptors are mainly expressed in the dorsal area in the SCN, which generally corresponds to the region of AVP positive neurons [8].
The timing of GABA synthesis, trafficking, and release are important for the modulation of circadian rhythms in the SCN. It was reported that mRNA levels of the GABA synthesizing enzyme GAD65 were higher in the light than in the dark period, but mRNA levels of GAD67 were not rhythmic in the SCN [38]. Another group also reported that both GAD65 and GAD67 mRNA showed circadian oscillations under constant darkness [13]. VGAT contents in the SCN did not exhibit circadian rhythms, but expression levels were attenuated under constant darkness compared with light dark conditions [18]. Furthermore, intercellular signals, such as VIP, could modulate GABA release in the SCN [24, 44].
Excitatory and/or inhibitory effects of GABA in the SCN
Although the effects of GABA on cellular activity in the SCN have been studied for more than 30 years, results remain controversial. For example, one study demonstrated that GABA had inhibitory effects on almost all SCN neurons [65], while several studies have reported that GABA has both excitatory and inhibitory effects in the SCN [15, 22, 57, 101, 116]. Wanger et al. reported that in SCN tissue slices, the application of GABA decreased firing frequency at night, and increased firing frequency during the day. This result indicates that the effects of GABA in SCN neurons are dependent on the circadian phase [116]. However, some investigators have reported no difference in the excitatory and inhibitory effects of GABA during the subjective day compared to night [15, 26, 57, 58, 65]. Similar to the application of GABA, GABA agonists, such as muscimol and baclofen, induce excitatory or inhibitory effects in the SCN [26, 39, 70], while GABA has also been shown to produce either excitatory or inhibitory effects on firing across the circadian day [22]. These effects were inhibited by the GABAA receptor antagonist bicuculline [2, 15, 22, 26, 65]. Regional differences in the effects of GABA in the SCN have also been reported. Short-term application of bicuculline increased neuronal activity in the ventral SCN, but decreased activity in dorsal SCN slices [2]. These pharmacological approaches have shown inconsistent observations regarding the acute effects of GABA in the SCN. Furthermore, these agents may represent non-specific pharmacological actions. For example, the GABAA receptor antagonist, bicuculline is generally known to block small-conductance calcium-activated potassium channels [45, 51] and does so in SCN neurons [110]. It is necessary to consider this non-specific effect of these drugs.
Coupling circadian oscillators in the SCN
Individual SCN neurons exhibit autonomous circadian rhythms even when isolated in culture [119, 120]. The circadian period, phase, and amplitude differ from each other in dispersed cell culture, although their rhythms are synchronized in SCN slices [33, 36, 79, 84] and in vivo [42, 122]. Due to the involvement of heterogeneous oscillators in the SCN, individual SCN cells must couple to each other. Synchronized circadian rhythms in the SCN entrain light–dark cycles to adapt to environmental light–dark conditions. It is thought that GABA may be involved in mediating circadian rhythm coupling in individual SCN neurons.
Synchronizer or destabilizer of cellular circadian rhythms
Liu and Reppert demonstrated that the application of GABA on dispersed SCN cultured cells completely inhibited spontaneous firing at all circadian phases. The application of GABA after the peak phase of neuronal activity rhythms induced a large phase delay. The GABAA agonist muscimol induced phase shifts of neuronal activity rhythms in SCN neurons, whereas the application of the GABAB agonist bacrofen had no effect, suggesting that this phase-dependent shift is mediated by GABAA receptors. Additionally, GABA was applied daily to dispersed SCN cultured cells to assess the effects of circadian rhythms in individual SCN neurons. After administration of GABA for 3 h every 24 h for 5 days, circadian rhythms of spontaneous firings in individual SCN neurons synchronized. This result has been interpreted as evidence that the daily application of GABA synchronized circadian firing rhythms in the dispersed SCN cell culture [60] (Fig. 3a). However, these data do not exclude the effects of other transmitters, such as neuropeptides.
Fig. 3.
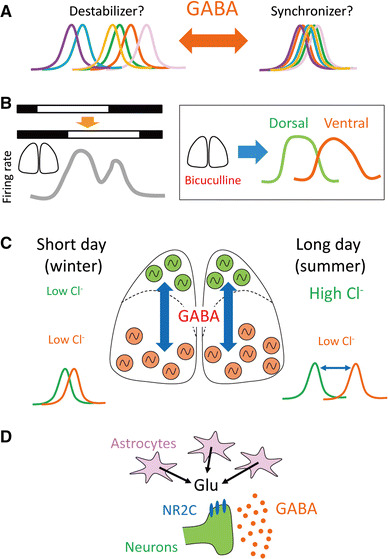
Possible roles of GABA in SCN cellular coupling. a GABA acts as both a synchronizer and destabilizer. b A phase delay shift results in bimodal neuronal activity in the intact SCN slice; however, after the application of GABAA receptor antagonists, circadian rhythms in the dorsal and ventral SCN are dissociated. c Long-day photoperiods change the intracellular chloride concentration in the dorsal SCN and decoupled circadian rhythms between the dorsal and ventral SCN. d Hypothetical model of the astrocytic-neuronal intercellular axis in the SCN
Monitoring gene expression or protein products of clock genes in the SCN using bioluminescence reporters has provided insights into the understanding of circadian rhythms [121, 123, 125]. Based on the evidence for the role of GABA in the synchronization of circadian rhythms in the SCN [60], antagonizing GABA receptors may induce desynchronization of cellular circadian rhythms. However, long-term application of GABAA (bicuculline) and GABAB (saclofen) receptor antagonists into the Per1 promoter-driven luciferase reporter (Per1-luc) SCN slices gradually increased the amplitude of circadian rhythms compared with controls at the tissue level [4]. This increased amplitude of circadian rhythms was due to increased amplitude of cellular circadian rhythms and decreased cycle-to-cycle period variation (increased precision of cellular rhythms). GABA receptor antagonists (bicuculline and saclofen) also increased the amplitude of circadian firing rhythms in dispersed cultured cells [4]. Similarly, blocking GABAA receptor signaling with the application of gabazine onto the Per2 protein fusion luciferase reporter (PER2::LUC) SCN slices decreased circadian period variability in individual cells compared to vehicle-treated controls [22]. These results suggest that GABA destabilizes circadian oscillations in the SCN (Fig. 3a).
Coupling of dorsal and ventral circadian oscillators
Constant light causes splitting of circadian behavioral rhythms [89]. The circadian rhythms of the shell and core, or left and right SCN, exhibited a 180° antiphase during splitting under constant light conditions [82, 124]. Another study showed that an abrupt change in the light–dark cycle disrupted synchronous oscillation of circadian components in the rat SCN [76]. After a phase delay shift of light–dark cycles, clock gene expression rhythms shifted rapidly in the ventrolateral SCN, whereas this shift occurred more slowly in the dorsomedial SCN [76]. Several researchers have demonstrated a role of GABA in the re-synchronization of the dorsal and ventral regions of the SCN after manipulation of environmental light–dark cycles.
Albus et al. revealed the role of GABA in coupling dorsal and ventral SCN circadian rhythms in acute SCN slices. Measurements of neuronal activity in the SCN following a 6-h phase delay in the light/dark schedule revealed bimodal patterns of activity rhythms. Furthermore, when the SCN was cut horizontally, separating the slice into dorsal and ventral areas, the peak phase of neuronal activity rhythms in the ventral SCN was significantly advanced compared with that in the dorsal SCN. Continuous application of a GABAA receptor antagonist (bicuculline) yielded similar results [2] (Fig. 3b). These results indicate that GABA is important for coupling circadian rhythms in the dorsal and ventral SCN.
Photoperiodic changes in SCN cellular networks
Environmental light–dark conditions change depending on the season. This photoperiodic change is important for seasonal reproduction in some animals [81, 126]. The long-day photoperiod also changes cellular networks in the SCN and decouples circadian oscillatory cell groups [30, 41, 77]. Recently, the role of GABAA receptors in coupling SCN dorsal and ventral circadian oscillators was reported to occur during the long-day photoperiod [19] (Fig. 3c). PER2::LUC reporter mice were exposed to 20 h of light and 4 h of dark (LD20:4), and the PER2::LUC bioluminescence was measured from the SCN slice. In these conditions, the circadian phase of the SCN core was advanced compared with that of the shell. This phase difference between the dorsal and ventral region in the SCN gradually returned to an organizational state similar to that observed under LD12:12 conditions. To investigate the role of GABAA signaling in the re-synchronization of dorsal and ventral oscillators after LD20:4 conditions, the GABAA receptor antagonist, bicuculline, was applied to the SCN slice. Bicuculline attenuated the re-synchronization when the circadian phase between dorsal and ventral regions was out of phase, but not when it was in-phase. These results were interpreted as evidence that GABAA signaling contributes to the synchronization of circadian rhythms between the dorsal and ventral SCN in a state-dependent manner. An alternative interpretation would suggest that GABA may acutely modulate firing without having much, if any, effect on circadian phase. These results also suggest that GABA is sufficient for the synchronization of dispersed SCN cells [60] and that the absence of GABA does not desynchronize cellular rhythms under steady-state networks in the SCN [4].
Intracellular chloride concentrations and cellular coupling
Long-day photoperiods also change the excitability [20], and levels of chloride transporter expression in SCN neurons. This determines the difference in circadian phase and period between the dorsal and ventral SCN [75] (Fig. 3c). It was observed that under long-day conditions, the phase difference in Bmal1 promoter-driven luciferase reporter (Bmal1-Eluc) circadian rhythms between the dorsal and ventral SCN were increased, and the circadian period of the dorsal SCN was decreased compared with the ventral SCN. Myung et al. also measured intracellular chloride concentrations in the SCN, using N-(ethoxycarbonylmethyl)-6-methoxyquinoliniumbromide fluorescence, and found that intracellular chloride concentrations were increased under long-day photoperiods. These results are due to a higher expression ratio of sodium/potassium/chloride cotransporter (NKCC1)/potassium/chloride cotransporters (KCC2), in the dorsal than the ventral SCN. Because NKCC1 is a chloride importer, a high ratio of NKCC1/KCC2 results in more GABA-induced excitation. KCC2 is expressed exclusively in VIP and GRP neurons, whereas NKCC1 is expressed in VIP, GRP, and AVP neurons within the SCN [7]. Recently, Klett and Allen reported that intracellular chloride concentrations were higher during the day than at night in both AVP- and VIP-positive neurons [52]. The prevalence of GABA excitation and inhibition is dependent on the level of chloride transporter expression and may affect the coupling of dorsal and ventral SCN circadian oscillations.
Astrocytes and GABA
In addition to neurons, astrocytes in the SCN are involved in circadian rhythms. Individual astrocytes display circadian rhythms entrained to daily temperature cycles [90]. Moreover, astrocytes co-cultured with adult SCN explants sustained the rhythms of astrocytes, suggesting that diffusible factors from the SCN are sufficient to entrain circadian oscillations in astrocytes [90]. Furthermore, VIP expressed in SCN neurons entrains astrocyte circadian rhythms [63]. It is known that astrocytes release ATP into the extracellular space and it has been shown that cultured astrocytes display daily oscillations of extracellular ATP concentrations that are under circadian control [64]. Astrocytes also regulate neuronal networks through the reuptake and release of various transmitters including glutamate [27, 88]; however, the functional mechanisms of these transmitters in astrocytes have yet to be identified. Recently, three groups reported that astrocytes regulate SCN and behavioral circadian rhythms [5, 10, 112]. Astrocyte-specific deletion of the Bmal1 gene led to reduced expression of astrocytic GABA transporter 1 and 3 (GAT1 and GAT3), suggesting a potential impairment in the clearance of extracellular GABA released by neurons [5]. Brancaccio et al. proposed a new model of circadian timekeeping in the SCN. They hypothesized that in the SCN, glutamate released from astrocytes maintains higher intracellular calcium levels, specifically in pre-synaptic terminals, through the activation of NMDA receptors (NR2C), which subsequently facilitates neuronal GABA release [10] (Fig. 3d). Because the peak phase of circadian calcium rhythms in astrocytes was observed at night, GABA release from neurons via glutamate release from astrocytes would increase at night, resulting in a decrease in neuronal activity at night.
Circadian outputs and GABA functions
Circadian rhythms in SCN neurons synchronize via several neurotransmitters, such as AVP, VIP, and GRP [3, 66, 67]. To regulate sleep/wake cycles, SCN circadian rhythms need to send outputs to peripheral circadian oscillators. Neuronal activity in the SCN is one of the most important input and/or output signals from molecular oscillations. Application of the sodium channel blocker, tetrodotoxin, into the SCN in vivo resulted in arrhythmicity in behavior without affecting the circadian oscillation [97]. In addition, optogenetic stimulation of the SCN in vivo modulated circadian behavioral rhythms [46]. GABA, therefore, may regulate the transmission of circadian output from the SCN by changing neuronal excitability. In contrast, it is also reported that GABA is involved in the synaptic plasticity changes in the retinohypothalamic tract-SCN synapses [69]. These results indicate that GABA modulates both input and output of circadian oscillation in the SCN.
Identification of SCN circadian outputs is important for understanding the mechanisms underlying the regulation of behavioral rhythms. Previously, SCN efferent projections have been investigated [50, 54, 104, 117, 118]. Neurons in the dorsal SCN project densely to the preoptic area, paraventricular nucleus (PVN), dorsomedial hypothalamus, and subparaventricular zone (SPVZ). In contrast, dense projections from the core are limited to the peri-suprachiasmatic area (PSCN), lateral SPVZ, and ventral tuberal area (VTU) [54]. Interestingly, the circadian rhythms of neuronal activity outside the SCN in vivo were in antiphase compared with the SCN in nocturnal animals [42, 80]. Importantly, in both diurnal and nocturnal animals, the peak phase of neuronal activity rhythms in the SCN is observed during the day. However, in diurnal animals, the peak phase of neuronal activity outside of the SCN is in-phase compared with that of the SCN [95], suggesting that circadian information from the SCN is transferred to output brain areas, and that this mechanism is different in nocturnal and diurnal animals. The mechanisms for switching day-night information from the SCN, however, have not been identified.
The PVN receives an efferent projection from the SCN. The PVN contains several neuropeptides, such as corticotrophin-releasing factor, oxytocin, and AVP, and regulates endocrine and autonomic functions. Electrical stimulation of the SCN evoked monosynaptic inhibitory postsynaptic potentials, as well as excitatory postsynaptic potentials in the PVN, indicating that GABA and glutamate are important mediators of fast monosynaptic transmission from the SCN to the PVN [31, 32]. Tousson and Meissl used a multi-electrode array dish to demonstrate that humoral factors are responsible for circadian rhythms in the PVN [111]. They observed circadian rhythms in PVN neuronal activity measured in brain slices containing both PVN and SCN. When the SCN was removed from the brain slice, PVN circadian rhythms disappeared and were subsequently restored by co-cultured SCN grafts.
The SCN regulates circadian rhythms of plasma melatonin concentrations via a multi-synaptic pathway, including the PVN, sympathetic preganglionic neurons of the spinal cord, and noradrenergic sympathetic neurons of the superior cervical ganglion [72]. The melatonin concentration is increased at night and decreased during the day. Blocking GABAergic transmission to the PVN results in inhibition of melatonin synthesis in the pineal gland [48, 49], suggesting that GABAergic signals from the SCN have an inhibitory effect on melatonin synthesis during the day. Importantly, melatonin administration suppressed spontaneous firing in the SCN [61, 99]. Neuronal and humoral regulation of PVN neuronal activity by the SCN is important for temporal integration of physiological events.
Variety of circadian recording methods
Several studies have attempted to understand the function of GABA in the SCN, using both ex vivo and in vivo methods (Fig. 4). Primary culture techniques (dispersed and slice) are useful for measuring SCN circadian rhythms. Typically, the SCN from neonatal animals is used for this experiment [34, 60, 79, 120]; however, acute SCN slices from adult animals have also been used for recording circadian rhythms [2, 25, 116]. Neonatal SCN tissue allows researchers to measure circadian rhythms over long periods; however, the cellular properties and neuronal networks in the SCN may not be the same in the adult. In general, NKCC1 expression predominates in immature neurons, in which the intracellular concentration of chloride ions is relatively high, whereas KCC2 expression predominates in mature neurons. These developmental changes of chloride transporters modulate excitability to GABA depending on age [9]. Developmental effects of GABA on SCN circadian oscillations have not been fully identified. Recently, however, we reported developmental changes of neuronal networks in the SCN, as Ono et al. demonstrated that Cryptochrome (Cry), a clock gene, is involved in cellular coupling in the adult SCN [84, 85]. GABA may have an important role in cellular coupling and circadian outputs in the SCN, depending on the developmental stage.
Fig. 4.
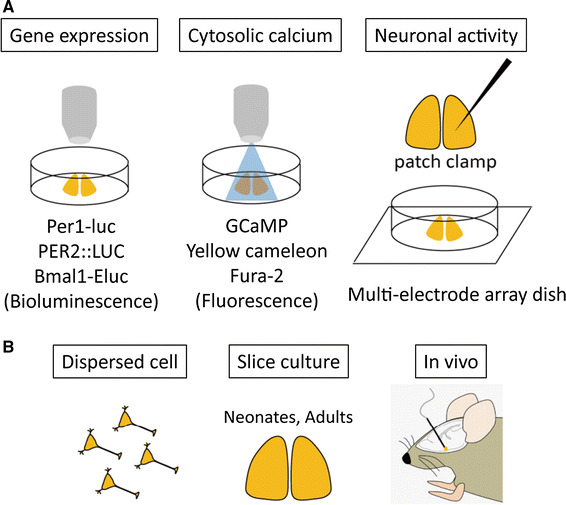
Variety of circadian rhythm recording techniques. a Different parameters can be measured using different methods. Clock gene expression rhythms can be measured with bioluminescence reporters. Cytosolic calcium concentrations can be measured with fluorescent probes. Neuronal activity can be recorded using patch clamp or multi-electrode array dish techniques. b SCN cell preparations for recording circadian rhythms. Individual SCN properties may depend on the coupling strength and the age of mice
GABA functions have been studied using various recording methods, such as neuronal activity recording, calcium imaging, and bioluminescence imaging [2, 4, 40, 43, 60, 101, 116]. These recording methods are useful for measuring circadian oscillations in the SCN. However, circadian oscillation in measurements such as gene expression, intracellular calcium levels, and neuronal activity do not show the same properties. For instance, Vansteensel et al. measured Per1-luc and neuronal activity rhythms in both acute SCN slices and in vivo, during a 6-h light/dark phase advance schedule. Per1 and neuronal activity rhythms in SCN slices demonstrated a phase shift immediately after the 6-h phase advance, whereas in vivo neuronal activity did not immediately shift, indicating that neuronal activity was dissociated between brain slices and in vivo [115]. Ono et al. also demonstrated that circadian rhythms of the clock genes, Per1 and Bmal1, were dissociated in cultured SCN slices [86]. We have successfully simultaneously measured Per1, Bmal1, cytosolic calcium ions, and neuronal activity in SCN slices and found that the circadian period of Bmal1-ELuc rhythms is shorter than that of Per1-luc rhythms. Furthermore, the circadian period of calcium and neuronal activity rhythms were intermediate between Per1 and Bmal1 rhythms. Simultaneous and multifunctional recording of circadian oscillation is a useful tool for understanding the hierarchal structure of SCN networks in addition to the roles of GABA within these networks.
Perspectives
All studies that have addressed the function of GABA in the SCN have only used pharmacological approaches. Because mice with genetic GABA deficiencies, such as VGAT- and GAD65/67-deficient mice, cannot survive after birth [47, 94], it is difficult to assess the roles of GABA in the SCN using a genetic approach. It is possible to measure SCN circadian rhythms obtained from embryos of VGAT- or GAD65/67-deficient mice. However, this slice culture approach is not enough to understand mechanisms of circadian rhythms as they relate to behavior. A conditional knockout method could potentially provide a solution to this problem. Recently, several Cre driver mice have been used to conditionally disrupt genes of interest in the SCN [6, 28, 55, 68]. Creating conditional KO mice is useful; however, there are currently no SCN-specific Cre driver mice. While Neuromedin-S is specifically expressed in the SCN, not all SCN neurons express this neuropeptide [55]. Recently, a Crispr/Cas9 genome editing method, a powerful tool for manipulating genes of interest in various species [17], was developed and has been used to manipulate signaling in specific cell types of the SCN [112]. Adeno-associated viral driven Crispr/Cas9 techniques may be applicable to the study of GABA function specifically in the SCN [92]. Since transfection and expression efficiency are not perfect, however, it is difficult to control viral diffusion outside of the SCN. Therefore, alternative methods are required for SCN-specific functional GABA deficiency.
To understand the roles of GABA in circadian physiology and behavior, it is important to identify neuronal networks and output pathways from the SCN. Several new methods have been developed to accomplish this. For example, the glycoprotein-deleted (DG) rabies virus is a useful tool for studies of neural circuits [87]. In combination with Cre mice, we can identify direct input and output pathways of specific neurons [98]. However, because neurons infected with the rabies virus are killed by approximately 14 days after infection [87], manipulations or recordings of neuronal activity are limited to this short time window. Recently, self-inactivating rabies virus has been developed, which may allow us to further understand neuronal brain networks [16]. AAV-mediated retrograde tracing methods have also been developed, which could allow us to label, manipulate, and measure retrograde-labeled neurons [109]. Identification of a local GABA circuit in the SCN and long-range brain networks may be important for the understanding of circadian physiology and behavior in the future.
Acknowledgements
This work was supported in part by the Uehara Memorial Foundation, the Nakajima Foundation, GSK Japan Research Grant 2015, the Project for Developing Innovation Systems of the MEXT, and Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, Ministry of Education, Culture, Sports, Science and Technology, Japan, and JSPS KAKENHI (Nos. 15H04679, 26860156, 15K12763).
Compliance with ethical standards
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 2.Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 3.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci USA. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, De Pietri Tonelli D. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nature Commun. 2017;8:14336. doi: 10.1038/ncomms14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedont JL, LeGates TA, Slat EA, Byerly MS, Wang H, Hu J, Rupp AC, Qian J, Wong GW, Herzog ED, Hattar S, Blackshaw S. Lhx1 controls terminal differentiation and circadian function of the suprachiasmatic nucleus. Cell Rep. 2014;7:609–622. doi: 10.1016/j.celrep.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belenky MA, Sollars PJ, Mount DB, Alper SL, Yarom Y, Pickard GE. Cell-type specific distribution of chloride transporters in the rat suprachiasmatic nucleus. Neuroscience. 2010;165:1519–1537. doi: 10.1016/j.neuroscience.2009.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 10.Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron. 2017;93(1420–1435):e1425. doi: 10.1016/j.neuron.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant DN, LeSauter J, Silver R, Romero MT. Retinal innervation of calbindin-D28 K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J Biol Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cagampang FR, Rattray M, Powell JF, Campbell IC, Coen CW. Circadian changes of glutamate decarboxylase 65 and 67 mRNA in the rat suprachiasmatic nuclei. NeuroReport. 1996;7:1925–1928. doi: 10.1097/00001756-199608120-00011. [DOI] [PubMed] [Google Scholar]
- 14.Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13:415–431. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- 15.Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim DY, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosc. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciabatti E, Gonzalez-Rueda A, Mariotti L, Morgese F, Tripodi M. Life-long genetic and functional access to neural circuits using self-inactivating rabies virus. Cell. 2017;170(382–392):e314. doi: 10.1016/j.cell.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science (New York, N.Y.) 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darna M, Schmutz I, Richter K, Yelamanchili SV, Pendyala G, Holtje M, Albrecht U, Ahnert-Hilger G. Time of day-dependent sorting of the vesicular glutamate transporter to the plasma membrane. J Biol Chem. 2009;284:4300–4307. doi: 10.1074/jbc.M805480200. [DOI] [PubMed] [Google Scholar]
- 19.Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron. 2013;80:973–983. doi: 10.1016/j.neuron.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farajnia S, van Westering TL, Meijer JH, Michel S. Seasonal induction of GABAergic excitation in the central mammalian clock. Proc Natl Acad Sci USA. 2014;111:9627–9632. doi: 10.1073/pnas.1319820111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francois-Bellan AM, Hery M, Faudon M, Hery F. Analysis of the inhibitory effect of oestradiol on functional GABA/5-HT relationship in the rat suprachiasmatic area. J Neuroendocrinol. 1989;1:415–422. doi: 10.1111/j.1365-2826.1989.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 22.Freeman GM, Jr, Krock RM, Aton SJ, Thaben P, Herzog ED. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron. 2013;78:799–806. doi: 10.1016/j.neuron.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science (New York, N.Y.) 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 24.Gompf HS, Irwin RP, Allen CN. Retrograde suppression of GABAergic currents in a subset of SCN neurons. Eur J Neurosci. 2006;23:3209–3216. doi: 10.1111/j.1460-9568.2006.04850.x. [DOI] [PubMed] [Google Scholar]
- 25.Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- 26.Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, de Jeu MT, Pennartz CM, Dudek FE. A reexamination of the role of GABA in the mammalian suprachiasmatic nucleus. J Biol Rhythms. 1999;14:126–130. doi: 10.1177/074873099129000515. [DOI] [PubMed] [Google Scholar]
- 27.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatori M, Gill S, Mure LS, Goulding M, O’Leary DD, Panda S. Lhx1 maintains synchrony among circadian oscillator neurons of the SCN. Elife. 2014;3:e03357. doi: 10.7554/eLife.03357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazlerigg DG, Ebling FJ, Johnston JD. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr Biol. 2005;15:R449–R450. doi: 10.1016/j.cub.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Hermes ML, Buijs RM, Renaud LP. Electrophysiology of suprachiasmatic nucleus projections to hypothalamic paraventricular nucleus neurons. Prog Brain Res. 1996;111:241–252. doi: 10.1016/s0079-6123(08)60412-4. [DOI] [PubMed] [Google Scholar]
- 32.Hermes ML, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. J Physiol. 1996;496(Pt 3):749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- 34.Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 35.Honma S. The mammalian circadian system: a hierarchical multi-oscillator structure for generating circadian rhythm. J Physiol Sci. 2018 doi: 10.1007/s12576-018-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honma S, Nakamura W, Shirakawa T, Honma K. Diversity in the circadian periods of single neurons of the rat suprachiasmatic nucleus depends on nuclear structure and intrinsic period. Neurosci Lett. 2004;358:173–176. doi: 10.1016/j.neulet.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma K. Circadian periods of single suprachiasmatic neurons in rats. Neurosci Lett. 1998;250:157–160. doi: 10.1016/s0304-3940(98)00464-9. [DOI] [PubMed] [Google Scholar]
- 38.Huhman KL, Hennessey AC, Albers HE. Rhythms of glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus. J Biol Rhythms. 1996;11:311–316. doi: 10.1177/074873049601100404. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda M, Toyoda H, Yamada J, Okabe A, Sato K, Hotta Y, Fukuda A. Differential development of cation-chloride cotransporters and Cl- homeostasis contributes to differential GABAergic actions between developing rat visual cortex and dorsal lateral geniculate nucleus. Brain Res. 2003;984:149–159. doi: 10.1016/s0006-8993(03)03126-3. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda M, Yoshioka T, Allen CN. Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17:58–70. doi: 10.1046/j.1460-9568.2003.02427.x. [DOI] [PubMed] [Google Scholar]
- 41.Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci USA. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- 45.Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- 46.Jones JR, Tackenberg MC, McMahon DG. Manipulating circadian clock neuron firing rate resets molecular circadian rhythms and behavior. Nat Neurosci. 2015;18:373–375. doi: 10.1038/nn.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakizaki T, Oriuchi N, Yanagawa Y. GAD65/GAD67 double knockout mice exhibit intermediate severity in both cleft palate and omphalocele compared with GAD67 knockout and VGAT knockout mice. Neuroscience. 2015;288:86–93. doi: 10.1016/j.neuroscience.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Kalsbeek A, Drijfhout WJ, Westerink BH, van Heerikhuize JJ, van der Woude TP, van der Vliet J, Buijs RM. GABA receptors in the region of the dorsomedial hypothalamus of rats are implicated in the control of melatonin and corticosterone release. Neuroendocrinology. 1996;63:69–78. doi: 10.1159/000126937. [DOI] [PubMed] [Google Scholar]
- 49.Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 50.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 51.Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- 52.Klett NJ, Allen CN. Intracellular chloride regulation in AVP+ and VIP+ neurons of the suprachiasmatic nucleus. Sci Rep. 2017;7:10226. doi: 10.1038/s41598-017-09778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 54.Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- 55.Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, Ikeda Y, Motoike T, Dixon S, Seinfeld JE, Takahashi JS, Yanagisawa M. Neuromedin S-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron. 2015;85:1086–1102. doi: 10.1016/j.neuron.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liou SY, Albers HE. Single unit response of neurons within the hamster suprachiasmatic nucleus to GABA and low chloride perfusate during the day and night. Brain Res Bull. 1990;25:93–98. doi: 10.1016/0361-9230(90)90257-z. [DOI] [PubMed] [Google Scholar]
- 58.Liou SY, Shibata S, Albers HE, Ueki S. Effects of GABA and anxiolytics on the single unit discharge of suprachiasmatic neurons in rat hypothalamic slices. Brain Res Bull. 1990;25:103–107. doi: 10.1016/0361-9230(90)90259-3. [DOI] [PubMed] [Google Scholar]
- 59.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 61.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 62.Mai JK, Kedziora O, Teckhaus L, Sofroniew MV. Evidence for subdivisions in the human suprachiasmatic nucleus. J Comp Neurol. 1991;305:508–525. doi: 10.1002/cne.903050312. [DOI] [PubMed] [Google Scholar]
- 63.Marpegan L, Krall TJ, Herzog ED. Vasoactive intestinal polypeptide entrains circadian rhythms in astrocytes. J Biol Rhythms. 2009;24:135–143. doi: 10.1177/0748730409332042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C. Circadian regulation of ATP release in astrocytes. J Neurosci. 2011;31:8342–8350. doi: 10.1523/JNEUROSCI.6537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mason R, Biello SM, Harrington ME. The effects of GABA and benzodiazepines on neurones in the suprachiasmatic nucleus (SCN) of Syrian hamsters. Brain Res. 1991;552:53–57. doi: 10.1016/0006-8993(91)90659-j. [DOI] [PubMed] [Google Scholar]
- 66.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 68.Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K, Honma S, Sakurai T. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron. 2015;85:1103–1116. doi: 10.1016/j.neuron.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Moldavan MG, Allen CN. GABAB receptor-mediated frequency-dependent and circadian changes in synaptic plasticity modulate retinal input to the suprachiasmatic nucleus. J Physiol. 2013;591:2475–2490. doi: 10.1113/jphysiol.2012.248047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moldavan MG, Irwin RP, Allen CN. Presynaptic GABA(B) receptors regulate retinohypothalamic tract synaptic transmission by inhibiting voltage-gated Ca2+ channels. J Neurophysiol. 2006;95:3727–3741. doi: 10.1152/jn.00909.2005. [DOI] [PubMed] [Google Scholar]
- 71.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 72.Moore RY, Klein DC. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71:17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- 73.Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- 74.Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 75.Myung J, Hong S, DeWoskin D, De Schutter E, Forger DB, Takumi T. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc Natl Acad Sci USA. 2015;112:E3920–E3929. doi: 10.1073/pnas.1421200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein E, Sehgal A, Shigeyoshi Y. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci. 2003;23:6141–6151. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naito E, Watanabe T, Tei H, Yoshimura T, Ebihara S. Reorganization of the suprachiasmatic nucleus coding for day length. J Biol Rhythms. 2008;23:140–149. doi: 10.1177/0748730408314572. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura TJ, Takasu NN, Nakamura W. The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci. 2016;66:367–374. doi: 10.1007/s12576-016-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- 80.Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD, Takumi T. In vivo monitoring of circadian timing in freely moving mice. Curr Biol. 2008;18:381–385. doi: 10.1016/j.cub.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 81.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 82.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 83.Okamura H, Berod A, Julien JF, Geffard M, Kitahama K, Mallet J, Bobillier P. Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: in situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neurosci Lett. 1989;102:131–136. doi: 10.1016/0304-3940(89)90067-0. [DOI] [PubMed] [Google Scholar]
- 84.Ono D, Honma S, Honma K. Cryptochromes are critical for the development of coherent circadian rhythms in the mouse suprachiasmatic nucleus. Nature Commun. 2013;4:1666. doi: 10.1038/ncomms2670. [DOI] [PubMed] [Google Scholar]
- 85.Ono D, Honma S, Honma K. Differential roles of AVP and VIP signaling in the postnatal changes of neural networks for coherent circadian rhythms in the SCN. Sci Adv. 2016;2:e1600960. doi: 10.1126/sciadv.1600960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ono D, Honma S, Nakajima Y, Kuroda S, Enoki R, Honma KI. Dissociation of Per1 and Bmal1 circadian rhythms in the suprachiasmatic nucleus in parallel with behavioral outputs. Proc Natl Acad Sci USA. 2017;114:E3699–E3708. doi: 10.1073/pnas.1613374114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Pittendrigh CS, Daan S. Functional-analysis of circadian pacemakers in nocturnal rodents. 5. Pacemaker structure—clock for all seasons. J Comp Physiol. 1976;106:333–355. [Google Scholar]
- 90.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science (New York, N.Y.) 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 92.Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu XB, Makarova KS, Koonin EV, Sharp PA, Zhang F. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:U186–U198. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 94.Saito K, Kakizaki T, Hayashi R, Nishimaru H, Furukawa T, Nakazato Y, Takamori S, Ebihara S, Uematsu M, Mishina M, Miyazaki J, Yokoyama M, Konishi S, Inoue K, Fukuda A, Fukumoto M, Nakamura K, Obata K, Yanagawa Y. The physiological roles of vesicular GABA transporter during embryonic development: a study using knockout mice. Mol Brain. 2010;3:40. doi: 10.1186/1756-6606-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato T, Kawamura H. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus) Neurosci Res. 1984;1:45–52. doi: 10.1016/0168-0102(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 96.Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scott FF, Belle MD, Delagrange P, Piggins HD. Electrophysiological effects of melatonin on mouse Per1 and non-Per1 suprachiasmatic nuclei neurones in vitro. J Neuroendocrinol. 2010;22:1148–1156. doi: 10.1111/j.1365-2826.2010.02063.x. [DOI] [PubMed] [Google Scholar]
- 100.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science (New York, N.Y.) 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 101.Shirakawa T, Honma S, Katsuno Y, Oguchi H, Honma KI. Synchronization of circadian firing rhythms in cultured rat suprachiasmatic neurons. Eur J Neurosci. 2000;12:2833–2838. doi: 10.1046/j.1460-9568.2000.00170.x. [DOI] [PubMed] [Google Scholar]
- 102.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 103.Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28 K cells in the hamster SCN express light-induced Fos. NeuroReport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- 104.Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6:2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- 105.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strecker GJ, Wuarin JP, Dudek FE. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J Neurophysiol. 1997;78:2217–2220. doi: 10.1152/jn.1997.78.4.2217. [DOI] [PubMed] [Google Scholar]
- 107.Sujino M, Masumoto KH, Yamaguchi S, van der Horst GT, Okamura H, Inouye ST. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr Biol. 2003;13:664–668. doi: 10.1016/s0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 108.Tanaka M, Hayashi S, Tamada Y, Ikeda T, Hisa Y, Takamatsu T, Ibata Y. Direct retinal projections to GRP neurons in the suprachiasmatic nucleus of the rat. NeuroReport. 1997;8:2187–2191. doi: 10.1097/00001756-199707070-00020. [DOI] [PubMed] [Google Scholar]
- 109.Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Teshima K, Kim SH, Allen CN. Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience. 2003;120:65–73. doi: 10.1016/s0306-4522(03)00270-7. [DOI] [PubMed] [Google Scholar]
- 111.Tousson E, Meissl H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J Neurosci. 2004;24:2983–2988. doi: 10.1523/JNEUROSCI.5044-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr Biol. 2017;27:1055–1061. doi: 10.1016/j.cub.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 114.VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 115.Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr Biol. 2003;13:1538–1542. doi: 10.1016/s0960-9822(03)00560-8. [DOI] [PubMed] [Google Scholar]
- 116.Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- 117.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 118.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 119.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science (New York, N.Y.) 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 122.Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci. 1998;18:10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science (New York, N.Y.) 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 124.Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci. 2005;25:9017–9026. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]


