Abstract
Antiretroviral therapy (ART) often results in painful peripheral neuropathy. Given that voluntary exercise has been shown to be beneficial in terms of modulating pain-like behaviors in various animal models of peripheral neuropathy, we have investigated the effects of voluntary wheel running on neuropathic pain induced by chronic ART. We first established an animal model of peripheral neuropathy induced by chronic 2′,3′-dideoxycytidine (ddC) treatment. We showed that mice receiving ddC (3 mg/kg/day) had increased mechanical and thermal sensitivity at 9 weeks after the onset of the treatment. We also found that voluntary wheel running attenuated or delayed the onset of ddC-induced peripheral neuropathy. This phenomenon was associated with the attenuation of dorsal root ganglion nociceptive neuron membrane excitability and reduction in the expression of the transient receptor potential cation channel subfamily V member 1 (TRPV1). Taken together, these results suggest that voluntary exercise is an effective strategy by which ART-induced peripheral neuropathy can be alleviated.
Keywords: Antiretroviral therapy, Peripheral neuropathy, Dorsal root ganglion, Nociceptive neuron, TRPV1
Introduction
Neuropathic pain is reported in 20–40% of patients infected with human immunodeficiency virus (HIV) [1] and seriously affects the patient’s quality of life. Hyperalgesia, allodynia, and spontaneous sensations, such as shooting and burning sensations, are the most common symptoms [2–5]. Antiretroviral therapy (ART) using nucleoside reverse transcriptase inhibitors (NRTIs) and other antiretroviral drugs has been demonstrated to be effective in controlling HIV infection, which has led to ART being widely accepted as first line treatment of HIV/acquired immunodeficiency syndrome (AIDS). However, one of the adverse side effects of ART is the development of painful peripheral neuropathy, as has been reported in about 15–30% of patients using such NRTIs as didanosine, stavudine, and zalcitabine (ddC) [6–8]. The development of painful peripheral neuropathy may lead to the patient discontinuing ART, resulting in worsening of the HIV infection. Many medications, such as antidepressants and topical pain killers, and various new strategies, such as the use of cannabinoid receptor agonists [10], have been used to manage neuropathic pain [9], but there is still a great need for safer non-pharmacological treatments to manage neuropathic pain in HIV-infected patients that have no adverse drug effects.
One possible approach to manage neuropathic pain in this patient population is to introduce physical exercise into the disease management program. Studies have shown that exercise has beneficial effects on the quality of life in HIV/AIDS patients [11], as well as on the relief of chemotherapy-induced pain in cancer patients [12, 13]. In animal models of pain, an extended swimming program has been found to attenuate the development of and to reverse nerve injury-induced thermal and mechanical hypersensitivity in rodents, as well as formalin-induced spontaneous pain in rats [14–16]. In addition, voluntary wheel running can decrease paw withdrawal frequency in mouse models of chronic muscle pain [17] and reverse mechanical and visceral hypersensitivity in a high-fat model of prediabetic neuropathy [18]. However, no study has been conducted to date with the aim to examine the effects of voluntary exercise paradigms on ART-induced neuropathic pain.
While the mechanisms underlying the attenuating effects of exercise on pain have not been extensively studied, the results of some studies indicate that physical activities can reduce the hyperexcitability of dorsal root ganglion (DRG) neurons associated with neuropathic pain. Studies on rats have shown that enhanced excitability of the small DRG neurons is associated with bone cancer pain [11]. In animals, injury affecting the axons or their soma in DRGs often causes hyperexcitability that leads to spontaneous firing and neuropathic pain [12, 13]. Enhanced excitability of nociceptive DRG neurons has also been observed in rodent models of inflammatory pain [19, 20]. Therefore, in the present study, we hypothesized that ART-induced neuropathic pain is associated with increased excitability of DRG neurons and voluntary exercise can alleviate ART-induced neuropathic pain.
Thus, we investigated whether chronic ddC treatment can increase mechanical and thermal sensitivity, indexes of peripheral neuropathic pain [21, 22], and whether repeated voluntary exercise sessions could alter nociceptive thresholds and sensory neuron excitability in a mice model of ART-induced neuropathic pain.
Materials and methods
Animals
Adult C57BL/6 J male mice (n = 84), aged 7–8 weeks at the time of initiation of the experiments, were obtained from the Shanghai Laboratory Animal Center, Shanghai, China. The mice were housed in their home cages under a 12:12 light:dark cycle, at 21–23 °C and 50–60% humidity. Food and water were provided ad libitum. All animal experiments were approved prior to the start of the study by the Institutional Animal Care and Use Committee of Daqing Oil Field General Hospital. The housing and treatment of the rats followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources, Commission on Life Sciences, 2011).
Animal model of ART-induced neuropathic pain
To establish an animal model of neuropathic pain induced by chronic ART, we administered the antiretroviral drug 2′,3′-dideoxycytidine [zalcitabine (ddC); Sigma-Aldrich, St. Louis, MO] to the mice for 13 weeks. ddC dissolved in normal saline was added to the drinking water (at 0.3–3 mg/kg/day) on the first day of the study. The daily dose corresponds to a standard human dosage (2.25 mg/day for a body weight of 60 kg on average) [23] adjusted for body surface area and was calculated on the basis of a daily water consumption of 5 mL. The ddC-containing water was replaced weekly during regular cage changes. All water bottles that contained ddC were covered with foil to avoid direct light exposure. Observations on fluid consumption, clinical signs and mortality were carried out daily. Body weights were recorded weekly. In general, the mice receiving drinking water supplemented with ddC did not show any signs of dehydration or atypical growth.
Behavioral studies
We conducted all behavioral assays between 8000 and 1700 hours once a week (i.e., always on a Friday). Briefly, prior to testing, mice were acclimated in plexiglass boxes to the testing apparatus for at least 30 min. For all behavioral testing, the experimenter was blinded to treatment conditions. To assess mechanical sensitivity, we also conducted von Frey assays in which mice were first allowed to acclimate on an elevated mesh grid, following which von Frey filaments were applied to the plantar hind paw using the up–down method as described previously [24]. Three trials on each paw were conducted, with a 10-min time interval between trials on opposite paws and a 15-min time interval between trials on the same paw. The average mechanical paw withdrawal thresholds of the three trials on both paws were used to determine the individual mechanical sensitivity. To assess the thermal nociception, we measured the reaction latencies (i.e., first sign of nociception, paw licking, flinching or jump response to avoid the heat) of mice to a hot plate (DL Naturgene Life Science, Beijing, China) at 55 ± 1 °C using a protocol described previously [25]. To avoid damage/injuries to the paws, we used a cut-off period of 20 s.
Voluntary exercise paradigms
Low-profile wireless running wheels were placed in individual cages (Med Associates Inc. Latham, NY) of both control and ddC-treated mice for 2 h (1800–200 hours) each night, 5 nights (Monday–Friday) a week for 13 weeks during the experimental period (duration of ddC treatment 13 weeks; dosage 3 mg/kg/day). Software provided by Med Associates Inc. was used to monitor the running distance. Identical running wheels, but locked, were placed into the cages of the control non-running animals. White noise was provided to modulate external noise during the 2-h training sessions. When not exercising, animals were housed in groups in their home cages.
Dissociating DRG neurons
After the last behavioral testing session, all mice were sacrificed via quick live decapitation. Lumbar (L) DRG (L3–L5) from each mouse were dissected, immediately placed in papain (45 U) and incubated for 30 min. After rinsing and incubation with collagenase (4.5 mg/200 μL) for an additional 30 min, the DRG were triturated to separate the neurons by passage through a 40-μm mesh filter. The dissociated cells were plated on poly-d-lysine/collagen-coated glass coverslips overnight and then tested.
Electrophysiology
Whole-cell patch-clamp recordings were made of current generation in cells approximately 20–30 μm in diameter, which increased the likelihood of obtaining recordings from nociceptive neurons. A P-97 micropipette puller (Sutter Instrument Co., Novato, CA) was used to prepare fire-polished glass pipettes with an open tip resistance of about 2.0–7.0 MΩ. Prior to recording, 291-mOsm pipettes were filled with an internal recording solution (120 mM K+ gluconate, 0.1 mM CaCl2, 2 mM MgCl2, 5 mM NaCl, 10 mM HEPES, 1.1 mM EGTA, 4 mM Na2ATP, 0.4 mM Na2GTP, 15 mM phosphocreatine; pH 7.3), During recording, the cells were continuously perfused with an external solution maintained at room temperature (145 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES, 7 mM glucose; adjusted to pH 7.4 with NaOH). For each recording, the series resistance was not higher than 20 MΩ. Patchmaster and the EPC10 USB amplifier (Heka Instruments Inc., Shanghai, China) were used to record the values from neurons. Data analysis was conducted using data from neurons with a resting membrane potential that was more negative than −45 mV.
Double immunofluorescence staining
Mice were intracardially perfused with saline followed by 4% paraformaldehyde. L3–L5 DRGs were immediately separated out and post-fixed using 4% paraformaldehyde, followed by submersion in 30% sucrose overnight for cryoprotection. The DRGs were then embedded in optimum cutting temperature compound and rapidly frozen at −20 °C. Frozen tissues were sectioned on a cryostat at a thickness of 15 μm, following which the DRG sections were co-incubated with transient receptor potential cation channel subfamily V member 1 (TRPV1) antibody (1:200; Abcam, Cambridge, UK) in 1% bovine serum albumin and 0.3% Triton-X100 in 0.01 mol/L phosphate-buffered saline (PBS) and one of the following antibodies: anti-neurofilament 200 (NF200) (1:1000; Sigma, St. Louis, MO), fluorescein isothiocyanate-labeled isolectin B4 (IB4-FITC, 10 μg/mL; Sigma), or anti-calcitonin gene-related peptide (CGRP, 1:1000; Sigma) overnight at 4 °C. After washing with PBS, the sections were incubated with rhodamine isothiocyanate (TRITC)-conjugated affinipure goat anti-rabbit immunoglobulin G (IgG; H+L; 1:2000; Jackson Immunoresearch Co., West Grove, PA) and fluorescein isothiocyanate-conjugated goat anti-mouse IgG (H+L; 1:2000; Jackson Immunoresearch Co.), except for IB4, for 40 min at 37 °C. Stained slides were viewed and photographed with a CCD camera under a fluorescent microscope (Leica DM IRB inverted research microscope; Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analyses
Data were analyzed using SPSS software (IBM Corp., Armonk, NY) and are presented as the mean ± standard error of the mean. Withdrawal latencies and thresholds were normalized to baseline prior to ART treatment. Repeated-measures analysis of variance (ANOVA) or one-way ANOVA was used, as appropriate. Specifically, to compare mechanical and thermal sensitivity, we used repeated-measures ANOVA, with week as the within-subject factor and treatment as the between-subject factor. DRG neuron membrane potential, spike frequency, spike accounts, and threshold were compared using one-way ANOVA. TRPV1 expression in DRG nociceptive neurons was compared using one-way ANOVA. Data distribution was tested for normality, and ANOVA results were corrected for variance homogeneity and sphericity as needed. In all analyses, a Bonferroni test was used to correct for multiple comparisons. P < 0.05 was considered to be significant.
Results
Chronic ddC treatment increased mechanical and thermal sensitivity
The mice on chronic ddC treatment developed peripheral neuropathy. Specifically, after 9 weeks of ddC (3 mg/kg/day) treatment mice exhibited increased mechanical sensitivity (Fig. 1a) and increased thermal sensitivity, as compared with control mice (Fig. 1b).
Fig. 1.
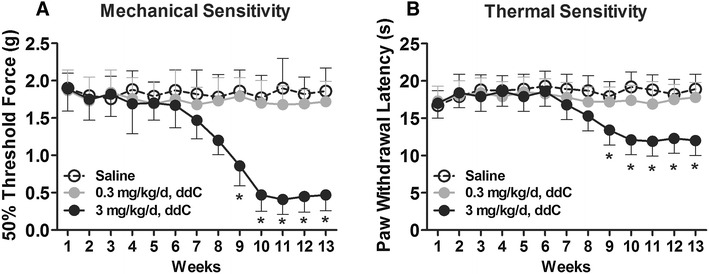
Chronic treatment with the nucleoside reverse transcriptase inhibitor zalcitabine (ddC) induced mechanical and thermal hypersensitivity. a Following 13 weeks of chronic ddC treatment (3 mg/kg/day), mechanical withdrawal thresholds were attenuated at treatment week 9 and remained attenuated thereafter. b Paw withdrawal latencies to heat stimuli were attenuated in mice on 13 weeks of chronic ddC treatment (3 mg/kg/day). Data are presented as the mean ± standard error of the mean (SEM). Bonferroni correction for multiple comparisons. Asterisks indicate a significant difference at P < 0.05 between the ddC-treated mice and the control mice (saline). n = 10 mice/group
Chronic ddC treatment increased DRG nociceptive neuron membrane excitability
To determine whether chronic ddC treatment alters membrane and cell excitability properties, we conducted whole-cell patch-clamp electrophysiology on dissociated L3–L5 DRG neuron cultures after completion of the last behavioral tests. Recordings were only conducted on small-diameter neurons (i.e., 20–30 μm diameter; Fig. 2a). Consistent with the results of the behavioral tests, 13 weeks of chronic ddC (3 mg/kg/day) treatment increased the resting membrane potential (Fig. 2c), promoted spike frequency (Fig. 2e) and spike counts (Fig. 2f), and decreased threshold potential (Fig. 2g).
Fig. 2.
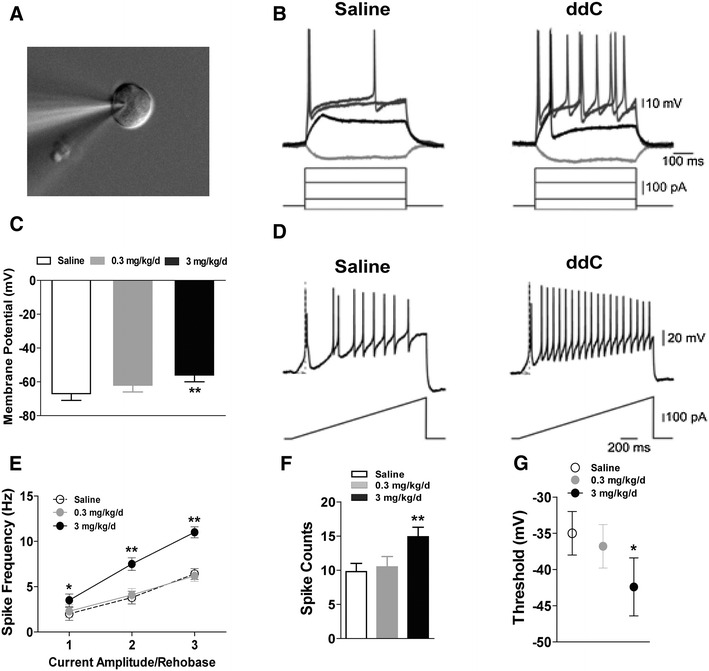
Chronic treatment with ddC altered the membrane excitability properties of dorsal root ganglion (DRG) neurons in mice. Whole-cell patch-clamp electrophysiology was performed on cultured small-diameter lumbar DRG neurons (n = 3 neurons/mouse). a Representative microphotograph of a patched neuron approximately 24 μm in diameter. b, d Representative traces of action potentials elicited by step (b) and ramp (d) current injections of rheobase. c Resting membrane potential was altered by chronic treatment with ddC (n = 10). e ddC treatment reduced first spike latency and increased the action potential (AP) frequency. Data presented as the mean ± SEM. Asterisks indicate a significant difference (*P < 0.05, **P < 0.01) in spike frequency between the ddC-treated mice and the control mice (saline). n = 10 per group. f Spike count measured from traces as in d. Note that the increase in the number of spikes (mean ± SEM) between the mice treated with ddC and the control (saline) mice is significant at **P < 0.01. n = 10. g AP thresholds for generation of APs, obtained by the phase plot analysis of AP (mean ± SEM), are significantly different (*P < 0.05) between ddC-treated mice and the saline control mice. n = 10. Bonferroni correction for multiple comparisons
In the whole-cell patch-clamp experiment, step (Fig. 2b) and ramp (Fig. 2d) current injections were used to analyze cell excitability properties. The rheobase was similarly altered in response to either step or ramp current injections by 13 weeks of chronic ddC (3 mg/kg/day) treatment. The results of this experiment showed that both DRG membrane and excitability properties were increased after 13 weeks of chronic ddC (3 mg/kg/day) treatment.
Voluntary wheel running attenuated mechanical and thermal sensitivity after chronic ddC treatment
Those mice which received did not perform voluntary wheel running during the period they received chronic ddC treatment developed peripheral neuropathy, which supports the results of the whole-cell patch-clamp electrophysiology experiments. Importantly, these mice exhibited increased mechanical sensitivity after 9 weeks of ddC (3 mg/kg/day) treatment, as compared with the control mice (Fig. 3a). Those mice which performed voluntary wheel running during the period they received chronic treatment of ddC exhibited increased mechanical sensitivity after 11 weeks of ddC (3 mg/kg/day) treatment, as compared with control mice (Fig. 3a). However, mice that performed voluntary wheel running while receiving chronic treatment of ddC exhibited less mechanical sensitivity than mice that did not perform voluntary wheel running (Fig. 3a).
Fig. 3.
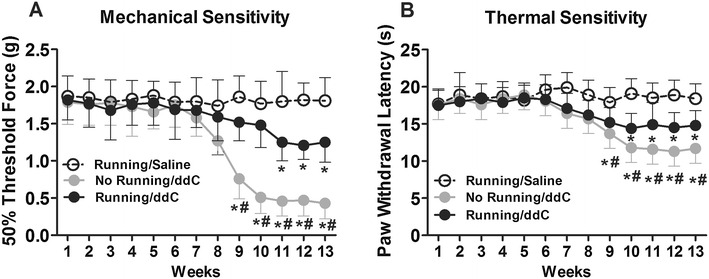
Voluntary wheel running reduced ddC treatment-induced mechanical and thermal hypersensitivity. a Following 13 weeks of chronic ddC treatment (3 mg/kg/day), mechanical hypersensitivity was attenuated by concurrent voluntary wheel running. b Similarly, following 13 weeks of chronic ddC treatment (3 mg/kg/day), thermal hypersensitivity was attenuated by concurrent voluntary wheel running. Data are presented as the mean ± SEM. Bonferroni correction for multiple comparisons. Asterisk indicates a significant difference (P < 0.05) between ddC-treated mice and the saline control mice. Hatching indicates a significant difference (P < 0.05) between running and non-running mice treated with ddC. n = 18/group
Similarly, during the chronic ddC treatment, mice exhibited increased thermal sensitivity after 9 weeks of ddC (3 mg/kg/day) treatment, as compared with control mice (Fig. 3b). Mice that performed voluntary wheel running during the period of chronic ddC treatment exhibited increased thermal sensitivity after 10 weeks of ddC (3 mg/kg/day) treatment, as compared with control mice (Fig. 3b). However, mice that performed voluntary wheel running during the chronic treatment of ddC exhibited less thermal sensitivity than mice that did not perform voluntary wheel running (Fig. 3b).
Taken together, these results suggest that voluntary wheel running alleviated and delayed the development of peripheral neuropathy after chronic ddC treatment.
Voluntary wheel running attenuated DRG nociceptive neuron membrane excitability after chronic ddC treatment
To determine whether voluntary wheel running alters membrane and cell excitability properties following chronic ddC treatment, we also conducted whole-cell patch clamp electrophysiology on dissociated L3–L5 DRG neuron cultures after completion of the behavioral tests. Consistent with the results of behavioral testing, 13 weeks of chronic ddC (3 mg/kg/day) treatment increased resting membrane potential (Fig. 4a), promoted spike frequency (Fig. 4b) and spike counts (Fig. 4c), and decreased threshold potential (Fig. 4d). However, mice that performed voluntary wheel running during the period of chronic treatment of ddC exhibited less membrane excitability properties than did those mice that did not perform voluntary wheel running.
Fig. 4.
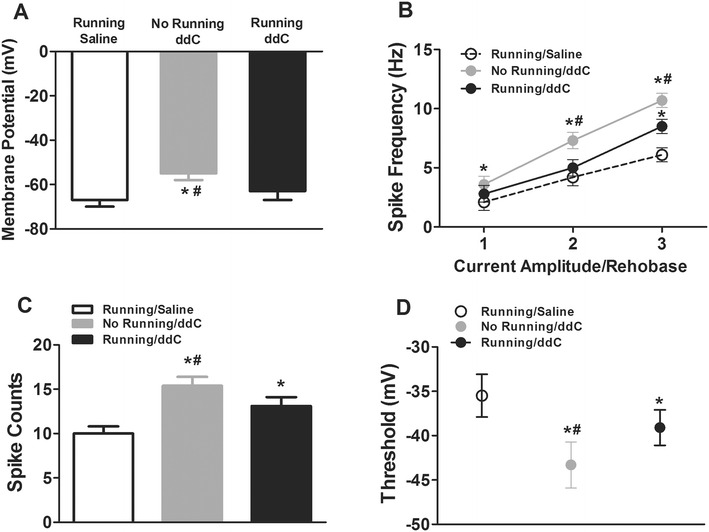
Voluntary wheel running reduced ddC treatment-induced increase in DRG neuron membrane excitability properties of mice. Whole-cell patch clamp electrophysiology was performed on small-diameter cultured lumbar DRG neurons (n = 3/mouse). a Resting membrane potential was altered by voluntary wheel running (mean ± SEM; n = 10). b Voluntary wheel running reduced AP frequency (mean ± SEM; n = 10) compared to the saline control group (*P < 0.05) and compared to the running/ddC group (# P < 0.05). c Number of spikes was attenuated (mean ± SEM; n = 10) in the no running/ddC group compared to the saline control group (*P < 0.05) and running/ddC group (# P < 0.05). d AP thresholds for generation of APs, obtained by the phase plot analysis of AP (mean ± SEM; n = 10) Significant difference at *P < 0.05 compared to saline and at # P < 0.05 compared to running/ddC group. Bonferroni correction for multiple comparisons
Voluntary wheel running attenuated TRPV-1 expression in DRG nociceptive neurons after chronic ddC treatment
Using specific neuronal markers, we showed that TRPV1 expression was upregulated in specific subtypes of DRG neurons, with TRPV1 expression clearly increased in distinct small DRG neurons. Specifically, TRPV1 increased within CGRP-positive neurons (Fig. 5b) and within IB4-positive neurons (Fig. 5c), most of which were C-fiber neurons. TRPV1 was not increased in NF200-positive neurons (Fig. 5a), suggesting that myelinated A-fiber neurons may not be involved in the increased expression of TRPV1. These results suggest that it is possible that C-fiber neurons play a great role than A-fiber neurons in the ART-induced expression of TRPV1, since the number of neurons double-stained with CGRP and TRPV1 and with IB4 and TRPV1, respectively, increased after chronic ddC treatment. These effects were reduced by voluntary wheel running.
Fig. 5.
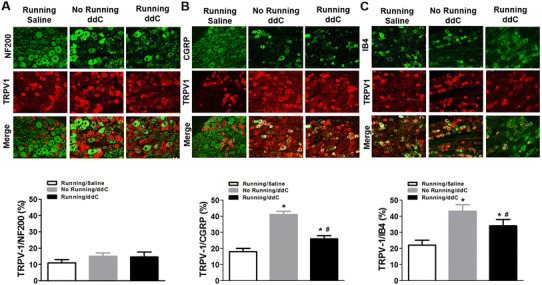
Voluntary wheel running reduced ddC treatment-induced increase in transient receptor potential cation channel subfamily V member 1 (TRPV1) expression in DRG nociceptive neurons. Top (a, b, c) Representative immunofluorescent images of DRG neurons expressing TRPV1 and DRG neuron markers. Bottom (a, b, c) Percentage of TRPV-1 based on anti-neurofilament 200- (NF200), anti-calcitonin gene-related peptide- (CGRP), and isothiocyanate-labeled isolectin B4- (IB4) positive nociceptive neurons in all counted DRG neurons. Significant difference at *P < 0.05 compared to saline and at # P < 0.05 compared to running/ddC group (n = 8/group)
Discussion
The aim of our study was to investigate the effects of voluntary wheel running on neuropathic pain induced by chronic ART in an animal model of peripheral neuropathy induced by chronic ddC treatment. Our results show that treatment with ddC at 3 mg/kg/day for 13 weeks resulted in increased mechanical and thermal sensitivity in mice at 9 weeks after the onset of the treatment. They also show that voluntary wheel running was able to attenuate or delay ddC-induced peripheral neuropathy, likely due to the attenuative effects of voluntary wheel running on DRG nociceptive neuron membrane excitability via the reduction of TRPV-1 expression. Taken together, we have demonstrated that voluntary exercise is an effective approach to partially alleviate ART-induced peripheral neuropathy.
In our study, we did not focus the effects of voluntary wheel running on nociceptive thresholds of the control animals (i.e., saline chronic treatment). Several previous studies have shown a lack of effects of voluntary exercise on nociceptive thresholds of naïve animals. For example, forced swim training in rats or wheel running in mice in home cage did not alter thermal and mechanical hind paw withdrawal thresholds [16, 17]. Human studies have consistently shown that athletes usually do not exhibit altered basal pain thresholds compared to non-athletes [26, 27]. Thus, we postulate that nociceptive thresholds were likely not altered in the control mice on the chronic saline treatment after voluntary wheel running.
Our findings that physical exercise (i.e., voluntary wheel running) can improve neuropathic pain responses induced by ART are consistent with those of several previous studies. For example, using a rat model, Kuphal et al. reported that 9 days of extended swimming can attenuate formalin-induced spontaneous pain [28]. In animal models of peripheral nerve and spinal cord injury, it has been reported that forced treadmill running can reverse thermal and mechanical hypersensitivity [29–33]. Consistent with these results, it has also been shown that a rigorous treadmill exercise program that was started 1 week before the administration of paclitaxel and continued throughout the treatment can partially alleviate chemotherapy-induced peripheral neuropathy in mice [34]. Furthermore, wheel running can normalize behavioral hypersensitivity associated with pre-diabetic neuropathy [18]. In humans, a recent study showed that a progressive-resisted exercise program has positive effects on health-related quality of life in people suffering from HIV/AIDS-related distal symmetrical poly-neuropathy [35]. The results of our study add to this body of literature, further suggesting that physical exercise may have a broader effect on regulating painful peripheral neuropathy induced by various pathological processes.
A critical factor that has to be considered in applying an exercise program to pain management is that forced exercise may involve different mechanisms than voluntary exercise. In this respect our study design differed from that of many other published studies in that we used voluntary exercise training as opposed to forced exercise training. It has been reported that forced exercise training is more stressful [36, 37]. For example, Leasure and Jones showed that forced—but not voluntary—wheel running can increase anxiety-like behavior in the open field test [38]. During our experiment, mice were moved in and out of cages which always were equipped with a running wheel, which may have increased the stress level in these animals. While control mice were also placed into cages with locked running wheel cages, environmental changes alone may also increase stress in animals. Therefore, we cannot rule out the effects of stress on mechanical and thermal sensitivity in ddC-treated mice after prolonged wheel running training. In fact, several studies have indicated that voluntary exercise may have anxiolytic effects [39–41]. While pain and anxiety are often associated with each other, few studies have examined the effects of anxiety levels on the regulation of neuropathic pain. Thus, an important aspect of future studies will be to explore whether anxiolytic drugs can promote the effects of voluntary exercise training on reducing ART-induced neuropathic pain.
Notably, exercise intensity also plays a critical role in reducing neuropathic pain in an animal model of neuropathic pain. Specifically, high-intensity exercise has been shown to be more effective than low-intensity exercise in reducing nerve injury-induced mechanical hypersensitivity in rats [31]. In our study, we found that mice which voluntarily used the training wheel (2 h/day) during the 13 weeks of chronic ddC treatment only exhibited partially alleviated neuropathic pain. However, we found no correlation between the total running distance (from week 1 to week 13) of individual mice and change in the mechanical paw withdrawal threshold with latency (data not shown). Therefore, it is likely that increasing the intensity of voluntary wheel running (i.e., longer time) in our study would not fully attenuate chronic ddC treatment-induced mechanical and thermal hypersensitivity. Nonetheless, our study indicates that a mild voluntary exercise program can be beneficial to pain management in patients receiving ART.
A number of studies have shown that voluntary exercise can induce physiological adaptions, including increased expression of endogenous opioids and altered expression of growth factors [14, 18, 31, 42–46]. Our study showed that chronic ddC treatment could enhance the expression of TRPV1 in the nociceptive DRG neurons and that voluntary wheel running can attenuate the expression of TRPV1 channels. TRPV1 has been shown to be critically involved in the regulation of mechanical and thermal sensitivity [47–50]. Furthermore, TRPV1 is highly expressed in DRG neurons, and activation of TRPV1 results in sodium and calcium influx, leading to cell depolarization [51, 52]. Therefore, our results suggest that the effects of voluntary exercise on the reduction of ART-induced peripheral neuropathy may be due to the attenuation of the expression of TRPV1 in the nociceptive DRG neurons. Given that studies have also suggested that other TRPV subtypes, such as TRPV4, are also important modulators of mechanical and thermal sensitivity, future studies should determine the role of specific TRPV subtypes in the regulation of ART-induced peripheral neuropathy.
Currently available treatments for neuropathic pain have only modest benefits and, consequently, interventions that prevent the development of neuropathic pain would potentially have a substantial effect on public health [21]. Our study was the first to report the effects of voluntary exercise on ART-induced neuropathic pain. Our results are consistent with those of many previous studies that have shown that voluntary exercise can improve pain-like behavior in various mouse models of peripheral neuropathy. These findings suggest that voluntary exercise may have overlapping mechanisms that contribute to attenuation of distinct types of pain. In fact, it should be noted that peripheral synaptic transmission from DRG neurons to the spinal cord dorsal horn is crucial for pain signaling [53, 54]. These signals are then transferred into the brain for processing the pain sensation and responses [55]. Thus, it is likely that central nervous system (CNS) sensitization due to augmented glutamate neurotransmission underlies the general effects of voluntary exercise on pain. It is important that future studies explore the effects of ART on adaptations in other regions of the CNS and dissect the putative role of voluntary exercise in modulations of these ART-induced neuroadaptations. This line of research will not only be important in advancing our knowledge in ART-induced peripheral neuropathy, but it may provide an effective non-pharmacological therapeutic intervention for treating ART-induced painful peripheral neuropathy in HIV/AIDS patients.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human/animal participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Funding
None.
Footnotes
Hong Ye and Xingguang Du contributed equally to this work.
References
- 1.Larue F, Fontaine A, Colleau SM. Underestimation and undertreatment of pain in HIV disease: multicentre study. BMJ. 1997;314:23–28. doi: 10.1136/bmj.314.7073.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 3.Pillay P, Wadley AL, Cherry CL, Karstaedt AS, Kamerman PR. Pharmacological treatment of painful HIV-associated sensory neuropathy. S Afr Med J. 2015;105:769–772. doi: 10.7196/SAMJnew.7908. [DOI] [PubMed] [Google Scholar]
- 4.Cherry CL, Wadley AL, Kamerman PR. Painful HIV-associated sensory neuropathy. Pain Manag. 2012;2:543–552. doi: 10.2217/pmt.12.67. [DOI] [PubMed] [Google Scholar]
- 5.Robinson-Papp J, Morgello S, Vaida F, Fitzsimons C, Simpson DM, Elliott KJ, Al-Lozi M, Gelman BB, Clifford D, Marra CM, McCutchan JA, Atkinson JH, Dworkin RH, Grant I, Ellis R. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain. 2010;151:732–736. doi: 10.1016/j.pain.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fichtenbaum CJ, Clifford DB, Powderly WG. Risk factors for dideoxynucleoside-induced toxic neuropathy in patients with the human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:169–174. doi: 10.1097/00042560-199510020-00009. [DOI] [PubMed] [Google Scholar]
- 7.Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19:481–494. doi: 10.2165/00002018-199819060-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 11.Ciccolo JT, Jowers EM, Bartholomew JB. The benefits of exercise training for quality of life in HIV/AIDS in the post-HAART era. Sports Med. 2004;34:487–499. doi: 10.2165/00007256-200434080-00001. [DOI] [PubMed] [Google Scholar]
- 12.Wonders KY, Whisler G, Loy H, Holt B, Bohachek K, Wise R. Ten weeks of home-based exercise attenuates symptoms of chemotherapy-induced peripheral neuropathy in breast cancer patients. Health Psychol Res. 2013;1(3):e28. doi: 10.4081/hpr.2013.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mary Collins M, Linda Abbott R, Julie Aschenbrenner R, Connie Hart B. Putting evidence into practice®: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2007;11:901. doi: 10.1188/07.CJON.901-913. [DOI] [PubMed] [Google Scholar]
- 14.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156:504–513. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 15.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Fox LE, Cheng J. Swim therapy reduces mechanical allodynia and thermal hyperalgesia induced by chronic constriction nerve injury in rats. Pain Med. 2013;14:516–525. doi: 10.1111/pme.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sluka KA, O’Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol. 2012;114:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154:2658–2667. doi: 10.1016/j.pain.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankarappa SA, Piedras-Rentería ES, Stubbs EB. Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem. 2011;118:224–236. doi: 10.1111/j.1471-4159.2011.07302.x. [DOI] [PubMed] [Google Scholar]
- 20.Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Practice. 2002;2:87–97. doi: 10.1046/j.1533-2500.2002.02011.x. [DOI] [PubMed] [Google Scholar]
- 21.Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16:S12–S20. doi: 10.1097/00002508-200006001-00004. [DOI] [PubMed] [Google Scholar]
- 22.Höke A. Animal models of peripheral neuropathies. Neurotherapeutics. 2012;9:262–269. doi: 10.1007/s13311-012-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N Engl J Med. 1996;334:1011–1018. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 24.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 25.Parvathy SS, Masocha W. Matrix metalloproteinase inhibitor COL-3 prevents the development of paclitaxel-induced hyperalgesia in mice. Med Princ Pract. 2013;22:35–41. doi: 10.1159/000341710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geva N, Defrin R. Enhanced pain modulation among triathletes: a possible explanation for their exceptional capabilities. Pain. 2013;154:2317–2323. doi: 10.1016/j.pain.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Tesarz J, Schuster AK, Hartmann M, Gerhardt A, Eich W. Pain perception in athletes compared to normally active controls: a systematic review with meta-analysis. Pain. 2012;153:1253–1262. doi: 10.1016/j.pain.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013;240:157–167. doi: 10.1016/j.expneurol.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168:273–287. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LGA, Santos ARS. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience. 2011;194:337–348. doi: 10.1016/j.neuroscience.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 33.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houlé JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Kim S, Hoke A. An exercise regimen prevents development paclitaxel induced peripheral neuropathy in a mouse model. J Peripher Nerv Syst. 2015;20:7–14. doi: 10.1111/jns.12109. [DOI] [PubMed] [Google Scholar]
- 35.Mkandla K, Myezwa H, Musenge E. The effects of progressive-resisted exercises on muscle strength and health-related quality of life in persons with HIV-related poly-neuropathy in Zimbabwe. AIDS Care. 2016;28:639–643. doi: 10.1080/09540121.2015.1125418. [DOI] [PubMed] [Google Scholar]
- 36.Carmody J, Cooper K. Swim stress reduces chronic pain in mice through an opioid mechanism. Neurosci Lett. 1987;74:358–363. doi: 10.1016/0304-3940(87)90324-7. [DOI] [PubMed] [Google Scholar]
- 37.Ke Z, Yip SP, Li L, Zheng X-X, Tong K-Y. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;6:e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 39.Binder E, Droste SK, Ohl F, Reul JMHM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salam J, Fox J, Detroy E, Guignon M, Wohl D, Falls W. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 42.Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86:1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Chen YW, Hsieh PL, Chen YC, Hung CH, Cheng JT. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116:482–490. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 44.Armada-da-Silva PA, Pereira C, Amado S, Veloso AP. Role of physical exercise for improving posttraumatic nerve regeneration. Int Rev Neurobiol. 2013;109:125–149. doi: 10.1016/b978-0-12-420045-6.00006-7. [DOI] [PubMed] [Google Scholar]
- 45.Belter JG. Effects of voluntary exercise and genetic selection for high activity levels on HSP72 expression in house mice. J Appl Physiol. 2004;96:1270–1276. doi: 10.1152/japplphysiol.00838.2003. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann P, Terenius L, Thorén P. Cerebrospinal fluid immunoreactive β-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat. Regul Pept. 1990;28:233–239. doi: 10.1016/0167-0115(90)90021-n. [DOI] [PubMed] [Google Scholar]
- 47.Tominaga M, Caterina M J, Rosen T A, Julius D. The capsaicin receptor. a heat- and proton-activated lon channel. Seibutsu Butsuri. 1999;39:159–164. doi: 10.2142/biophys.39.159. [DOI] [Google Scholar]
- 48.Eid SR, Cortright DN (2009) Transient receptor potential channels on sensory nerves. Handb Exp Pharmacol 194:261–281. doi:10.1007/978-3-540-79090-7_8 [DOI] [PubMed]
- 49.Luo H, Cheng J, Han J-S, Wan Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund’s adjuvant in rats. Neuroreport. 2004;15:655–658. doi: 10.1097/00001756-200403220-00016. [DOI] [PubMed] [Google Scholar]
- 50.Luo H, Xu IS, Chen Y, Yang F, Yu L, Li GX, Liu FY, Xing GG, Shi YS, Li T, Han JS, Wan Y. Behavioral and electrophysiological evidence for the differential functions of TRPV1 at early and late stages of chronic inflammatory nociception in rats. Neurochem Res. 2008;33:2151–2158. doi: 10.1007/s11064-008-9751-4. [DOI] [PubMed] [Google Scholar]
- 51.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 52.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 53.Wood JN. Molecular mechanisms of nociception and pain. Handb Clin Neurol. 2006;81:49–59. doi: 10.1016/S0072-9752(06)80009-6. [DOI] [PubMed] [Google Scholar]
- 54.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 55.Cregg R, Momin A, Rugiero F, Wood JN, Zhao J. Pain channelopathies. J Physiol. 2010;588:1897–1904. doi: 10.1113/jphysiol.2010.187807. [DOI] [PMC free article] [PubMed] [Google Scholar]


