Abstract
We hypothesized that the role of stroke volume (SV) in the metaboreflex-induced cardiac output (CO) increase was blunted when the metaboreflex was stimulated by exercise muscle ischemia (EMI) compared with post-exercise muscle ischemia (PEMI), because during EMI heart rate (HR) increases and limits diastolic filling. Twelve healthy volunteers were recruited and their hemodynamic responses to the metaboreflex evoked by EMI, PEMI, and by a control dynamic exercise were assessed. The main finding was that the blood pressure increment was very similar in the EMI and PEMI settings. In both conditions the main mechanism used to raise blood pressure was a CO elevation. However, during the EMI test CO was increased as a result of HR elevation whereas during the PEMI test CO was increased as a result of an increase in SV. These results were explainable on the basis of the different HR behavior between the two settings, which in turn led to different diastolic time and myocardial performance.
Keywords: Blood pressure, Exercise, Impedance cardiography, Myocardial contractility, Cardiac preload
Introduction
The muscle “metaboreflex”, a reflex evoked by those afferent nerve endings sensitive to accumulation of muscle metabolic end-products, is attracting growing interest in the scientific community because it is thought to contribute significantly to cardiovascular regulation during exercise by providing continuous feedback to the cardiovascular control areas about the metabolic status of contracting muscles [4, 26, 30, 31, 35, 36].
Our group has recently conducted several experiments on humans to investigate the hemodynamic effect of the metaboreflex recruitment by using the method of post-exercise muscle ischemia (PEMI) after dynamic exercise [6–10]. From these experiments it became apparent that, at least for healthy individuals, the blood pressure increment occurring during the metaboreflex obtained by means of PEMI is mainly the result of a flow-mediated mechanism, i.e. a rise in cardiac output (CO), whereas peripheral vasoconstriction has little or no effect. Rather, the latter mechanism is predominant when no further reserve of myocardial contractility is available, for example in heart failure patients [8]. The CO elevation is, in turn, the consequence of an increased stroke volume (SV), which takes place because of an improvement in myocardial performance and cardiac filling. Moreover, from our experiments and from results from other investigators [19, 25, 26, 37] it seems well established that in the PEMI setting heart rate (HR) cannot participate in the flow-mediated response because withdrawal of central command at the end of exercise and increased baroreflex activity enhance the parasympathetic tone and counterbalance the increased sympathetic outflow, thus preventing HR from increasing in response to the PEMI maneuver.
However, to date there is little understanding of how SV and HR combine to affect CO during the metaboreflex. It is possible that the increase in SV we described in our previous reports was a consequence of HR behavior, because it is likely that the lack of cardio-acceleration in response to PEMI led to a prolonged diastolic time (DT), which, in turn, improved ventricular filling, thereby recruiting the Frank–Starling mechanism and enhancing myocardial performance and SV [1, 6, 9, 33]. In fact, it is well known that the role of SV varies with the possibility of elevating HR, because tachycardia reduces DT and consequently ventricular filling. Thus, changes in HR and SV may oppose each other [15] and their combined effect should therefore be investigated. Actually, in our previous investigations we never found any difference in HR behavior between PEMI and control recovery, whereas we often found enhanced myocardial performance and SV. Thus, it may be that our results were affected by the particular HR behavior induced by the PEMI maneuver, i.e. it may be that the relative bradycardia induced by PEMI may have improved cardiac pre-load and increased SV.
Thus, we wondered whether the SV response observed in our previous experiments would have been the same if the metaboreflex was recruited when an HR increment was still possible. This particular situation can be investigated when the metaboreflex is elicited during dynamic exercise, i.e. by trapping the circulation of the working muscle. In this setting the central command is still operating and HR has been reported to be capable of increasing in response to the metaboreflex recruitment [26], whereas, to the best of our knowledge, no one has ever reported that the central command activation improves myocardial performance and/or cardiac filling. Hence, during exercise ischemia the central command and the metaboreflex may exert opposite effects on SV, because the former may reduce DT and cardiac filling, thereby limiting the possibility of increasing SV, whereas the latter may improve both cardiac performance and cardiac filling.
This study was designed to address the hypothesis that the SV response differs depending on the setting of the metaboreflex activation. In particular, we hypothesized that, if the metaboreflex was stimulated during dynamic exercise then the role of SV in the CO increment would be blunted compared with the metaboreflex elicited by means of the PEMI maneuver, because in this setting it was likely that tachycardia limited ventricular filling.
Methods
Study population
Twelve healthy males between the ages of 26 and 45 (mean ± standard error of the mean (SEM) 33.2 ± 1.8 years), whose height and body mass were 174.5 ± 1.4 cm and 73 ± 2.4 kg, respectively, volunteered as subjects. All were physically active and none had any history of cardiac or respiratory disease or was taking any medication and none showed any abnormalities on physical examination. The study was performed according to the Declaration of Helsinki and was approved by a local ethics committee. All subjects gave written informed consent.
Experimental design
All experiments were carried out in a temperature-controlled air-conditioned room (temperature set at 22°C and relative humidity 50%). Each subject performed the following rest-exercise-recovery sessions on three separate days (the interval between tests was at minimum 2 days), randomly assigned to eliminate any order effect:
Post-exercise muscle ischemia session (PEMI session): 3 min of resting, followed by 3 min of exercise, consisting of a rhythmic handgrip achieved by squeezing the balloon of a sphygmomanometer (30 squeezes/min) at 30% of the predetermined maximum capacity, followed by 3 min of PEMI on the exercised arm. At the end of the strain PEMI was induced by rapidly (in <3 s) inflating an upper arm biceps tourniquet to 50 mmHg above peak exercise systolic pressure. The cuff was kept inflated for 3 min. Three minutes of recovery were further allowed after the cuff was deflated, for a total of 6 min of recovery. We chose this procedure (mild dynamic arm exercise followed by PEMI) to recruit the metaboreflex because in previous studies [6, 8] it was demonstrated to be able to evoke the hemodynamic adjustments we were seeking in this investigation, i.e. a flow-mediated blood pressure response during the metaboreflex engagement. Moreover, this mild effort enabled exercising during muscle ischemia, which was applied in the protocol session described below.
Exercise muscle ischemia (EMI) session: the same rest-exercise–recovery procedure used for PEMI was performed. However, in this session muscle ischemia was induced in the exercising arm by inflating the biceps tourniquet to 50 mmHg above systolic pressure at the start of exercise. Then, at the end of effort the tourniquet was deflated and 6 min of recovery was allowed.
Control exercise-recovery session (CER session): the same rest-exercise–recovery procedure used for PEMI and EMI was performed without tourniquet inflation. The CER session was introduced to gather control hemodynamic data in a situation which did not recruit the metaboreflex.
As can be inferred, the length of each procedure was the same (i.e. 12 min).
Assessment of physiological variables
Hemodynamic data were measured by using the impedance method, which was previously used in similar experiments dealing with the metaboreflex, with good reproducibility and accuracy [6–10]. In the last few years we have developed a method for processing impedance data during which we eliminated from hemodynamic calculation traces affected by impedance artefacts, thus obtaining reliable and reproducible hemodynamic estimates. This data-acquisition procedure is described in detail elsewhere [5–10]. Briefly, by using an impedance cardiograph (NCCOM 3; BoMed, Irvine, CA, USA) we obtained analog traces of electrocardiogram, thorax impedance (Z 0), and Z 0 first derivative that were stored by means of a digital chart recorder (ADInstruments, PowerLab 8sp, Castle Hill, Australia). The Sramek–Bernstein equation [3] was then used to calculate beat-to-beat SV from stored impedance traces. Also calculated was the pre-ejection period/left ejection time ratio (PEP/VET), which has been reported to be inversely correlated with the angiographic ejection fraction (r = −0.90) and is an inverse index of cardiac performance [22]. Moreover, we assessed DT by subtracting the sum of PEP and VET from the cardiac cycle total period, and, by dividing SV by DT, obtained the ventricular filling rate (VFR), which is a measure of the mean rate of diastolic blood flux [5, 10, 14]. HR was calculated as the reciprocal of the electrocardiogram R–R interval and CO was obtained by multiplying SV × HR.
Subjects were also connected to a manual sphygmomanometer to measure systolic and diastolic blood pressure, which was taken by the same physician throughout all protocol sessions. Mean blood pressure (MBP) was calculated by use of the formula of Moran and co-workers [23], which takes into account changes in the diastolic and systolic periods caused by exercise tachycardia. Systemic vascular resistance (SVR) was calculated by multiplying the MBP/CO ratio by 80, where 80 is a conversion factor to change units to standard resistance units.
Pulmonary ventilation (V E), oxygen uptake (VO2), and carbon dioxide production (VCO2) were measured throughout tests by use of a breath-by-breath metabolic measurement system (MedGraphics Breeze, St Paul, MN, USA) calibrated immediately before each protocol session. To obtain an index of metabolite production, we also calculated the excess CO2 production (CO2excess) as follows:
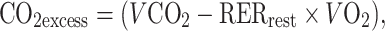 |
where RERrest is the respiratory exchange ratio at rest. CO2excess is an index of H+ and lactic acid production, because, at tissue pH, lactic acid dissociates and produces H+, which is buffered by HCO3 − and other cell buffers. The quantity buffered by HCO3 − leads to H2CO3, which in turn, dissociates into H2O and CO2 [2] Thus, an excess of CO2 is produced and is superimposed on the CO2 which normally results from aerobic metabolism.
Data analysis
Beat-to-beat hemodynamic and breath-by-breath ventilatory data were averaged for 3 min. Data are reported as the mean ± SEM percentage changes from corresponding rest values. We chose to perform statistics on percentage changes instead of absolute data because the mild exercise performed by subjects was expected to cause only slight changes in hemodynamics. Thus, percentage changes enabled curtailing of inter-individual variance and highlighting of small perturbations better than absolute values. Because rest values of CO2excess were clearly equal to 0, for this variable we reported absolute values instead of percentage changes from rest. Commercially available software (GraphPad Prism 4) was used for statistical calculations. Comparisons were performed using two-way analysis of variance (ANOVA) for repeated measures (factors: condition and time) followed by the Bonferroni post-hoc test when appropriate to compare corresponding time points between conditions, whereas the Dunnett post-hoc test was used to compare variables’ response with corresponding rest values. Statistical significance was set at a p value of <0.05 in all cases.
Results
All subjects completed the protocol and none complained of unbearable pain or discomfort during the periods of arm circulatory occlusion. No significant differences between rest variables before the three tests were observed (Table 1), thus tests started from similar hemodynamic and ventilatory conditions. Figures 1, 2 and 3 depict circulatory and ventilatory percentage changes from baseline during each protocol session.
Table 1.
Mean values ± SEM of variables during rest preceding handgrip tests
| PEMI test | CER test | EMI test | p | |
|---|---|---|---|---|
| HR (beats min−1) | 65.6 ± 2 | 63.8 ± 2.3 | 61.6 ± 2.5 | NS |
| SV (ml) | 71.8 ± 2.9 | 68.5 ± 2.2 | 69 ± 2.5 | NS |
| CO (l min−1) | 4.7 ± 0.2 | 4.3 ± 0.2 | 4.2 ± 0.1 | NS |
| PEP/VET | 0.49 ± 0.02 | 0.51 ± 0.02 | 0.51 ± 0.03 | NS |
| DT (ms) | 495 ± 28.7 | 528.6 ± 32.4 | 570.8 ± 41.3 | NS |
| VFR (ml s−1) | 148.7 ± 11.3 | 135.8 ± 10.2 | 129.2 ± 9.3 | NS |
| MAP (mmHg) | 85.6 ± 1.9 | 85.5 ± 2.9 | 88.5 ± 2.1 | NS |
| SVR (dynes s cm−5) | 1488.6 ± 73.2 | 1606.9 ± 106.1 | 1702.6 ± 69.3 | NS |
| VO2 (ml min−1) | 267.6 ± 22 | 248.9 ± 18.8 | 271.1 ± 26.9 | NS |
| VCO2 (ml min−1) | 231.7 ± 22.4 | 211.6 ± 18.8 | 235.5 ± 26.8 | NS |
| V E (l min−1) | 8.62 ± 0.68 | 8.28 ± 0.74 | 8.56 ± 1.03 | NS |
Fig. 1.
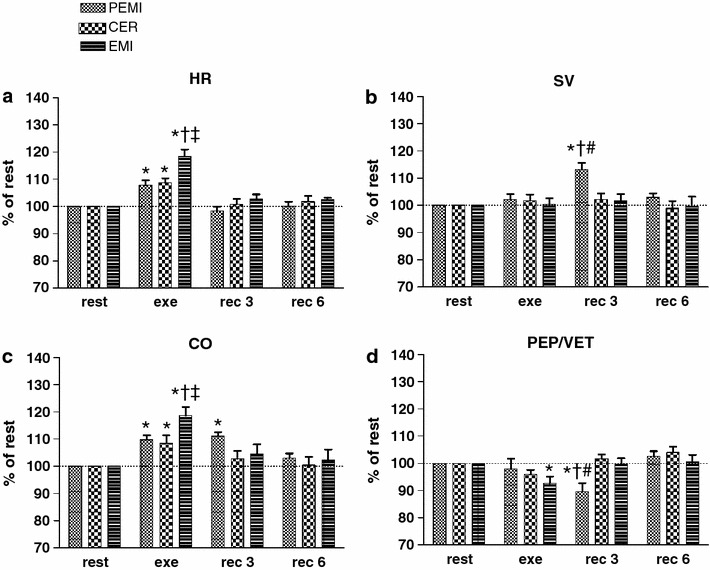
Time courses of heart rate (HR, a), stroke volume (SV, b), cardiac output (CO, c), and the inverse of myocardial contractility (PEP/VET, d) during rest, exercise, and recovery in all protocol sessions. Horizontal dashed lines identify the variables’ resting levels. Values are mean ± SEM percentage changes from the corresponding rest values. *p < 0.05 versus rest; † p < 0.05 versus CER test; ‡ p < 0.05 versus PEMI test; # p < 0.05 versus EMI test
Fig. 2.
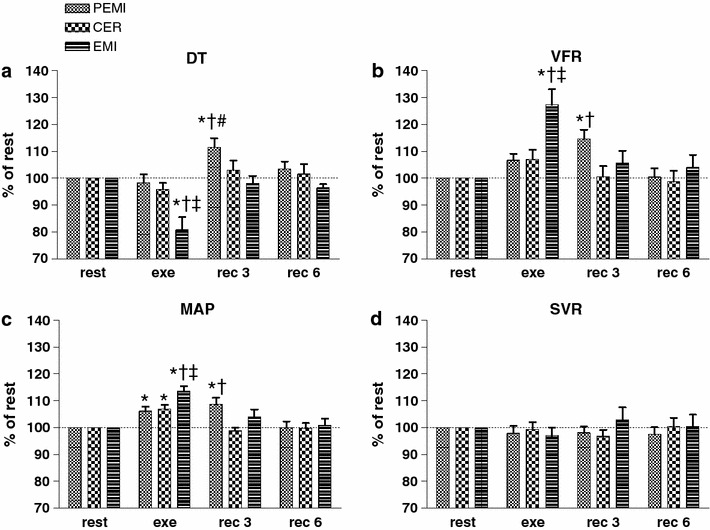
Time courses of diastolic time (DT, a), ventricular filling rate (VFR, b), mean arterial pressure (MAP, c), and systemic vascular resistance (SVR, d) during rest, exercise, and recovery in all protocol sessions. Horizontal dashed lines identify variables’ resting levels. Values are mean ± SEM percentage changes from the corresponding rest values. *p < 0.05 versus rest; † p < 0.05 versus CER test; ‡ p < 0.05 versus PEMI test; # p < 0.05 versus EMI test
Fig. 3.
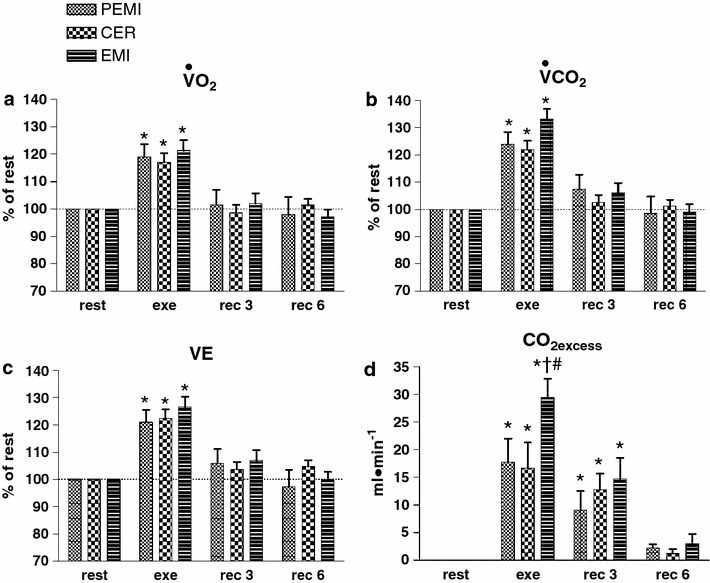
Time courses of oxygen uptake (VO2, a), carbon dioxide production (VCO2, b), and pulmonary ventilation (V E, c) during rest, exercise, and recovery in all protocol sessions. Horizontal dashed lines identify variables’ resting levels. Values are mean ± SEM percentage changes from the corresponding rest values. *p < 0.05 versus rest; † p < 0.05 versus CER test; # p < 0.05 versus EMI test
In detail, Fig. 1a shows that HR increased during all exercise sessions compared with the rest level. However, this increment was more evident during the EMI test, when HR reached higher levels than at the corresponding time-points of both the PEMI and the CER tests. Then, during the recovery period, HR returned to values no different from baseline. SV (Fig. 1b) was stable throughout all protocol sessions with the remarkable exception of the period of circulatory occlusion of the PEMI test, when SV rose in comparison with the rest level and also compared with the corresponding time-points of both the EMI and CER tests. Figure 1c depicts the time course of CO, which increased during all exercise tests compared with baseline. This elevation was more pronounced during the EMI test than in the PEMI and the CER tests. During the recovery periods from both the EMI and the CER tests, CO decreased toward the pre-exercise level. Differently, during the period of metaboreflex stimulation of the PEMI test CO remained higher than the rest value. PEP/VET (inversely related to myocardial performance) significantly decreased (i.e. cardiac performance increased) compared with baseline during the exercise period of the EMI test and during the period of circulatory occlusion of the PEMI test (Fig. 1d).
Figure 2a shows that DT significantly dropped during the exercise phase of the EMI test compared both with baseline and with the corresponding time-points of both the PEMI and the CER tests. Furthermore, DT rose during the phase of metaboreflex stimulation of the PEMI test. VFR (Fig. 2b) was enhanced during the period of metaboreflex stimulation of the EMI test in comparison with rest level and the other two tests. VFR also increased compared with baseline during the metaboreflex stimulation of the PEMI test. In all protocol sessions, during the handgrip strains MAP increased from rest level (Fig. 2c), but this increment was more pronounced during the EMI test than during the other protocol sessions. Moreover, during the period of muscle ischemia of the PEMI test MAP was elevated compared both with rest and with the CER test. Figure 2d reveals that SVR was not statistically different among the protocol periods.
Finally, in all exercise runs VO2, VCO2, and V E increased similarly compared with the rest values (Fig. 3a–c, respectively), without any detectable differences among conditions. CO2excess (Fig. 3d) increased during the exercise phases of all tests. However, this increment was higher during the EMI test in comparison with the other protocol sessions. The time course of CO2excess was no different between tests, and CO2excess returned to baseline during the last minutes of recovery.
Discussion
In this study of healthy humans we evoked a typical hemodynamic response (i.e. a blood pressure increase) to the muscle metaboreflex recruitment by applying two different approaches—EMI and post-exercise muscle ischemia. The blood pressure increase was very similar between these two conditions and in both cases the main mechanism used to achieve the target blood pressure was a flow-mediated mechanism, i.e. an increase in CO; peripheral vasoconstriction was not involved. This result is not novel; previous work has revealed that cardiovascular adjustments to metaboreflex stimulation mainly rely on a flow-mediated mechanism rather than on vasoconstriction [3, 6–8, 27, 32, 34].
The major new finding of this study is that the role of SV in the described CO increase varies depending on the possibility of elevating HR. In fact, when HR could not increase then the metaboreflex-induced CO elevation mainly relied on the SV response, whereas when an HR response occurred SV remained stable and the CO increment was the consequence of HR elevation. To the best of our knowledge this is the first study dealing with metaboreflex in humans which has investigated on the combined effect of SV and HR. Moreover, this is the first study reporting that during the metaboreflex the role of SV varies with the possibility of elevating HR.
Accordingly to the initial hypothesis, we believe that the results obtained in this work provide sufficient evidence that the SV response during the metaboreflex depends on the setting of the metaboreflex stimulation: if the metaboreflex is stimulated by the PEMI maneuver, SV increases; in contrast, if the metaboreflex is stimulated by EMI, then SV does not increase. This result seems to be explainable, at least in part, by the different behavior of HR between the two methods of metaboreflex stimulation, which in turn affected DT and myocardial performance. Indeed, the PEMI maneuver was not able to stimulate cardiac chronotropism whereas during the EMI test clear HR elevation occurred compared with the control test.
As stated in the Introduction, the lack of HR response during the PEMI maneuver is not a novel phenomenon, because it has previously been described in several studies dealing with the metaboreflex conducted in both animals and humans [1, 6, 26, 30]. The current consensus is that in the PEMI setting an increment in the parasympathetic tone takes place because of two phenomena: the intense arterial baroreflex stimulation because of the sustained blood pressure and the withdrawal of the central command at the cessation of exercise. The consequence is that the parasympathetic activity increases and obscures the effect of the metaboreflex-induced sustained sympathetic activity upon the sinus node, thus explaining the lack of an effect of PEMI upon HR [19, 25, 26]. The results obtained in this work are in good accordance with this scenario. Different HR behavior, on the other hand, was observed when the metaboreflex was evoked by means of EMI. In this situation HR increased in comparison with the other sessions of the protocol, which meant that the EMI maneuver was capable of eliciting a chronotropic stimulation, possibly because the central command was still operating and reduced the parasympathetic tone which, in turn, could not counteract the enhanced sympathetic activity towards the sinus node.
The described different HR behavior between the PEMI and the EMI conditions had a remarkable consequence on DT: it decreased during the metaboreflex period of EMI, because of the cardio-acceleration, whereas it increased during the PEMI test, because HR decreased towards baseline. As a consequence of the DT elongation, during the PEMI test more efficient cardiac filling was likely to have occurred. Actually, VFR increased during the PEMI compared with the CER test and this recruited the Frank–Starling mechanism and enhanced cardiac performance, thereby increasing SV.
The fact that during PEMI an improvement in myocardial performance occurred also arises from the analysis of PEP/VET (inversely related to myocardial performance and ejection fraction) which decreased during the PEMI test compared with the CER test. It should, however, be noted that the PEP/VET ratio is sensitive to enhancements of both cardiac inotropism and preload [13, 22]. That is, it decreases when there is an increase in cardiac pre-load and/or when an enhancement in cardiac inotropism occurs. Thus, we could not know whether, along with the described improvement in myocardial performance, an increase in myocardial contractility was also involved in the PEP/VET shortening. However, we believe this was not unlikely. That myocardial contractility may be enhanced by the metaboreflex is apparent from previous studies of dogs [27, 32]. In particular Sala-Mercado and co-workers [32] concluded that during dynamic exercise the metaboreflex increases myocardial contractility, and this sustained SV despite the reduction in VFR because of tachycardia. Hence, it can be hypothesized that the PEP/VET reduction occurring during the PEMI test resulted from the combination of both the increased myocardial performance and the enhanced myocardial contractility.
In contrast with the PEMI test, during the EMI test DT decreased markedly and SV remained stable, without showing any detectable increase. This fact (i.e. stable SV notwithstanding the DT reduction) was the consequence of the enhanced VFR that occurred during the EMI manoeuvre (Fig. 2b), which maintained diastolic volume despite the reduced time for filling. The VFR increment during the EMI test was noteworthy and was even higher than during the period of circulatory occlusion of the PEMI test. Thus, it is conceivable that, to defend cardiac pre-load from the reduced DT and to maintain SV, the cardiovascular regulatory mechanisms caused mobilization of visceral blood volume towards the heart. This phenomenon is in accordance with studies reporting that the metaboreflex is capable of eliciting substantial central blood volume mobilization [1, 33, 34]. Moreover, it is possible that, similarly to what was described for the PEMI test, enhancement of myocardial contractility also occurred during the EMI condition and protected SV from the reduced DT.
In the light of the findings of this study it is possible to hypothesize that the metaboreflex-induced enhancement in SV we found in our previous work [6–10] was, at least in part, the consequence of the blunted HR response induced during the PEMI maneuver. The results of this investigation also suggest that HR is just an indirect estimate of CO and that SV measurement should be performed whenever possible. Furthermore, the hemodynamic scenario depicted for the PEMI and the EMI tests leads us to speculate that the cardiovascular regulatory mechanisms operate with remarkable plasticity and involve very complex integration between the various components of the circulatory response (namely: chronotropism, pre-load, after-load, and inotropism), and a variety strategies can be used to achieve the target blood pressure. In detail, when a chronotropic response is not possible (for example during PEMI) the cardiovascular apparatus compensates for it by increasing DT and cardiac filling and/or inotropism to increase SV and CO in order to achieve the target blood pressure. The capacity of the cardiovascular system to operate with plasticity during the metaboreflex also arises from animal studies reporting that the metaboreflex obtained by EMI was capable of increasing SV under constant HR condition [27, 29].
However, animals suffering from heart failure were unable to increase SV in response to EMI because of their inability to increase ventricular performance [29]. These results were confirmed for humans also. In heart failure patients, who had reduced or absent contractility reserve and limited pre-load reserve, it was shown that their response to the metaboreflex was shifted towards vasoconstriction and that the SV and CO increase was virtually absent [8]. In the light of the complex integration of chronotropism, pre-load, after-load, and inotropism apparent from this work and, accordingly, from the results in our previous paper and from the cited studies conducted on animals, we can speculate that in heart failure patients there is disruption in the normal plasticity of circulatory adjustments to the metaboreflex described herein. Because they cannot rely on the reserve of cardiac performance and/or pre-load (which are exhausted), they rely on vasoconstriction and/or HR elevation to increase blood pressure during the metaboreflex.
Comparison of previous studies and this work
The mechanism responsible for the blood pressure increment during the metaboreflex activation is still the subject of debate among scientists. In contrast with the findings in this work, some previous experiments on humans revealed no significant changes in SV and CO during PEMI [24, 34], whereas substantial peripheral vasoconstriction occurred [1, 16]. These studies concluded that the pressor response during PEMI was a result of peripheral vasoconstriction. In contrast with these findings and in accordance with our results, other studies on humans and animals support the concept that the metaboreflex-induced increase in blood pressure relies on a flow-mediated mechanism [1, 27, 32, 34]. Moreover, very recently it was found that the mechanisms involved in the muscle metaboreflex response in dogs are continuously dependant on whether a rise in CO occurs, i.e. whether vasoconstriction occurs depends on whether CO rises [18]. Thus, our findings seem to accord with the concept that the rise in blood pressure during the metaboreflex may be not solely the consequence of a neurally induced increase in peripheral vascular resistance, rather the elevation in CO is responsible for the phenomenon [28].
It has recently been proposed [20, 21] that the baroreflex counteracts the metaboreflex-induced increase in blood pressure by buffering the sympathetic activity directed toward vascular tone regulation so that the muscle metaboreflex-induced vasoconstriction could be inhibited. According to this scenario, we believe that our finding of an unchanged SVR in the EMI and the PEMI settings is explainable by the intense baroreflex stimulation during these conditions. Hence, our data seem in good accordance with previous reports demonstrating that the arterial baroreflex buffers the muscle metaboreflex-induced increase in sympathetic tone mainly by inhibition of peripheral vasoconstriction and by increasing parasympathetic tone at the sinus node, whereas the increase in cardiac performance because of the metaboreflex is not counteracted [20, 21].
Limitations of the study
One weak point of this research is that we did not measure metabolite concentrations during the metaboreflex phases of the EMI and the PEMI tests to verify whether or not muscle ischemia induced accumulation of different metabolic end-products in these two settings. It was, in fact, to be expected that the circulatory occlusion during the EMI test caused higher metabolite concentrations compared with PEMI, because in the former condition the muscle was contracting during ischemia whereas in the latter situation ischemia was applied when the muscle was not working. Many putative substances are thought to be responsible for the metaboreflex, namely H+, lactate, diprotonated phosphate, ATP, adenosine, arachidonic acid, prostaglandins, potassium, bradykinin, sodium, acetylcholine, histamine, serotonin, and others, and for none of these substances, to the best of our knowledge, is there conclusive evidence of its importance or real involvement in the metaboreflex phenomenon. Determining the amounts of such a large number of substances would be complex, expensive, sometimes invasive, and far beyond the capacity of our laboratory. Thus, we could not know whether or not the metaboreflex was activated to the same extent during of the EMI and the PEMI tests. To obtain an index of metabolite production we used a respiratory index (CO2excess) which reflects H+ and lactic acid accumulation. Actually, H+ and lactic acid are putative end-products believed to induce metaboreflex activation [24]. The statistical analysis indicated that this index was higher in the EMI test than under the other protocol conditions, in turn indicating that in this setting accumulation of more metabolites probably occurred. It should, however, be considered that the MAP increase was no different during the metaboreflex phases of the EMI and PEMI tests (+13.5% ± 1.9 and +8.5% ± 2.4, respectively, compared with rest; p = 0.12), which seems to suggest the level of metaboreflex stimulation was no different between the two settings. Hence, although the EMI test is likely to lead to greater metabolite concentrations than the PEMI test, it is a matter of fact that MAP did not seem to be affected by this occurrence; this result leads us to speculate that the metaboreflex may have been stimulated to a similar extent in the two settings. Alternatively, it is possible that, in response to the metaboreflex, a more intense baroreflex stimulation occurred which successfully buffered the increased sympathetic tone and limited the MAP elevation.
Yet, another explanation for the phenomenon may arise from studies performed during hypoxia. Interestingly, it has been found during the metaboreflex after handgrip that neither muscle sympathetic nerve activity nor MAP were enhanced by hypoxia compared with handgrip alone, whereas HR did increase [17]. Furthermore, it was reported that hypoxia has only a trivial effect on oxidative stress and on the discharge of group III and IV muscle afferents during contraction [11, 16]. Taken together these findings seem to suggest that hypoxia is a minor stimulus to afferents that sense a mismatch between blood supply and demand, and this could help to explain why we did not observe any MAP difference between the metaboreflex phases of the EMI and the PEMI tests. Probably, the main mechanism responsible for the metaboreflex activation during the circulatory arrest maneuvers was the interruption of metabolites washout rather than hypoxia.
Another fact deserving consideration is that output from the mechanoreflex could have been more intense during EMI than during the other protocol sessions and this could have affected the hemodynamic response during the EMI test. Indeed, it has been suggested that muscle mechanoreceptors are sensitized by venous congestion and accumulation of metabolites within muscle [12], and as a consequence the muscle mechanoreflex could have been more activated during EMI than during the exercise phase of the CER and the PEMI tests. Thus, the muscle mechanoreflex could have been involved in the hemodynamic response during EMI, although our study protocol cannot answer this question.
To summarize, our results suggest that the mechanisms involved in the muscle metaboreflex depend on whether or not an increase in HR occurs. In normal individuals, who have a reserve of both HR and myocardial performance, there is a shift in the mechanisms via which the metaboreflex-induced CO response is achieved. When an HR response is possible, the CO increase is mainly achieved by means of HR, whereas when HR cannot increase cardiovascular controlling mechanisms manage to enhance myocardial performance and SV. Thus, it is possible to speculate that during physiological and clinical situations that do not allow increasing HR (such as in subjects with atrio-ventricular block, during therapy with beta-blockers, and during intense baroreflex stimulation, for example in the PEMI setting, etc.) subjects rely on cardiac performance and SV to reach the target CO. In contrast, when cardiac performance cannot be improved (for example in heart failure, and in subjects who cannot centralize blood volume, for example individuals with peripheral sympathetic dysfunction or paraplegia) subjects rely on HR to elevate CO. Thus, future studies dealing the metaboreflex in these particular conditions are warranted to fully elucidate the hemodynamics of the metaboreflex in humans.
As a further layer of complexity it should be considered that from previous studies it seems that work intensity affects the extent of metaboreflex stimulation, i.e. the greater the workload the higher the metaboreflex stimulation [7, 9]. This has a profound effect on the circulatory response, which arises from the complex interaction between the metaboreflex and the simultaneous baroreflex activation. For instance, recent investigations indicate that the arterial baroreflex capacity to buffer the metaboreflex is impaired in heart failure [21].
Considering the complexity of the hemodynamic response which arises from these facts, it is our opinion that the setting of the metaboreflex stimulation (PEMI or EMI), the kind of exercise (static or dynamic), the intensity of the workload, and the clinical status of subjects are all critical factors determining the HR and the SV response during the metaboreflex. Hence, the setup condition should be carefully evaluated before drawing conclusions from studies dealing with this topic.
In conclusion, this investigation provides evidence that the blood pressure response to the metaboreflex activation obtained both during and after mild arm exercise is mainly the consequence of a CO increase. However, the hemodynamic scenario is quite different between the two settings: when the metaboreflex is stimulated during exercise the flow-mediated response is the consequence of HR elevation, whereas when the metaboreflex is recruited during recovery the hemodynamic response is achieved by means of an increase in SV, because in this setting HR response is blunted. Thus, it seems that the systems controlling the cardiovascular apparatus operate with remarkable plasticity and that there is complex modulation of the chronotropic status of the heart, cardiac performance, and ventricular filling with the purpose of reaching the target blood pressure.
Acknowledgments
This study was supported by the University of Cagliari and the Italian Ministry of Scientific Research. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
References
- 1.Bastos GB, Williamson JW, Harrelson T, Nôbrega ACL. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med Sci Sports Exerc. 2000;32:1114–1118. doi: 10.1097/00005768-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Beaver WL, Wasserman K, Whipp BJ. Bicarbonate buffering of lactic acid generated during exercise. J Appl Physiol. 1986;60:472–478. doi: 10.1152/jappl.1986.60.2.472. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DP. A new stroke volume equation for Thoracic Electrical Bioimpedance: theory and rationale. Crit Care Med. 1986;14:904–909. doi: 10.1097/00003246-198610000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol. 1978;45:574–580. doi: 10.1152/jappl.1978.45.4.574. [DOI] [PubMed] [Google Scholar]
- 5.Crisafulli A, Orrù V, Melis F, Tocco F, Concu A. Hemodynamics during active and passive recovery from a single bout of supramaximal exercise. Eur J Appl Physiol. 2003;89:209–216. doi: 10.1007/s00421-003-0796-4. [DOI] [PubMed] [Google Scholar]
- 6.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc. 2003;35:221–228. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 7.Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu A. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol (Heart Circ Physiol) 2006;291:H3035–H3042. doi: 10.1152/ajpheart.00221.2006. [DOI] [PubMed] [Google Scholar]
- 8.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol (Heart Circ Physiol) 2007;292:H2988–H2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- 9.Crisafulli A, Milia R, Lobina A, Caddeo M, Tocco F, Concu A, Melis F. Hemodynamic effect of metaboreflex activation in men after running above and below the velocity of the anaerobic threshold. Exp Physiol. 2008;93:447–457. doi: 10.1113/expphysiol.2007.041863. [DOI] [PubMed] [Google Scholar]
- 10.Crisafulli A, Milia R, Vitelli S, Caddeo M, Tocco F, Melis F, Concu A. Hemodynamic responses to metaboreflex activation: insights from spinal cord-injured humans. Eur J Appl Physiol. 2009;106:525–533. doi: 10.1007/s00421-009-1045-2. [DOI] [PubMed] [Google Scholar]
- 11.Dousset E, Steinberg JG, Faucher M, Jammes Y. Acute hypoxemia does not increase the oxidative stress in resting and contracting muscle in humans. Free Radic Res. 2002;36:701–704. doi: 10.1080/10715760290029146. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JP, White MJ. Muscle afferent contributions to the cardiovascular response to isometric exercise. Exp Physiol. 2004;89:639–646. doi: 10.1113/expphysiol.2004.028639. [DOI] [PubMed] [Google Scholar]
- 13.Garrad CL, Weissler AM, Dodge HT. The relationship of alterations in systolic time intervals to ejection fraction in patients with cardiac disease. Circulation. 1970;42:455. doi: 10.1161/01.cir.42.3.455. [DOI] [PubMed] [Google Scholar]
- 14.Gledhill N, Cox D, Jamnik R. Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc. 1994;26:1116–1121. [PubMed] [Google Scholar]
- 15.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 16.Hill JM, Pickar JG, Parrish MD, Kaufman MP. Effect of hypoxia on the discharge of group III and IV muscle afferents in cats. J Appl Physiol. 1992;73:2524–2529. doi: 10.1152/jappl.1992.73.6.2524. [DOI] [PubMed] [Google Scholar]
- 17.Houssiere A, Najem B, Ciarka A, Velez-Roa S, Naeje R, van de Borne P. Chemoreflex and metaboreflex control during static hypoxic exercise. Am J Physiol (Heart Circ Physiol) 2005;288:H1724–H1729. doi: 10.1152/ajpheart.01043.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li ZH, Ichinose TK, Dawe E, O’Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol (Heart Circ Physiol) 2009;298:H245–H250. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise. Circulation. 1999;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O’Leary DS. The arterial baroreflex alters the strength and mechanisms of the muscle metaboreflex pressor response during dynamic exercise. Am J Physiol (Heart Circ Physiol) 2005;288:H1374–H1380. doi: 10.1152/ajpheart.01040.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O’Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol (Heart Circ Physiol) 2005;289:H2416–H2423. doi: 10.1152/ajpheart.00654.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lewis RP, Rittgers SE, Forester WF, Boudoulas A. A critical review of the systolic time intervals. Circulation. 1977;56:146–158. doi: 10.1161/01.cir.56.2.146. [DOI] [PubMed] [Google Scholar]
- 23.Moran D, Epstein Y, Keren G, Laor A, Sherez J, Shapiro Y. Calculation of mean arterial pressure during exercise as a function of heart rate. Appl Hum Sci. 1995;14:293–295. doi: 10.2114/ahs.14.293. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyasu T, Ueno H, Nishiyasu M, Tan N, Morimoto K, Morimoto A, Deguchi T, Murakami N. Relationship between mean arterial pressure and muscle cell pH during forearm ischemia after sustained handgrip. Acta Physiol Scand. 1994;151:143–146. doi: 10.1111/j.1748-1716.1994.tb09731.x. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyasu T, Nobusuke T, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol. 1994;77:2778–2783. doi: 10.1152/jappl.1994.77.6.2778. [DOI] [PubMed] [Google Scholar]
- 26.O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol (Heart Circ Physiol) 1998;275:H220–H224. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary DS, Joyner MJ. The muscle metaboreflex does/does not restore blood flow to contracting muscles. J Appl Physiol. 2006;100:357–361. doi: 10.1152/japplphysiol.01222.2005. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary DS, Sala-Mercado JA, Augustyniak RA, Hammond RL, Rossi NF, Ansorge EJ. Impaired muscle metaboreflex-induced increases in ventricular function in heart failure. Am J Physiol (Heart Circ Physiol) 2004;287:H2612–H2618. doi: 10.1152/ajpheart.00604.2004. [DOI] [PubMed] [Google Scholar]
- 30.Piepoli M, Clark AL, Coats AJS. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol (Heart Circ Physiol) 1995;269:H1428–H1436. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- 31.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 32.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O’Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol (Heart Circ Physiol) 2006;290:H751–H757. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 33.Sheriff DD, Augstyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol (Heart Circ Physiol) 1998;275:H767–H775. doi: 10.1152/ajpheart.1998.275.3.H767. [DOI] [PubMed] [Google Scholar]
- 34.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in females. J Appl Physiol. 2007;103:228–233. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 35.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 36.Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe K, Ichinose M, Fujii N, Matsumoto M, Nishiyasu T. Individual differences in the heart rate response to activation of the muscle metaboreflex in humans. Am J Physiol (Heart Circ Physiol) 2010;299:H1708–H1714. doi: 10.1152/ajpheart.00255.2010. [DOI] [PubMed] [Google Scholar]


