Abstract
Emulsified isoflurane (EIso) preconditioning can induce cardioprotection. We investigated whether EIso application after ischemia protects hearts against reperfusion injury and whether it is mediated by the inhibition of apoptosis. Rats were subjected to 30-min coronary occlusion followed by 180-min reperfusion. At the onset of reperfusion, rats were intravenously administered saline (sham, control group), 30 % intralipid (IL group) or 2 ml kg−1 EIso (EIso group) for 30 min. After reperfusion, infarct sizes, myocardial apoptosis and expression of Bcl-2, Bax and caspase-3 proteins were determined. Hemodynamic parameters were not different among groups. Compared with control and intralipid group, EIso limited infarct size, inhibited apoptosis, increased the expression of Bcl-2, decreased the expression of Bax, cleaved caspase-3, and enhanced Bcl-2/Bax ratio. EIso protects hearts against reperfusion injury when administered at the onset of reperfusion, which may be mediated by the inhibition of apoptosis via modulation of the expression of pro- and anti-apoptotic proteins.
Keywords: Emulsified isoflurane, Ischemia and reperfusion injury, Cardioprotection
Introduction
It has been demonstrated that volatile anesthetics produces preconditioning against myocardial ischemia and reperfusion (I/R) injury [1–4] and also confers marked protection when administered at the onset of or during early reperfusion [5–9].
Emulsified isoflurane (EIso) is a new formulation that enables an intravenous [10, 11] or oral [12] (rather than the traditional inhalation) route of administration for this anesthetic. We and other researchers have demonstrated that pretreatment with EIso produced strong cardioprotection against infarction, I/R injury or hypoxia/reoxygenation injury in rabbits [13, 14], rats [1, 15–17] and neonatal cardiac myocytes [18]. Several studies have recently shown that administration of isoflurane at early reperfusion provides cardioprotection against I/R injury [6, 8, 9]. Whether EIso administered after ischemia protects hearts against reperfusion injury remains unknown. Thus, the primary aim of the present study was to evaluate the cardioprotective effect of EIso when administered at the onset of reperfusion.
The potential mechanisms underlying anesthetic induced postconditioning have not completely been clarified. However, substantial evidence has shown that anesthetic-induced preconditioning and postconditioning share similar signal transduction pathways and that prevention of apoptosis might be a primary target of volatile anesthetics in the protection of the myocardium against I/R injury [19]. Emerging evidence indicates that isoflurane may inhibit cardiac myocyte apoptosis in various animal models [20–23]. These findings prompted us to postulate that EIso administered after ischemia may also protect the myocardium against I/R injury by inhibiting apoptosis, thereby precluding untimely cell death.
Therefore, the aims of the current study were to investigate whether administering EIso at the onset of reperfusion protects the heart against I/R injury and whether this effect is associated with inhibition of apoptosis.
Materials and methods
The study was approved by the Institutional Animal Care and Use Committee of Sichuan University (Sichuan, China) (Permit Number: 20100318) and was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Preparation of emulsified isoflurane
Details of the EIso preparation have been described previously [10, 11]. EIso (8 % vol./vol.) and 30 % intralipid were provided by Huarui Pharmaceutical Co. (Chengdu, China).
General preparation
Infarct or sham operations were performed on 200–250 g Sprague–Dawley male rats (approximately 10 weeks old, 8 per group) kept in a 12-h light–dark cycle under controlled conditions. Details of the surgical implantation of instruments have been described previously [17]. In brief, all rats were anesthetized by a single intraperitoneal injection of 1 % sodium pentobarbital (5 ml kg−1). A segment of the left anterior descending coronary artery (LAD) was isolated and a 6-0 silk ligature was placed around the vessel for production of coronary artery occlusion and reperfusion. Rats in the sham group underwent the same procedure except that the suture passed under the coronary artery without ligation. Coronary artery occlusion was verified by the presence of epicardial cyanosis and regional dyskinesia in the ischemic zone, as well as ST segment change. Reperfusion was achieved by releasing the snare and confirmed by visual observation of reactive hyperemia. Temperature was maintained with a heating blanket.
Experimental protocol
The group allocation of rats was randomized. After instrumentation was completed, all rats were stabilized for 20 min and subjected to 30 min of coronary occlusion (except for sham-operated group), followed by 3 h of reperfusion. Immediately at the onset of reperfusion, in separated groups, rats were intravenously administered isovolumetric continuous infusion of physiological saline (time-matched aerobic perfusion with sham operation and control group [sham and CON]), 30 % intralipid (IL group) and 2 ml kg−1 EIso (8 % vol./vol., EIso group) at a constant rate of 4 ml kg−1 h−1 for 30 min. Eight rats per group were used. In the second set of experiments, four experimental groups of rats (n = 8, each group) were subjected to the same experimental procedures. At the end of 180 min reperfusion, hearts were collected and kept in liquid nitrogen until further processed (for Western blot) or were fixed in paraffin (for immunohistochemical and apoptosis assay).
Measurements of hemodynamics
Hemodynamic parameters including heart rate (HR), left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and maximum rate of increase/decrease in left ventricular pressure (±dP/dt max) were continuously monitored by the connected calibrated pressure transducer with physiologic recorder (Biolap 420E+, Taimeng, Chengdu, China) throughout the experiment.
Measurement of myocardial infarct size
After 3 h of reperfusion, the coronary artery was re-occluded and 1 % Evans blue (Sigma Chemical Co., St. Louis, MO, USA) was injected into the left ventricular cavity to delineate the area at risk (AAR) within left ventricle (LV) as a non-stained area. Hearts were then frozen and sliced perpendicularly to the long axis from apex to base in 1-mm-thick sections. Sections were incubated for 20 min at 37 °C in 1 % 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma Chemical Co., St. Louis, MO, USA) in 0.1 M phosphate buffer adjusted to pH 7.4. After overnight fixation by 4 % paraformaldehyde, infarcted and noninfarcted myocardium within the AAR were separated and weighed. Infarct size was expressed as a percentage of the AAR.
Tissue preparation
In additional experiments, sham-operated hearts and infarcted hearts were evaluated for morphologic changes and biochemical markers of apoptosis at the protein level (n = 8). After 3 h of reperfusion, 2 ml of 1 % Evans blue dye was injected into the heart to delineate the LV AAR from the normal area. Hearts were then cut into 2-mm-thick slices, parallel to the atrioventricular. The atria and right ventricle were removed. Transverse sections of the hearts were immersed in 4 % paraformaldehyde overnight at room temperature, then, fixed in formaldehyde, dehydrated, and embedded in paraffin. In each heart, 5-µm-thick consecutive sections, including the left ventricular walls and the septums, were sliced for immunohistochemical analysis and apoptosis measurement. Two researchers blinded to the treatment group performed all analysis and measurements of pro- and anti-apoptotic protein expression and apoptosis.
Determination of myocardial apoptosis
Myocardial apoptosis was qualitatively analyzed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay according to the manufacturer’s protocol (Roche Diagnostics, USA). More than 20 random different fields from each heart were chosen. The apoptotic index (AI) was determined as a percent of the number of positively TUNEL-stained cardiomyocyte apoptotic nuclei/total cardiomyocyte nuclei population. Images were obtained using a CAST system (Olympus A/S, Denmark) and analyzed with Image-pro plus (Media Cybernetics Inc., Carlsbad, CA, USA). Eight hearts were studied in each group. Assays were performed in a blinded manner.
Immunohistochemical analysis of the expression of apoptotic proteins
The expression levels of Bcl-2, Bax and caspase-3 protein were visualized using an immunohistochemical method. Heart sections of non-ischemic and ischemic left ventricles were deparaffinized in xylene and isopropanol. Primary antibodies containing Bcl-2, Bax and cleaved caspase-3 (Santa Cruz Biotechnology, USA) and secondary antibodies containing biotinylated goat anti-rabbit IgG (Bcl-2, caspase-3) or goat anti-mouse IgG (Bax) (Santa Cruz Biotechnology, USA) were used for Bcl-2, Bax and cleaved caspase-3 immunohistochemical staining, followed by the addition of SP (streptomyces anti-biotin protein-peroxidase) solution. 3,3-Diaminobenzene (DAB) (Beijing Zhongshan Golden Bridge Biotechnology Co., China) solution was used to stain the tissue, followed by counterstaining with hematoxylin for microscopy. Phosphate buffer solution was used as a negative control. An inverted microscope (CAST system, Olympus A/S, Denmark) was used to capture the digital images. Brown staining in the cytoplasm was evaluated as positive expression. Ten optical sights were randomly selected per slide for image analysis with Image-pro plus (Media Cybernetics Inc., Carlsbad, CA, USA). Statistical value was calculated by the ratio of optical density of area positively stained to mean optical density, i.e. positive expressive index (PEI).
Western blot analysis
Heart biopsies were homogenized in ice-cold lysis buffer. The homogenate was then centrifuged at 6,000 rpm for 15 min at 4 °C to remove cellular debris. Protein concentrations were determined using the BCA method (Pierce, Rockford, IL, USA). Equivalent amounts (20 μg) of protein in the supernatant were loaded and separated for SDS–polyacrylamide gel electrophoresis followed by transfer to a nitrocellulose membrane (Bio-Rad). After blocking, membranes were incubated for 2 h at room temperature with the following antibodies: Bcl-2, Bax, caspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and GAPDH (Abcam, Cambridge, MA, USA). The caspase-3 antibody detects endogenous levels of full-length caspase-3 (32 kDa) and the large fragment of caspase-3 resulting from cleavage (17 kDa). The membranes were incubated with horseradish-peroxidase-conjugated secondary antibodies containing either anti-mouse (1:5,000; Pierce) or anti-rabbit (1:10,000; Pierce) for 1 h at room temperature. Peroxidase activity was visualized by a Supersignal chemiluminescence detection kit (Pierce). A Kodak X-ray Processor 102 (Eastman Kodak, Rochester, NY, USA) was used to expose the film and individual band density was analyzed with Image J 1.440 software (Wayne Rasband, NIH, USA).
Statistical analysis
Data were analyzed with SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL, USA). The values are given as means and 95 % CIs or SDs. Homogeneity of variance was tested by Levene’s test. Two-way repeated-measures analysis of variance (ANOVA) was used to evaluate the statistical difference of hemodynamic changes over time, with group as a between-subjects factor and time as a within-subjects factor. Group × time interactions were also tested. Mauchly’s test was used to assess the sphericity assumption. If not applicable, a Greenhouse–Geisser correction was used to adjust the degrees-of-freedom [24]. The Bonferroni correction procedure was used for multiple comparisons at individual time points between groups when statistical differences were identified for group-time interactions. Otherwise, global conclusions were drawn and comparisons between times were performed. Statistical analyses of infarct size, protein expression, and TUNEL assay were performed by one-way ANOVA. If variances determined by a homogeneity-of-variance assumption were equal, the Bonferroni test was examined post hoc for multiple comparisons; otherwise, Dunnett’s T3 test was applied. Statistical significance was defined as P < 0.05.
The primary endpoint of this study was the infarct size evaluation. The criterion for significance was set at 0.05. A sample size of 8 rats in each group provided 80 % power to detect a conservative 20 % reduction in infarct size with a SD of 5 %.
Results
Systemic hemodynamics
Table 1 shows the time course of hemodynamics during the experiments. A total of 32 rats were randomized in the study. Statistically significant differences among sham, CON, IL and EIso groups in HR (P < 0.001), LVSP (P < 0.001), LVEDP (P < 0.001), dP/dt max (P < 0.001) and −dP/dt max (P < 0.001) were found over the period of measurement by repeated-measures analysis of variance. Significant group × time interactions were observed for HR (P < 0.001), LVSP (P < 0.001), LVEDP (P < 0.001), dP/dt max (P < 0.001) and −dP/dt max (P = 0.005). There was no difference in the baseline characteristics between groups with respect to any of these hemodynamic parameters before the coronary occlusion. During the reperfusion period, heart rate decreased in CON, IL and EIso groups. After 30-min ischemia, LVSP and dP/dt max showed a marked reduction, accompanied by an increase in LVEDP and −dP/dt max, compared with the sham group and respective baseline values. However, there were no significant group-related differences in the hemodynamic parameters throughout the occlusion and reperfusion period among CON, IL and EIso groups.
Table 1.
Hemodynamics
| Variable | Baseline | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 30 min | 60 min | 120 min | 180 min | |||
| HR (beats/min) | |||||||
| Sham | 409 ± 42 | 400 ± 44††,‡‡ | 397 ± 40††,‡‡ | 399 ± 43††,‡‡ | 391 ± 48††,‡‡ | 393 ± 41††,‡‡ | 389 ± 39††,‡‡ |
| CON | 435 ± 14 | 382 ± 18** ,## | 363 ± 27** ,## | 361 ± 29** ,## | 359 ± 27** ,## | 352 ± 33** ,## | 318 ± 39** ,## |
| IL | 411 ± 42 | 336 ± 39* ,## | 319 ± 40** ,## | 329 ± 43** ,## | 313 ± 48** ,## | 302 ± 50** ,## | 300 ± 43** ,## |
| EIso | 422 ± 47 | 349 ± 37* ,## | 336 ± 48** ,## | 319 ± 38** ,## | 348 ± 50* ,# | 351 ± 40** ,## | 332 ± 40** ,## |
| LVSP (mmHg) | |||||||
| Sham | 152 ± 7 | 143 ± 10††,‡‡ | 145 ± 11††,‡‡ | 147 ± 8††,‡‡ | 148 ± 9††,‡‡ | 148 ± 8††,‡‡ | 147 ± 9††,‡ |
| CON | 164 ± 7 | 118 ± 19** ,## | 113 ± 14** ,## | 124 ± 15** ,## | 127 ± 13** ,## | 128 ± 9** ,## | 124 ± 20** ,## |
| IL | 156 ± 14 | 115 ± 11** ,## | 111 ± 11** ,## | 122 ± 10** ,## | 122 ± 9** ,## | 126 ± 12**, ## | 120 ± 18** ,# |
| EIso | 163 ± 6 | 120 ± 18** ,## | 113 ± 16**, ## | 115 ± 9** ,## | 125 ± 13**, ## | 129 ± 15** ,## | 123 ± 16**, ## |
| LVEDP (mmHg) | |||||||
| Sham | −2 ± 2 | −1 ± 1†,‡ | −1 ± 1††,‡‡ | −2 ± 1††,‡ | −2 ± 1†,‡‡ | −2 ± 1††,‡‡ | −2 ± 1††,‡ |
| CON | −2 ± 2 | 5 ± 3** ,# | 5 ± 2** ,## | 3 ± 2** ,# | 3 ± 1** ,# | 4 ± 2** ,## | 4 ± 1** ,## |
| IL | −1 ± 1 | 6 ± 2** ,# | 6 ± 1** ,## | 3 ± 1** ,# | 4 ± 2**, ## | 4 ± 1** ,## | 4 ± 2** ,# |
| EIso | −1 ± 1 | 7 ± 2** ,## | 7 ± 2**, ## | 6 ± 1* ,## | 5 ± 4*, # | 5 ± 2** ,## | 4 ± 2**, # |
| dP/dt max (mmHg/s) | |||||||
| Sham | 6943 ± 732 | 6354 ± 884††,‡‡ | 6633 ± 749††,‡‡ | 6678 ± 757††,‡ | 6699 ± 725††,‡‡ | 6733 ± 685††,‡ | 6522 ± 811††,‡‡ |
| CON | 7971 ± 858 | 4748 ± 1064** ,## | 4342 ± 1115**, ## | 5066 ± 1239** ,## | 5492 ± 1437**, ## | 5811 ± 1378* ,## | 5054 ± 1322** ,## |
| IL | 7039 ± 683 | 4231 ± 822** ,## | 3813 ± 691** ,## | 4618 ± 699** ,# | 4792 ± 651**, ## | 5070 ± 784** ,# | 4359 ± 649** ,## |
| EIso | 7877 ± 958 | 5797 ± 1228**, # | 5385 ± 1147** ,## | 5584 ± 909** ,# | 6081 ± 996* ,## | 6176 ± 1004* ,## | 6118 ± 958* ,# |
| −dP/dt max (mmHg/s) | |||||||
| Sham | −6634 ± 1998 | −5806 ± 1501†,‡ | −6237 ± 1770††,‡‡ | −6262 ± 1935††,‡‡ | −6318 ± 1895††,‡‡ | −6355 ± 1870††,‡ | −6189 ± 1999††,‡ |
| CON | −7845 ± 1032 | −4967 ± 1045** ,# | −4871 ± 707** ,## | −5277 ± 565** ,## | −5294 ± 681** ,## | −5355 ± 493** ,## | −4492 ± 992** ,## |
| IL | −6786 ± 1429 | −3858 ± 632** ,# | −3680 ± 847** ,## | −4529 ± 1134**, ## | −4524 ± 1140**, ## | −4704 ± 1155* ,# | −4238 ± 1290**, # |
| EIso | −7096 ± 907 | −4291 ± 1754**, # | −4159 ± 1371**, ## | −4210 ± 1530** ,## | −4733 ± 916** ,## | −4882 ± 669** ,## | −4649 ± 615** ,## |
Data are mean values ± SD
LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; ±dP/dt max, maximum rate of increase/decrease in left ventricular pressure. EIso, 2 ml/kg emulsified isoflurane; CON, control; IL, 30 % intralipid. n = 8 in each group
* P < 0.05, ** P < 0.01 vs baseline; † P < 0.05, †† P < 0.01 vs CON; ‡ P < 0.05, ‡‡ P < 0.01 vs IL; # P < 0.05, ## P < 0.01 vs sham
Emulsified isoflurane reduces infarct size
Myocardial infarct size expressed as a percentage of the AAR differed significantly among groups (F = 10.07, P = 0.001) (Fig. 1). EIso administered at the onset of reperfusion reduced infarct size from 32.5 ± 3.6 % (95 % CI 29.5–35.5 %; P = 0.001) in CON and 29.5 ± 3.3 % (95 % CI 26.7–32.3 %; P = 0.048) in IL group to 25.3 ± 2.8 % (95 % CI 22.9–27.6 %). However, intralipid has no effect on infarct size (P = 0.236 vs CON).
Fig. 1.
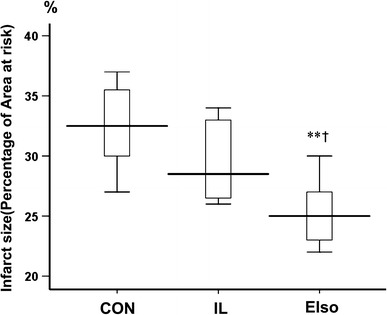
Myocardial infarct size expressed as a percentage of left ventricular area at risk in rats receiving no postconditioning stimuli (CON), 30 % intralipid (IL) or 2 ml kg−1 emulsified isoflurane (EIso). The upper and lower edges of each box plot represent the 25th percentile and the 75th percentile, respectively. The solid horizontal line within the box indicates the median value for the variable. Data are expressed as mean ± SD (n = 8 per group). **Significantly (P < 0.01) different from CON. †Significantly (P < 0.05) different from IL group
Emulsified isoflurane decreases myocardial apoptosis
TUNEL positive cardiomyocytes were not detected in the sham-operated group, nor in the non-ischemic zones in either group after I/R injury. However, they were prevalent in the left ventricular area at risk (Fig. 2). Administration of EIso reduced the ratio of apoptotic cardiomyocytes to total number of cardiomyocytes from 24.9 ± 2.4 % (CI: 22.9–26.8 %, P < 0.001) in CON and 22.3 ± 2.1 % (CI: 20.5–24.0 %, P = 0.057) in IL group to 19.5 ± 2.0 % (CI 17.8–21.2 %). Treatment with intralipid had no effect on cardiomyocyte apoptosis (P = 0.073 vs CON). Caspase-3 normally exists in an inactive state, which is cleaved into a biologically active P17 subunit when an apoptotic stimulus occurs [25]. We determined the expression of cleaved caspase-3 protein by immunohistochemistry and Western blotting. As shown in Fig. 3a, after I/R injury, cleaved caspase-3 protein expression was significantly increased in the CON, IL and EIso groups, compared with the sham-operated group (P < 0.01). EIso significantly decreased the expression level of cleaved caspase-3 (P = 0.022 vs CON). Indeed, although treatment with EIso did not alter the expression of total caspase-3, conversion of caspase-3 to the active cleaved form increased significantly after reperfusion injury in CON, IL and EIso groups compared with the sham group (Fig. 3b). However, EIso inhibited the increase in cleaved caspase-3 after 3 h of reperfusion (P < 0.001 vs CON, P = 0.007 vs IL) Fig. 3b.
Fig. 2.
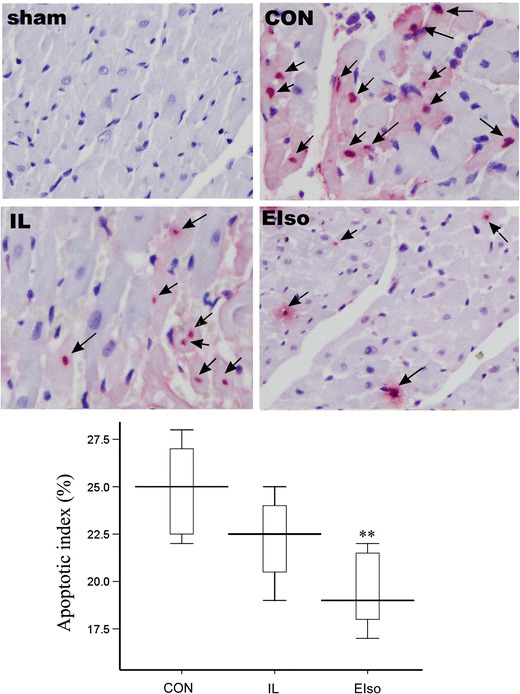
Apoptosis determined by measurement of terminal transferase dUTP nick end labeling (TUNEL) positive cardiomyocyte nuclei in the area at risk of myocardium obtained from rats receiving saline (sham, CON), 30 % intralipid (IL) or 2 ml kg−1 emulsified isoflurane (EIso) during early reperfusion after coronary artery occlusion. Arrows denote TUNEL positive nucleus. Positive cells were not detected in non-ischemic zone after ischemia and reperfusion. Magnification 400×. Sham, Sham operated group; CON, control group; IL, 30 % intralipid group; EIso, emulsified isoflurane group of 2 ml kg−1. Box-plot graph illustrates the average percentage of TUNEL-positive ventricular myocytes in the ischemic regions of LVs. Each group n = 8. Data are expressed as mean ± SD. **P < 0.01 compared with CON
Fig. 3.
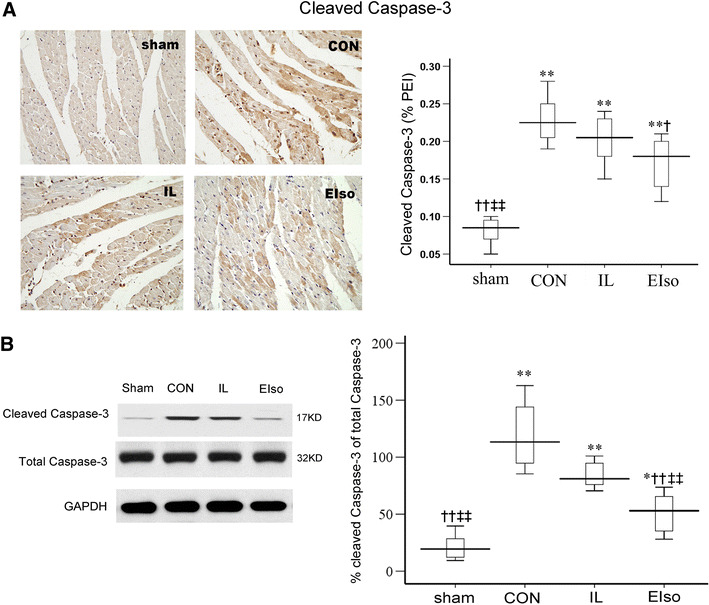
Caspase-3 protein expression and cleavage in myocardium of rats in each group. a Cleaved caspase-3 immunoreactivity in the cytoplasm of myocytes of hearts. Magnifications, 400×. b Western blot analysis of total and cleaved caspase-3 protein expression in hearts subjected to ischemia/reperfusion and receiving different treatments. Representative blots of total and cleaved caspase-3 (left). Data are quantified as percentage of cleaved caspase-3 of total caspase-3 and displayed using a box-plot. Data are presented as mean ± SD. Sham, Sham operated group; CON, control group; IL, 30 % intralipid group; EIso, emulsified isoflurane group of 2 ml kg−1. Each group n = 8. PEI, positive expressive index. *P < 0.05, **P < 0.01 vs sham; † P < 0.05, †† P < 0.01 vs CON; ‡‡ P < 0.01 vs IL
Expression of Bcl-2 and Bax proteins
Western blotting and immunohistochemistry confirmed the expression of Bcl-2 and Bax protein in cardiomyocytes of rats. As shown in Fig. 4, treatment using EIso significantly upregulated the expression of Bcl-2 protein (P = 0.001 vs CON) and downregulated the expression of Bax proteins (P = 0.001 vs CON, P = 0.038 vs IL), with the increase of Bcl-2/Bax ratio (P < 0.001 vs CON, P = 0.004 vs IL). Expression of Bcl-2 and Bax protein in the myocardium was further determined by Western blot analysis (Fig. 5), showing that significant difference was found among groups (Bcl-2: F = 13.97, P < 0.001 Bax: F = 13.36, P < 0.001). Compared with the sham-operated group, I/R injury in CON group significantly reduced Bcl-2 expression (P < 0.001) and increased Bax expression (P < 0.001). However, in EIso-treated hearts, this downregulation of Bcl-2 and upregulation of Bax expression was attenuated (Bcl-2: P = 0.006 vs CON; Bax: P < 0.001 vs CON), accompanied by the increase of Bcl-2/Bax ratio (P < 0.001 vs CON). Although the administration of intralipid did not alter the expression level of Bcl-2 and Bax, Bcl-2/Bax ratio was increased in IL group (compared with CON, P = 0.031: immunohistochemistry; P = 0.017: Westernblot).
Fig. 4.
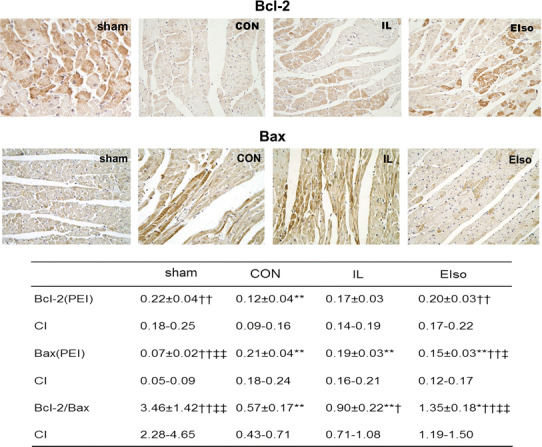
Myocardial Bcl-2 and Bax protein immunostaining. Rats were subjected to 3 h of sham ischemia and reperfusion or 30 min of myocardial ischemia followed by 3 h of reperfusion. Sham, Sham operated group; CON, control group; IL, 30 % intralipid group; EIso, emulsified isoflurane group of 2 ml kg−1. Magnification 400×. Data are presented as mean ± SD. Each group n = 8. PEI, positive expressive index; CI, 95 % confidence interval. *P < 0.05, **P < 0.01 vs sham; † P < 0.05, †† P < 0.01 vs CON; ‡ P < 0.05, ‡‡ P < 0.01 vs IL
Fig. 5.
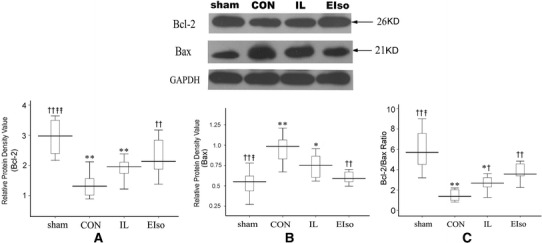
Effect of emulsified isoflurane on expression of Bcl-2 and Bax proteins visualized by Western blot analysis. Representative Western blot bands of Bcl-2 (26 kD), Bax (21kD) expression in the ischemic myocardium 3 h after reperfusion are shown. Corresponding GAPDH band as loading controls is shown in the upper panels. The box-plot graphs in the lower panel show quantification of the Western blot analysis for the respective proteins of Bcl-2 (a), Bax (b) and Bcl-2/Bax ratio (c) compared with GAPDH, respectively. Data are given as mean ± SD (n = 8, each group). *P < 0.05, **P < 0.01 vs sham; † P < 0.05, †† P < 0.01 vs CON; ‡ P < 0.05, ‡‡ P < 0.01 vs IL
Discussion
The major findings of the current study are as follows. First, we demonstrated that 2 ml kg−1 EIso (8 % vol./vol.) administered at the onset of early reperfusion reduced infarct size and attenuated myocardial apoptosis after I/R injury. Second, EIso exerted its cellular protection by modulation the expression of pro- and anti-apoptotic proteins, specifically Bcl-2, Bax and caspase-3.
In the previous studies, EIso, a formulation that enables an intravenous or oral [12] (rather than the traditional inhalation) route of administration for this anesthetic [10, 11], has already been shown to have a strong preconditioning effect on rabbits [13, 14, 26], rats [1, 15, 16] and neonatal rat cardiac myocytes [18]. However, because of the unpredictability of the precise timing of coronary artery occlusion for the majority of patients with acute myocardial infarction, a more practical strategy of administering postconditioning mimicking agents, including volatile anesthetics, may have the clinically attractive possibility of successfully reducing ischemic damage. There is evidence that the administration of isoflurane [6, 8] during early reperfusion protects the myocardium against I/R injury in various animal models and in patients undergoing coronary artery surgery with cardiopulmonary bypass [27]. Our present study confirmed and extended these observations, demonstrating that the administration of EIso during early reperfusion induces cardioprotection against I/R injury. Additionally, our results indicate that the beneficial effect of EIso may result from the inhibition of cardiac apoptosis. Our findings are concordant with the studies concerning the anti-apoptosis mechanism underlying isoflurane induced postconditioning, which may involve the activation of the apoptotic-related PI3K-Akt signal pathway [26] and inhibition of the mitochondrial permeability transition pore (mPTP) [8].
EIso used in our study is prepared by adding liquid isoflurane to 30 % intralipid [10, 11]. We used identical anesthetic protocols and an isovolumetric intravenous continuous infusion protocol for all the test drugs in all experimental groups. We showed that EIso (but not intralipid) could successfully reduce infarct size compared with IL and CON groups. Our results are contrary to a very recent study by Rahman et al. [28] showing that a single bolus injection of 20 % intralipid (5 ml/kg body weight) 5 min before reperfusion reduced infarct size in rat hearts. This discrepancy may be due to the fact that the entire drug was present in the myocardium before and after reperfusion in their study, while the infusion of EIso or intralipid started only at the onset of reperfusion and lasted for 30 min in our study. Furthermore, the drug concentration and administration strategy required to protect the myocardium may be specific for a given pharmaceutical stimulus; it may differ depending on the infusion speed, method or the drug exposure time. In line with our observations are findings from our previous [14, 16, 17] and other studies [13] using a similar infusion protocol, and confirming that intralipid alone did not decrease infarct size and that only EIso reduced infarct size and ameliorated myocardial damage. Interestingly, from our TUNEL staining result, EIso inhibited apoptosis and there is no difference of apoptosis between IL and EIso groups. Although intralipid failed to decrease infarct size, it could slightly increase Bcl-2/Bax ratio, indicating intralipid alone also showed an anti-apoptosis effect to a certain extent. Consistently, our lab previously found that intralipid postconditioning could inhibit cardiac myocyte apoptosis in isolated rat hearts [29]. Taken together, intralipid may affect the myocardial protective effects during reperfusion, however, in the current study, using an isovolumetric infusion protocol at a constant rate of 4 ml kg−1 h−1 for 30 min and a concentration of 30 %, intralipid alone failed to ameliorate permanent myocardial damage after reperfusion. Halogenated ether is dissolved in the lipid phase in the present study. We showed that EIso could effectively decrease infarct size and inhibit apoptosis. Emulsified anesthetics may enhance their cytoprotective actions by recruiting the large organic–aqueous interface between ether-loaded micelles and lipid rafts [30]. The emulsified ethers remodel lipid rafts over the large interfacial area. They reassemble key signaling molecules and translate the signal to subcellular targets such as nuclei and mitochondria. Although the exact mechanisms of EIso-induced cardioprotection are not clear, the interaction of the organic/aqueous interface with halogenated ether might be of particular interest.
Apoptosis, a highly regulated, controlled process whereby the cell commits suicide without inducing an inflammatory response, plays an important role in I/R injury [31]. Apoptosis is controlled by the complex interaction of numerous pro-survival and pro-death signals, among which, the Bcl-2 family exerts its effect primarily at the level of mitochondria, where they regulate cytochrome c release, caspase activation and calcium release from the endoplasmic reticulum. The Bcl-2 family is composed of anti-apoptotic (Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1), and pro-apoptotic proteins (Bax, Bak, Bok), all of which possess Bcl-2 homology regions which form a hydrophobic groove [32]. Bcl-2/Bax ratio has also been suggested to determine survival or death after I/R injury [33, 34]. A limited number of previous studies have suggested that the reduction in apoptosis contributes to the cardioprotective effects of isoflurane induced preconditioning [20] or post-conditioning [21] [23] in animal models of I/R injury. Our current study extends these findings by demonstrating that EIso induced cardioprotection against I/R injury occurs concomitantly with increases in Bcl-2 protein expression and decreases in Bax protein expression, resulting in an increased Bcl-2/Bax ratio. Caspases are a family of cysteine proteases that remain in an inactive state until hydrolysed at a specific aspartate [35]. Caspases are essential effector molecules in mediating apoptotic cell death, among which, caspase-3 is the crucial downstream effector that is cleaved and activated in response to apoptotic stimuli by the upstream initiator caspases [36]. The presence of activated caspase-3 is a very reliable or complementary marker to TUNEL in detecting apoptotic cells [37]. In the present study, we assessed apoptosis by TUNEL staining and measured activated caspase-3 tissue abundance after 3 h of reperfusion. We found that cleaved caspase-3 expression in the myocardium at risk is attenuated in the EIso group. In agreement with our findings, another research group also found that isoflurane preconditioning is associated with decreased expression of cleaved caspase-3 either in mice after I/R injury [38] or in adult and neonatal rat ventricular myocytes after hypoxia [22].
The mechanisms responsible for the beneficial effects of anesthetics-induced postconditioning remains poorly understood. However, a signaling cascade may contribute to the recruitment of multiple endogenous cardioprotective pathways to reduce reperfusion injury. The “crosstalk” among these pathways has been shown in different studies. Activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway, which is a major component of the RISK (reperfusion injury salvage kinase) pathway, prevents cardiac myocyte apoptosis and protects the myocardium from I/R injury [39]. Administration of isoflurane before and after reperfusion attenuates postischemic damage through a PI3K/Akt-dependent mechanism [26]. In addition, isoflurane postconditioning protects hearts against reperfusion by preventing opening of the mitochondrial permeability transition pore (mPTP) via PKB/Akt-GSK3β signaling in rabbits [8]. The JAK-STAT pathway has also been shown to be essential to the cardioprotection induced by ischemia postconditioning. Goodman et al. [40] demonstrated that both JAK-STAT and RISK signaling pathways need to be activated in postconditioning. JAK-STAT signaling may serve as an upstream initiator of RISK via PI3K-Akt activation, while only JAK-STAT signaling activation (without PI3K-Akt activation) is insufficient for producing cardioprotection. EIso treatment may elicit the activation of reperfusion injury salvage kinases. In a recent study, Yan et al. [41] showed that the cardioprotective effect of emulsified isoflurane may result from the activation of the JAK-STAT pathway. Furthermore, it has been proved that JAK2-STAT3 signaling has emerged as a critical regulator of apoptosis. Postconditioning may reduce myocardial apoptosis during prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. In the current study, although our results confirmed that apoptosis is involved in mediating postconditioning induced by emulsified isoflurane, the communication between signaling pathways and other components involved in EIso-induced cardioprotection needs to be further investigated.
The current results must be interpreted within the constraints of several potential limitations. (1) Blood concentrations of isoflurane and intralipid during or after infusion as well as end-tidal concentrations of isoflurane were not measured in this investigation; therefore, the estimated in vivo concentrations of isoflurane and intralipid are unknown and an unequal dose of drug administered to the rats cannot be absolutely excluded as a factor involved in I/R injury. (2) In the current experiment, the pH, PO2, and PCO2 were not monitored. However, blood gas permits the diagnosis of oxygenation, gas exchange, ventilation and acid–base homeostasis; thus it remains unknown whether reactive oxygen species would produce or release during and after the infusion of EIso. (3) We used only one dose of EIso and one infusion protocol in the study, researches regarding the combination of different administration protocols and dose responses deserve further investigation.
The present study has shown, for the first time, that when intravenously administered at the onset of reperfusion after prolonged ischemia, EIso attenuates myocardial apoptosis and reduces infarction size. This effect is mediated by modulating of the expression of pro- and anti-apoptotic proteins, especially Bcl-2, Bax and caspase-3. From a clinical point of view, post-ischemic treatment with pharmaceutical stimuli is particularly promising, because it is not readily feasible to predict the onset of ischemia for the majority of patients with acute myocardial infarction. Future clinical studies need to be done to determine its intrinsic protective effects, however, it is reasonable to extrapolate that intravenous administration of EIso will also have a cardioprotective effect on patients either undergoing coronary artery surgery or even outside of the perioperative setting.
Acknowledgments
This study was supported by Grant No. 30901412 to Zhaoyang Hu from the National Natural Science Foundation of China.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hu ZY, Liu J. Mechanism of cardiac preconditioning with volatile anaesthetics. Anaesth Intensive Care. 2009;37:532–538. doi: 10.1177/0310057X0903700402. [DOI] [PubMed] [Google Scholar]
- 2.Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 4.Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Weber NC, Preckel B, Schlack W. The effect of anaesthetics on the myocardium–new insights into myocardial protection. Eur J Anaesth. 2005;22:647–657. doi: 10.1017/S0265021505001080. [DOI] [PubMed] [Google Scholar]
- 6.Ge ZD, Pravdic D, Bienengraeber M, Pratt PF, Jr, Auchampach JA, Gross GJ, Kersten JR, Warltier DC. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85. doi: 10.1097/ALN.0b013e3181c4a607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagel PS. Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth. 2008;22:753–765. doi: 10.1053/j.jvca.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg. 2005;101:1590–1596. doi: 10.1213/01.ANE.0000181288.13549.28. [DOI] [PubMed] [Google Scholar]
- 10.Zhou JX, Luo NF, Liang XM, Liu J. The efficacy and safety of intravenous emulsified isoflurane in rats. Anesth Analg. 2006;102:129–134. doi: 10.1213/01.ane.0000189612.24147.07. [DOI] [PubMed] [Google Scholar]
- 11.Yang XL, Ma HX, Yang ZB, Liu AJ, Luo NF, Zhang WS, Wang L, Jiang XH, Li J, Liu J. Comparison of minimum alveolar concentration between intravenous isoflurane lipid emulsion and inhaled isoflurane in dogs. Anesthesiology. 2006;104:482–487. doi: 10.1097/00000542-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Lin HX, Luo NF, Liu J, Yang J, Li Q. The sedative and hypnotic effects and safety of oral emulsified isoflurane in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41(862–864):887. [PubMed] [Google Scholar]
- 13.Chiari PC, Pagel PS, Tanaka K, Krolikowski JG, Ludwig LM, Trillo RA, Jr, Puri N, Kersten JR, Waltier DC. Intravenous emulsified halogenated anesthetics produce acute and delayed preconditioning against myocardial infarction in rabbits. Anesthesiology. 2004;101:1160–1166. doi: 10.1097/00000542-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Rao Y, Wang YL, Zhang WS, Liu J. Emulsified isoflurane produces cardiac protection after ischemia-reperfusion injury in rabbits. Anesth Analg. 2008;106:1353–1359. doi: 10.1213/ane.0b013e3181679347. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Zhang W, Liu S, Yanfang C, Li T, Liu J. Cardioprotection afforded by St Thomas solution is enhanced by emulsified isoflurane in an isolated heart ischemia reperfusion injury model in rats. J Cardiothorac Vasc Anesth. 2010;24:99–103. doi: 10.1053/j.jvca.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Hu ZY, Liu J. Effects of emulsified isoflurane on haemodynamics and cardiomyocyte apoptosis in rats with myocardial ischaemia. Clin Exp Pharmacol Physiol. 2009;36:776–783. doi: 10.1111/j.1440-1681.2009.05138.x. [DOI] [PubMed] [Google Scholar]
- 17.Hu ZY, Luo NF, Liu J. The protective effects of emulsified isoflurane on myocardial ischemia and reperfusion injury in rats. Can J Anaesth. 2009;56:115–125. doi: 10.1007/s12630-008-9016-3. [DOI] [PubMed] [Google Scholar]
- 18.Yang MC, Chen YP, Cao DJ, Qian X, Li P. The optimal concentration for the protective effect of emulsified isoflurane on the neonatal rat cardiac myocytes hypoxia/reoxygenation injury of primary culture. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:1075–1077. [PubMed] [Google Scholar]
- 19.Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ, Dobson GP. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol. 2007;103:1441–1448. doi: 10.1152/japplphysiol.00642.2007. [DOI] [PubMed] [Google Scholar]
- 20.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Therap. 2006;318:186–194. doi: 10.1124/jpet.105.100537. [DOI] [PubMed] [Google Scholar]
- 21.Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Analg. 2005;101:942–949. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- 22.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–1014. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Venkatapuram S, Wang C, Krolikowski JG, Weihrauch D, Kersten JR, Warltier DC, Pratt PF, Jr, Pagel PS. Inhibition of apoptotic protein p53 lowers the threshold of isoflurane-induced cardioprotection during early reperfusion in rabbits. Anesth Analg. 2006;103:1400–1405. doi: 10.1213/01.ane.0000240903.63832.d8e. [DOI] [PubMed] [Google Scholar]
- 24.Keselman HJ, Keselman JC. The analysis of repeated measures designs in medical research. Stat Med. 1984;3:185–195. doi: 10.1002/sim.4780030211. [DOI] [PubMed] [Google Scholar]
- 25.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 26.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 27.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, ten Broecke PW, De Blier IG, Stockman BA, Rodrigus IE. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299–310. doi: 10.1097/00000542-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M. Phosphorylation of GSK-3beta mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology. 2011;115:242–253. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu SL, Liu J. Apoptosis mechanism of intralipid postconditioning to reduce ischemia reperfusion injury of isolated rat hearts. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:663–666. [PubMed] [Google Scholar]
- 30.Lucchinetti E, Schaub MC, Zaugg M. Emulsified intravenous versus evaporated inhaled isoflurane for heart protection: old wine in a new bottle or true innovation? Anesth Analg. 2008;106:1346–1349. doi: 10.1213/ane.0b013e31816d1661. [DOI] [PubMed] [Google Scholar]
- 31.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. doi: 10.1161/01.RES.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 32.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 33.Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, Guyton RA, Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45:651–660. doi: 10.1016/S0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, Vinten-Johansen J. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res. 2000;94:133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- 35.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/S0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 37.Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–228. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsumi YM, Patel HH, Lai NC, Takahashi T, Head BP, Roth DM. Isoflurane produces sustained cardiac protection after ischemia-reperfusion injury in mice. Anesthesiology. 2006;104:495–502. doi: 10.1097/00000542-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Feng J, Lucchinetti E, Fischer G, Xu L, Pedrazzini T, Schaub MC, Zaugg M. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Goodman MD, Koch SE, Fuller-Bicer GA, Butler KL. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol. 2008;295:H1649–H1656. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan L, Jiang X, Tai W, Shi E. Emulsified isoflurane induces postconditioning against myocardial infarction via JAK-STAT pathway. J Surg Res. 2012;178:578–585. doi: 10.1016/j.jss.2012.06.007. [DOI] [PubMed] [Google Scholar]


