Abstract
GnRH neurons form a final common pathway for the central regulation of reproduction. Although the involvement of acetylcholine in GnRH secretion has been reported, direct effects of acetylcholine and expression profiles of acetylcholine receptors (AChRs) still remain to be studied. Using immortalized GnRH neurons (GT1-7 cells), we analyzed molecular expression and functionality of AChRs. Expression of the mRNAs were identified in the order α7 > β2 = β1 ≧ α4 ≧ α5 = β4 = δ > α3 for nicotinic acetylcholine receptor (nAChR) subunits and m4 > m2 for muscarinic acetylcholine receptor (mAChR) subtypes. Furthermore, this study revealed that α7 nAChRs contributed to Ca2+ influx and GnRH release and that m2 and m4 mAChRs inhibited forskolin-induced cAMP production and isobutylmethylxanthine-induced GnRH secretion. These findings demonstrate the molecular profiles of AChRs, which directly contribute to GnRH secretion in GT1-7 cells, and provide one possible regulatory action of acetylcholine in GnRH neurons.
Keywords: Acetylcholine, Acetylcholine receptors, Gonadotropin-releasing hormone, GnRH neurons, GT1-7
Introduction
Acetylcholine is the first neurotransmitter identified in the nervous systems and plays pivotal roles in a wide variety of physiological processes such as muscle contraction, transmission of autonomic signaling, and learning and memory. There are two categories of receptors that bind acetylcholine and transmit its signaling: nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors (mAChRs) named after the effective agonists, nicotine and muscarine, respectively [1, 2].
NAChRs belong to the cis-loop family of ligand-gated ion channels that include ionotropic GABA, glycine, and 5-HT3 receptors. Sixteen subunits of nAChR (α1–7, 9, 10, β1–4, γ, ε, and δ) have been identified in the mammals and assemble into a variety of receptor subtypes each containing five homologous subunits [3, 4]. nAChRs in the nervous system are formed from αβ combination of α2–α6 and β2–β4 subunits. In addition, α7 and α9 subunits are capable of forming homomeric nAChRs, and α10 forms a heteromer with α9. These ion channels are permeable to Na+ and K+, and in some neuronal types to Ca2+. Some subunit compositions such as α7 homomeric receptors are highly permeable to Ca2+ [5]. mAChRs are G-protein coupled receptors, and are classified as metabotropic receptors. There are five muscarinic acetylcholine receptor subtypes, designated m1-5. They are further divided into two broader families based on coupling to different G-proteins. m1, m3, and m5 receptors are coupled to Gq/11-proteins and activate phospholipase C, while m2 and m4 receptors are coupled to Gi/o-proteins and inhibit adenylyl cyclases [6].
Gonadotropin-releasing hormone (GnRH) neurons represent the final common pathway for the central regulation of reproduction. A prior study using the bovine median eminence implicated the involvement of acetylcholine in GnRH release [7]. However, relatively few studies have reported a physiological role of acetylcholine in function of GnRH neurons [8–11]. Richardson et al. [12] documented stimulation of GnRH release by acetylcholine from hypothalamic organ cultures, which was blocked by a nAChR antagonist, hexamethonium and not by a mAChR antagonist, atropine, suggesting the involvement of nAChRs in GnRH release. A recent immunohistochemical study of Turi et al. [13] demonstrated the existence of cholinergic afferents to GnRH neurons of the preoptic area in the rat. DNA microarray analysis of Todman et al. [14] documented the presence of nicotinic β1, β2, and γ subunits and muscarinic m1 subtype in mouse GnRH neurons. However, direct effects of acetylcholine on GnRH neurons and detailed expression profiles of acetylcholine receptors in the neurons still remain to be analyzed.
GnRH neurons are relatively a few in numbers, and are scattered in regions including the diagonal band of Broca, medial septum, medial preoptic area and suprachiasmatic nucleus, anterior and lateral hypothalami making complex neural network with other neurons [15]. These in vivo characteristics greatly hinder the endocrinological and morphological investigation that directly target GnRH neurons. An immortalized cell line, GT1-7, derived from mouse hypothalamus synthesizes and releases GnRH [16]. Furthermore, owing to plentiful cell numbers and the ability to manipulate the cells in vitro, this cell line provides a suitable model for GnRH neurons. GT1-7 cells also express functional neurotransmitter receptors, which permits their molecular and pharmacological properties to be studied. A pioneering study by Krsmanovic et al. [17] pharmacologically demonstrated the presence of nAChRs and mAChRs coupled with both Gq/11- and Gi/o-proteins in GT1-7 cells, and characterized modulatory effects of acetylcholine on GnRH release from GT1-7 cells. Determination of subunit expression and the detailed composition of acetylcholine receptors in GnRH neurons are required to further the understanding of the modulatory effects of acetylcholine on GnRH secretion; however, such studies have been limited.
Therefore, as a preliminary study to investigate acetylcholine receptors in GnRH neurons, this paper aimed to determine the expression profiles of nAChR subunits and mAChR subtypes and their functional characteristics in GT1-7 cells. We comprehensively analyzed the expression of nAChR subunit and mAChR subtype mRNAs in GT1-7 cells using RT-PCR. Expression levels were quantified using real-time PCR. The functional characteristics of nAChRs were evaluated from Ca2+ influx using Ca2+ imaging, while mAChRs were assessed by measurement of cAMP formation using enzyme immunoassays (EIAs). In addition, the effects of both nAChR and mAChR agonists on GnRH secretion were assessed using GnRH assay.
Materials
Animals
Adult male and pregnant female C57BL/6 J mice were purchased from CLEA Japan (Tokyo, Japan). The mice were maintained for 1–2 weeks under a 12-h light/dark illumination with free access to food and water. Ten-week-old male and neonatal mice were used. The animals were deeply anesthetized and killed by decapitation. Adult and neonatal mouse skeletal muscles and adult mouse brains were removed. The brain regions containing the preoptic area and hypothalamus were dissected. The skeletal muscles and brain sections were frozen in liquid nitrogen until use. Experiments using the animals were conducted in adherence to the Guidelines for the Care and Use of Laboratory Animals of Nippon Medical School. The experimental procedures were approved by the Committee for Animal Experimentation of Nippon Medical School.
Cell culture
GT1-7 cells [16] (kindly provided by Dr. Richard Weiner) were grown in Dulbecco’s modified Eagle’s medium (DMEM), containing 10 % fetal bovine serum (Equitech-Bio, Kerrville, TX, USA), 4.5 g/l d-glucose, 586 mg/l l-glutamine, 2.0 g/l NaHCO3 and 110 mg/l sodium pyruvate. Cells were cultured at 37 °C under 5 % CO2, 95 % atmosphere. The culture medium was changed every 3–4 days, and the cells were passaged every 1 week, and used in experiments within ten passages.
RT-PCR
Total RNA was extracted from GT1-7 cells, the skeletal muscles, and the brain sections using RNAiso plus (Takara Bio, Tokyo, Japan) following the manufacturer’s instructions. Total RNA was treated with Turbo DNase (Thermo Fisher Scientific, Waltham, MA, USA) and re-purified. RNA concentration was quantified by absorption at 260 nm. Total RNA was reverse-transcribed with oligo-dT primers to produce first-strand cDNA. The reaction mixtures (total volume, 20 μl) contained 5 μg of total RNA, 1 × RT buffer, 1 mM dNTP mixture, 500 ng oligo-(dT)15, 20 U RNA inhibitor (Promega, Madison, WI, USA) and 100 U ReverTra Ace (Toyobo, Osaka, Japan). The reaction was carried out at 42 °C for 60 min, and stopped by heating to 75 °C for 15 min. cDNA was treated with 4 U of RNase H (Takara Bio).
PCR was performed in 25 μl of reaction mixtures comprising cDNA corresponding to 200 ng of total RNA, 1 × PCR buffer, 0.2 mM dNTP mixture, 0.2 μM forward and reverse primers, and Blend Taq polymerase (Toyobo). PCR conditions consisted of an appropriate number of cycles (25–33 cycles for acetylcholine receptor subunits and subtypes, 16 cycles for Gnrh1, and 17 cycles for Actb and Gapdh) of 95 °C for 30 s, 60 °C for 20 s and 72 °C for 30 s, with an initial denaturation at 95 °C for 5 min and a final elongation at 72 °C for 5 min. The sequences of oligonucleotide primers used for conventional RT-PCR are listed in Table 1.
Table 1.
Oligonucleotide primers used for conventional RT-PCR
| Acetylcholine receptor class | Gene | Direction | Oligonucleotide sequence (5′ to 3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| Nicotinic acetylcholine receptor | Chrna1 | Forward | 5’-TTAACCCGGAAAGTGACCAG-3’ | 397 | NM_007389 |
| Reverse | 5′-ATGATGGACGCAATGACAAA-3′ | ||||
| Chrna2 | Forward | 5′-TCCCCTCAGAGATGATCTGG-3′ | 368 | NM_144803 | |
| Reverse | 5′-GTAGGTGACGTCGGGGTAGA-3′ | ||||
| Chrna3 | Forward | 5′-CTACCAAGGGGTGGAGTTCA-3′ | 197 | NM_145129 | |
| Reverse | 5′-ACGGGAAGTAGGTCACATCG-3′ | ||||
| Chrna4 | Forward | 5′-GGACCCTGGTGACTACGAGA-3′ | 409 | NM_015730 | |
| Reverse | 5′-GGCGTAGGTGATGTCAGGAT-3′ | ||||
| Chrna5 | Forward | 5′-GTGGATCCCAGACATCGTTT-3′ | 284 | NM_176844 | |
| Reverse | 5′-CGCTCATGATTTCCCATTCT-3′ | ||||
| Chrna6 | Forward | 5′-CAAGACAAAGGAGGCAGGAG-3′ | 371 | NM_021369 | |
| Reverse | 5′-GAAATAGCCCCACAGTTCCA-3′ | ||||
| Chrna7 | Forward | 5′-CATTCCACACCAACGTCTTG-3′ | 357 | NM_007390 | |
| Reverse | 5′-TGAGCACACAAGGAATGAGC-3′ | ||||
| Chrna9 | Forward | 5′-CCCTTGCGTCCTCATATCGT-3′ | 402 | NM_001081104 | |
| Reverse | 5′-GACCCTGGAAGTTTGCCATA-3′ | ||||
| Chrna10 | Forward | 5′-CAGGGCCTGTTGCTTTACAT-3′ | 210 | NM_001081424 | |
| Reverse | 5′-TGTCTGCCACTGGTCTCAAG-3′ | ||||
| Chrnb1 | Forward | 5′-TTCTACCTCCCACCAGATGC-3′ | 168 | NM_009601 | |
| Reverse | 5′-GGAGAAGGTGACAAGGACCA-3′ | ||||
| Chrnb2 | Forward | 5′-GTACCGCTGGTGGGAAAGTA-3′ | 541 | NM_009602 | |
| Reverse | 5′-TGGTCCCAAAGACACAGACA-3′ | ||||
| Chrnb3 | Forward | 5′-GAGTTCTGGTCGCTTTCCTG-3′ | 287 | NM_027454 | |
| Reverse | 5′-GCAGCCCTCAGTTCTAGGTG-3′ | NM_173212 | |||
| Chrnb4 | Forward | 5′-CCTGCCACTCCTAAGTCTGC-3′ | 315 | NM_148944 | |
| Reverse | 5′-AACACGCTGGGTAGCCTAGA-3′ | ||||
| Chrnd | Forward | 5′-TGGTGGTGGTGATCTGTGTC-3′ | 256 | NM_021600 | |
| Reverse | 5′-CGCTCTGATTGCTTCTCAAA-3′ | ||||
| Chrne | Forward | 5′-TTGCCCAGAAAATTCCAGAG-3′ | 151 | NM_009603 | |
| Reverse | 5′-GGGGATGTAGCATGAGTCGT-3′ | ||||
| Chrng | Forward | 5′-TCCTCCTGCTCCATCTCTGT-3′ | 171 | NM_009604 | |
| Reverse | 5′-CCCATTCTCTGTGAAAGCCT-3′ | ||||
| Muscarinic acetylcholine receptor | Chrm1 | Forward | 5′-GACCCTACAGACCCCTCTCC-3′ | 233 | NM_007698 |
| Reverse | 5′-GCAGGTTGCCTGTCACTGTA-3′ | ||||
| Chrm2 | Forward | 5′-CACTGGGAGAAGTGGAGGAG-3′ | 430 | NM_203491 | |
| Reverse | 5′-GATCCAGCCACAAGGACAAT-3′ | ||||
| Chrm3 | Forward | 5′-ACAGTCGCTGTCTCCGAACT-3′ | 642 | NM_033269 | |
| Reverse | 5′-TGCCACAATGACAAGGATGT3′ | ||||
| Chrm4 | Forward | 5′-GCTTTGACCGCTATTTCTGC-3′ | 369 | NM_007699 | |
| Reverse | 5′-TCAGAGGGCTCTTGAGGAAA-3′ | ||||
| Chrm5 | Forward | 5′-TTGGCTTGCACTCGACTATG-3′ | 261 | NM_205783 | |
| Reverse | 5′-GTGGGCTCAGAGAGGAACTG-3′ | ||||
| Gnrh1 | Forward | 5′-CCTGGGGGAAAGAGAAACACT-3′ | 246 | NM_008145 | |
| Reverse | 5′-TCACAAGCCTCAGGGTCAATG-3′ | ||||
| Actb | Forward | 5′-CCTAAGGCCAACCGTGAAAAGATG-3′ | 430 | NM_007393 | |
| Reverse | 5′-ACCGCTCGTTGCCAATAGTGATG-3′ | ||||
| Gapdh | Forward | 5′-TGAAGGTCGGTGTGAACGGATTTG-3′ | 359 | NM_008084 | |
| Reverse | 5′-GGCGGAGATGATGACCCTTTTG-3′ |
PCR products (5 μl) were separated by electrophoresis on 1.8 % of agarose gels, and visualized by ethidium bromide staining under UV irradiation. Gel images were captured using an ASTEC Gel Scene System (ASTEC, Fukuoka, Japan).
DNA sequencing
PCR products were extracted from agarose gels using a Wizard SV gel and PCR Clean-up system (Promega), and cloned into pGEM-T Easy vectors (Promega). Sequencing reactions were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). Fluorescent signals were detected using ABI Prism 3100 or 3130 Genetic Analyzers (Thermo Fisher Scientific).
Real-time PCR
Quantitative PCR was conducted using an ABI 7300 Real Time PCR System (Thermo Fisher Scientific) and SYBR GreenER qPCR SuperMix kits (Thermo Fisher Scientific) according to the manufacturers’ instructions. The reaction mixtures (25 μL) contained 1 × SYBR GreenER qPCR SuperMix, 0.2 μM each forward and reverse primer, and an appropriate amount (equivalent to 20 ng of total RNA for Actb, Gapdh, and Gnrh1, or equivalent to 200 ng for acetylcholine receptors) of cDNA or 102–108 copies of plasmid DNA. The PCR conditions consisted of 40 cycles of 95 °C for 15 s and 60 °C for 35 s with initial steps of 50 °C for 2 min and 95 °C for 10 min. The sequences of oligonucleotide primers used for quantitative PCR are listed in Table 2.
Table 2.
Oligonucleotide primers used for quantitative PCR
| Acetylcholine receptor class | Gene | Direction | Oligonucleotide sequence (5′ to 3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| Nicotinic acetylcholine receptor | Chrna3 | Forward | 5′-CTACCAAGGGGTGGAGTTCA-3′ | 197 |
| Reverse | 5′-ACGGGAAGTAGGTCACATCG-3′ | |||
| Chrna4 | Forward | 5′-TAACCAAAGCCCACCTGTTC-3′ | 296 | |
| Reverse | 5′-GGCGTAGGTGATGTCAGGAT-3′ | |||
| Chrna5 | Forward | 5′-GTGGATCCCAGACATCGTTT-3′ | 284 | |
| Reverse | 5′-CGCTCATGATTTCCCATTCT-3′ | |||
| Chrna7 | Forward | 5′-CCGGAGTGAAAAATGTTCGT-3′ | 110 | |
| Reverse | 5′-CAAGACGTTGGTGTGGAATG-3′ | |||
| Chrnb1 | Forward | 5′-TGAAGGAGGACTGGCAGTTT-3′ | 145 | |
| Reverse | 5′-GGTCCTCAAGGGAAAGGTTC-3′ | |||
| Chrnb2 | Forward | 5′-CCCCTGCGTACTCATCACCT-3′ | 172 | |
| Reverse | 5′-GTACTTTCCCACCAGCGGTA-3′ | |||
| Chrnb4 | Forward | 5′-CCTGCCACTCCTAAGTCTGC-3′ | 315 | |
| Reverse | 5′-AACACGCTGGGTAGCCTAGA-3′ | |||
| Chrnd | Forward | 5′-GACCGTCTCTGCCTGTTTGT-3′ | 143 | |
| Reverse | 5′-ATGAAGCGCTTGTCCTGTTC-3′ | |||
| Muscarinic acetylcholine receptor | Chrm2 | Forward | 5′-AGAAAGCTCCAACGACTCCA-3′ | 275 |
| Reverse | 5′-GCTGCTTGGTCATCTTCACA-3′ | |||
| Chrm4 | Forward | 5′-TCCTCACCTGGACACCCTAC-3′ | 154 | |
| Reverse | 5′-TTGAAAGTGGCATTGCAGAG-3′ | |||
| Gnrh1 | Forward | 5′-CCTGGGGGAAAGAGAAACACT-3′ | 246 | |
| Reverse | 5′-TCACAAGCCTCAGGGTCAATG-3′ | |||
| Actb | Forward | 5′-CTGGCTCCTAGCACCATGAAGA-3′ | 198 | |
| Reverse | 5′-GTAAAACGCAGCTCAGTAACAGTC-3′ | |||
| Gapdh | Forward | 5′-ACAGTCCATGCCATCACTGCC-3′ | 266 | |
| Reverse | 5′-GCCTGCTTCACCACCTTCTTG-3′ |
Ca2+ imaging
GT1-7 cells were seeded on poly-d-lysine-coated glass-bottom dishes at a concentration of 2 × 105 cells/dish and cultured for 48 h. The cells were washed with DMEM and loaded for 45 min with 3 μM Fluo-4-AM (Dojindo, Kumamoto, Japan) in DMEM containing 0.04 % Pluronic F-127 (Thermo Fisher Scientific). After loading, the medium was replaced with recording solution [126 mM NaCl, 5 mM KCl, 10 mM CaCl2, 0.8 mM MgCl2, 10 mM glucose, 20 mM HEPES, 0.6 mM NaHCO3, and 0.1 % bovine serum albumin (BSA) pH 7.4]. The cells were incubated at 37 °C for 15–30 min, and then analyzed for fluorescence using a confocal laser scanning microscope (LSM 710 Carl Zeiss, Jena, Germany) and LSM Software ZEN 2009 (Carl Zeiss). Acetylcholine (Sigma-Aldrich, St. Louis, MO, USA) and nicotine (Wako, Osaka, Japan) were bath-applied. When an α7 nAChR antagonist, methyllycaconitine (MLA, Sigma-Aldrich), was employed, the cells were incubated with 10–1000 nM MLA for 5–15 min before injecting 1 mM acetylcholine. For the measurement of green fluorescence, a 489 nm diode laser and 493–622 nm filter were used. Stacks of images (one image/s, 512 × 512 pixel) were recorded for 5 min using a 20 × lens and ZEN 2009. Regions of interest were created that exactly enclosed a group of GT1-7 cells (n = 100–200). To measure the relative intensity, the mean of fluorescence intensity before the injection (about 6 s) was calculated as the basal fluorescence intensity, and the change of fluorescence intensity was collected. The drug-evoked responses were obtained as a percentage of the maximum change of fluorescence intensity in comparison to the value of the basal fluorescence intensity. The mean fluorescence in regions of interest were collected from three or four dishes for each treatment (N = 3–4).
Enzyme immunoassay
To measure cAMP concentration, GT1-7 cells were seeded onto 24-well culture plates at a concentration of 1 × 106 cells/ml and cultured for 48 h. The cells were washed twice with DMEM and incubated in DMEM with 250 μM isobutylmethylxanthine (IBMX, Wako) for 10 min. The cells were then stimulated with 30 μM forskolin (Wako) and treated with 100 nM acetylcholine, 100 nM muscarine or a vehicle control for 10 min. 100 nM atropine or a vehicle control was added 5 min before stimulation. After treatment, the medium was aspirated, and the cells were lysed in 0.05 M acetate buffer (pH 5.8) containing 0.25 % dodecyltrimethylammonium and 0.02 % BSA. The concentration of cAMP in the lysates was determined using a cAMP Complete ELISA kit (Enzo Life Sciences, Plymouth Meeting, PA, USA). All samples were assayed in duplicate. The intra-assay coefficient of variation was less than 5 %. According to the manufacturer’s description, the minimum sensitivity of the assay is 0.30 pmol/ml, and an inter-assay coefficient of variation is less than 13.7 %.
To examine the effect of acetylcholine via nAChRs, GT1-7 cells were seeded onto six-well culture plates at a concentration of 2 × 106 cells/ml and cultured for 72 h. The cells were washed twice with DMEM then incubated in DMEM with 0.1 % BSA for 15 min. The cells were stimulated with nicotine (300 μM or 1 mM), or a vehicle control for 40 min. MLA or a vehicle control was added 5 min before stimulation. To evaluate the muscarinic effect on GnRH release, the cells were cultured in 75 cm2 flasks at 80 % confluency. The cells were washed twice with DMEM and incubated in supplemented DMEM with and/or without 250 μM IBMX and/or 100 nM muscarine. Dimethyl sulfoxide (0.1 %) was used as a vehicle. The medium was collected at different times (6, 12, and 24 h) after stimulation. The collected medium was stored at –80 °C until use.
The concentration of GnRH in the medium was determined using a Luteinizing Hormone-Releasing Hormone EIA Kit (Phoenix Pharmaceuticals, Burlingame, CA, USA). All samples were assayed in duplicate. The intra-assay coefficient of variation was less than 6 %. According to the manufacturer’s description, the minimum sensitivity of the assay is 0.04 ng/ml, and inter-assay coefficient of variation is less than 15 %.
Statistical analysis
Data are presented as mean ± SEM. Statistical differences were analyzed by one-way analysis of variance followed by the Tukey–Kramer multiple comparison test. A value of p < 0.05 was considered statistically significant.
Results
Expression of nAChR subunits and mAChR subtypes in GT1-7 cells
Expression profiles of nAChR subunit and mAChR subtype mRNAs in GT1-7 cells were analyzed using RT-PCR (Fig. 1). The analysis showed expression of α3, α4, α5, α7, β1, β2, β4, and δ nAChR subunits. The mRNA transcripts for m2 and m4 subtypes were also detected.
Fig. 1.
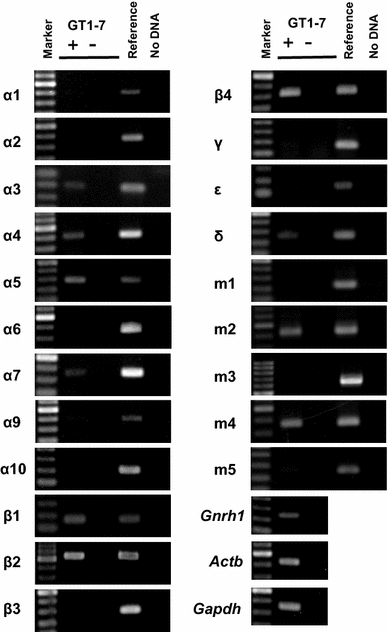
Expression of nAChR (α1–7, 9, 10 and β1–4, γ, ε, δ) subunits and mAChR subtypes (m1–m5) in GT1-7 cells. Total RNA from GT1-7 cells was subject to RT-PCR analysis. Expression of nAChR (α1–7, 9, 10 and β1–4, γ, ε, δ) subunit and mAChR subtype (m1–m5) transcripts was examined. Gnrh1 is used as a marker of GnRH neurons. Actb and Gapdh are used as internal controls. Marker 100 bp DNA ladder marker, + reverse-transcribed cDNA, − total RNA without reverse transcriptase, Reference reference organ (the brain section containing the preoptic area and hypothalamus for α2–7, 9, 10, β1–4, γ, ε, and δ, adult skeletal muscle for α1, ε, and δ and neonatal skeletal muscle for γ). The same results were obtained in four separately prepared samples
Real-time PCR analysis was applied to quantify the expression levels of nAChR subunits and mAChR subtypes detected by conventional RT-PCR (Fig. 2). The rank order of expression levels of nAChR subunit mRNAs was α7 > β2 = β1 ≧ α4 ≧ α5 = β4 = δ > α3, with > reflecting significant difference and = a lack of significant difference among pairs (Fig. 2a). The expression level of the α7 subunit is one to four orders of magnitude higher than those of the other subunits. The levels of mAChR mRNA transcripts were also quantified in the following order m4 > m2 (Fig. 2b).
Fig. 2.
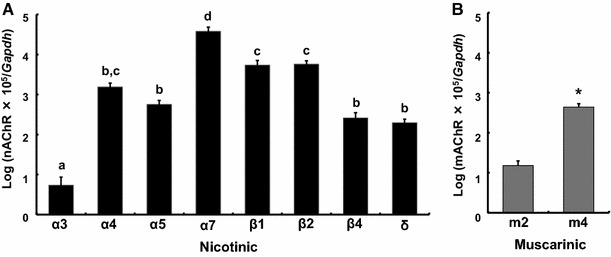
Quantification of nAChR subunit and mAChR subtype mRNA levels in GT1-7 cells. Expression levels of nAChR subunit and mAChR subtype mRNAs were quantitatively analyzed using real-time PCR. The expression levels of the target genes were normalized with that of the Gapdh gene. The different letters (a) and the asterisk (b) on the columns indicate statistically significant differences (p < 0.05). The data are represented as the mean ± SEM of four separately prepared samples
Effects of nAChR agonists on intracellular Ca2+ concentration ([Ca2+]i)
Activation of nAChRs induces membrane depolarization resulting in the opening of voltage-dependent Ca2+ channels. Furthermore, some neuronal nAChRs have high Ca2+ permeability. To confirm the functionality of nAChRs in GT1-7 cells, we conducted Ca2+ imaging using a Fluo-4-AM Ca2+ indicator. Application of relatively high concentrations (300 μM and 1 mM) of nicotine induced an increase of [Ca2+]i in GT1-7 cells (Fig. 3a, b). GT1-7 cells responded to 1 mM acetylcholine in the presence but not absence of extracellular Ca2+ (Fig. 3c), indicating the lack of a Gq/11-coupled mAChR in GT1-7 cells.
Fig. 3.
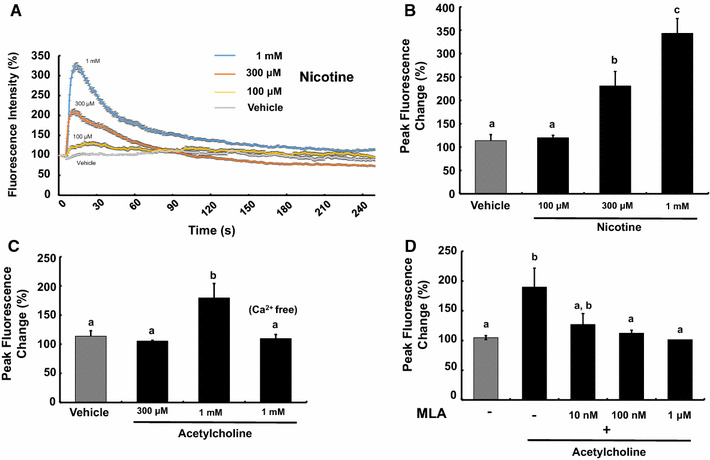
Nicotine- and acetylcholine-induced [Ca2+]i increase in GT1-7 cells. a Representative traces of intracellular calcium measured as Fluo-4 fluorescence with different concentrations (100–1000 μM) of nicotine. GT1-7 cells showed [Ca2+]i increase in response to nicotine. b Effects of nicotine on the peak fluorescence changes of the Ca2+ indicator in GT1-7 cells. c Effects of acetylcholine on the peak fluorescence changes of the Ca2+ indicator in GT1-7 cells. d Inhibitory effects of MLA on the peak fluorescence changes of the Ca2+ indicator induced by acetylcholine in GT1-7 cells. The different letters on the columns indicate statistically significant differences (p < 0.05). The data are represented as the mean ± SEM obtained from four separately prepared samples
It is to be noted that the high-doses of nicotine and acetylcholine were required to induce [Ca2+]i increase in GT1-7 cells. Considering the sensitivity to the agonists and the above quantification of the mRNA levels, the predominant nAChR in GT1-7 cells was deduced to be a α7 homomeric receptor. Application of an α7 homomeric nAChR antagonist, MLA (100 nM and 1 μM), inhibited the acetylcholine-induced [Ca2+]i increase (Fig. 3D).
Reduction of forskolin-induced cAMP formation by mAChR agonists
Functional expression of Gi/o-coupled mAChRs was evaluated using a cAMP EIA assay. Acetylcholine and muscarine attenuated forskolin-induced cAMP formation in GT1-7 cells (Fig. 4). The inhibitory effect of acetylcholine was blocked by treatment with a mAChR antagonist, atropine.
Fig. 4.
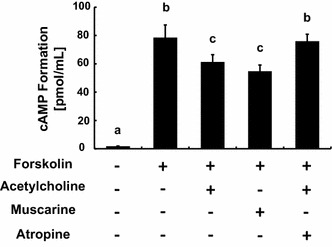
Effects of mAChR agonists on forskolin-induced cAMP formation in GT1-7 cells. GT1-7 cells were incubated in DMEM containing IBMX for 10 min, followed by forskolin and agonist stimulation for 10 min. cAMP concentrations were evaluated using cAMP EIAs. Forskolin-induced cAMP formation was inhibited by 100 nM acetylcholine and 100 nM muscarine. Atropine (100 nM) antagonized the effect of acetylcholine. Dimethyl sulfoxide (0.1 %) was used as a vehicle control. The different letters on the columns indicate statistically significant differences (p < 0.05). The data are represented as the mean ± SEM obtained from four separately prepared samples
Nicotine-induced GnRH release from GT1-7 cells
GnRH assays were performed to evaluate nicotine-induced GnRH release from GT1-7 cells (Fig. 5a). High-dose applications of nicotine (300 μM and 1 mM) induced GnRH release, and co-treatment with MLA inhibited the release, indicating the involvement of α7 nAChRs in GnRH release.
Fig. 5.
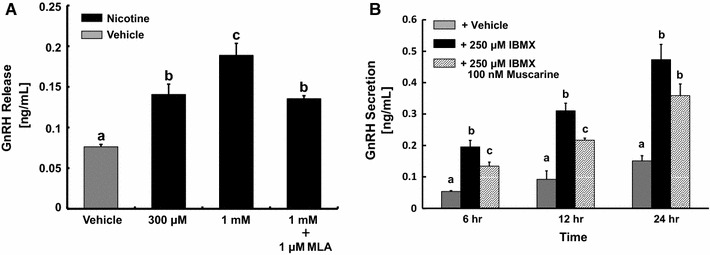
Effects of nAChRs and mAChRs on GnRH secretion from GT1-7 cells. a Effect of nicotine on GnRH release from GT1-7 cells was assessed using GnRH EIAs. GT1-7 cells were incubated in DMEM with 0.1 % BSA for 15 min and then stimulated by nicotine for 30 min. b Effect of muscarine on IBMX-induced GnRH secretion from GT1-7 cells was assessed using GnRH EIAs. GT1-7 cells were treated with 250 μM IBMX and stimulated by 100 nM muscarine for 6, 12, and 24 h. The different letters on the columns indicate statistically significant differences (p < 0.05). The data were represented as the mean ± SEM obtained from four separately prepared samples
Reduction of IBMX-induced GnRH secretion by muscarine
Effect of mAChRs on IBMX-induced GnRH secretion from GT1-7 cells was examined using GnRH EIA kits. IBMX is a phosphodiesterase inhibitor, and long-term incubation with the reagent increases basal cAMP concentration in treated cells. The treatment succeeded to stimulate GnRH secretion, and GT1-7 cells showed significantly lower GnRH secretion at 6 and 12 h after application of muscarine, but not at 24 h (Fig. 5b). This result indicates the involvement of mAChRs in suppressing GnRH secretion.
Discussion
In the present study, we demonstrated that (1) GT1-7 cells express nAChR subunits (α3, α4, α5, α7, β1, β2, β4, and δ), and mAChR subtypes (m2 and m4), (2) nicotine and acetylcholine elevated [Ca2+]i via functional nAChRs, predominantly in the form of α7 homomeric receptors, (3) nAChR agonist induced GnRH release from GT1-7 cells, and (4) mAChR agonist suppressed forskolin-induced cAMP formation and IBMX-induced GnRH secretion.
The concentration of acetylcholine in the preoptic region was reported to synchronize with the estrus cycle and to drastically change from the proestrus morning to afternoon [18]. Estrogens alter mRNA expression and activity of acetylcholine-related enzymes in the hypothalamus [19–21]. Therefore, acetylcholine and acetylcholine receptors are believed to play certain roles in the central regulation of reproduction. Recently, Turi et al. [13] documented the direct innervation of cholinergic axons to GnRH neurons. However, there are only a few studies that have reported the potential involvement of acetylcholine and acetylcholine receptors in GnRH secretion. Sano et al. [22] demonstrated that intravenous administration of nicotine inhibited GnRH pulse generator activity. This inhibitory action of nicotine was indirectly mediated through inhibitory GABAergic neurons [23]. The direct effects of GABA on GnRH neurons have already been established to be excitatory [24–26]. Hence, the inhibitory action of GABA is deduced to be indirect. On the other hand, in vitro analyses using hypothalamic slices [12] and cell cultures [17] demonstrated that acetylcholine induced GnRH release through activation of nAChRs and that muscarine suppressed GnRH secretion.
The α4β2 and α7 nAChRs are expressed in the hypothalamus [5, 27]. The null mouse mutant of the α4 nAChR subtype showed normal reproduction [28], whereas mice deficient in the α7 subtype exhibited some reduction in fertility [29]. A subsequent study by Morley and Rodriguez-Sierra [30] reported that α7-null mutant mice had asynchronous estrus cycles, suggesting that the gene is an obligatory factor for the synchronization of the female ovulatory cycle. Our quantitative analysis demonstrated that the α7 subunit is a prevailing nAChR subunit in GT1-7 cells. Membrane depolarization and subsequent Ca2+ influx are critical factors in GnRH release from GnRH neurons [31–34]. In particular, activation of L-type and R-type voltage-gated Ca2+ channels is strongly involved in GnRH release [35]. We observed nicotine and acetylcholine-induced Ca2+ influx and inhibition of the influx by an α7 homomeric nAChR antagonist, MLA. In addition, nicotine induced GnRH release, which was inhibited by MLA. These results indicate that Ca2+ influx resulted from direct opening of nAChRs, predominantly α7 nAChRs, and activation of voltage-gated Ca2+ channels by membrane depolarization, which subsequently participated in GnRH release.
cAMP production is also known to be related to GnRH secretion. In the present study, we observed the expression of the Gi/o-coupled mAChRs, m4 and to lesser extent m2, and did not detect the expression of Gq/11-coupled mAChR subtypes. In addition, we showed no functional activity of Gq/11-coupled mAChR subtypes by agonist stimulation using Ca2+ imaging in the absence of extracellular Ca2+. Treatment of hypothalamic neurons and GT1-7 cells with muscarine inhibited GnRH release [17]. Koren et al. [36] also reported that selective muscarinic antagonists for Gi/o-coupled mAChRs stimulated release of GnRH from the median eminence, and they deduced that the release was mediated via the m4 mAChR subtype. Furthermore, Frattarelli et al. [37] demonstrated that cAMP formation did not participate in pulsatile GnRH secretion but increased basal GnRH secretion. Therefore, our results are in good accordance with these prior studies, and, to our knowledge, this is the first report that basal GnRH secretion is directly modulated by the m4 mAChR subtype. However, Krsmanovic et al. [17] pharmacologically analyzed the expression of acetylcholine receptors in GT1-7 cells and demonstrated the presence of nAChRs, and Gq/11-coupled M1 and Gi/o-coupled M2 mAChR subtypes. Morales et al. [38] also reported the expression of Gq/11-coupled acetylcholine receptors. These disparities of mAChR expression patterns may be because of differences in cell culture conditions and techniques, or differentiation of cell characteristics over time. Todman et al. [14] examined expression of neurotransmitter receptor mRNAs in mouse native GnRH neurons using DNA microarrays and reported limited expression profiles of nicotinic and muscarinic receptor subtypes. Therefore, more detailed and comprehensive analyses using native GnRH neurons will be required to establish physiological roles of acetylcholine and its receptors within the GnRH neuronal network.
Direct effect of acetylcholine on other type of GnRH neurons was described by Kawai et al. [39]. They found generation of rebound burst potential via mAChRs in the pace-making terminal nerve GnRH neurons of the goldfish (Carassius auratus). On the other hand, Tanaka et al. [34] reported that hyperpolarization current pulse induced only a single rebound action potential but not a burst of action potentials in rat GnRH neurons, implying no involvement of mAChRs in the generation of rebound burst potential in rodent GnRH neurons.
The half maximal effective concentration of acetylcholine for α7 nAChRs (130 μM [40]) is three to four orders of magnitude higher than those for Gi/o-coupled mAChRs (39.8 nM for m2 and 162 nM for m4 [41]). In our studies, activation of nAChRs required a high concentration of acetylcholine, while mAChRs responded to a low dose. The effective dose of acetylcholine via nAChRs on Ca2+ influx and GnRH release seemed extremely high, while that via mAChRs was relatively low which seems to fit in the physiological range. The EC50 values of acetylcholine and nicotine on nAChRs is diverse depending on subtype formation. Among them, α7 nAChR, which is a predominant from in GT1-7 cells, marked one to two order of magnitude higher EC50 of acetylcholine than the representative neuronal nAChR, α4β2. Since physiologically maximum concentration of acetylcholine in the cholinergic synapses seems to be estimated as sub-micromolar to millimolar order [42–45], the agonist-demanding feature may be interpreted that the α7 nAChR subtype exerts the locally restricted transient effect of acetylcholine. On the other hand, relatively low-dose sensitive mAChRs are considered to function on a steady basis. The concentration of acetylcholine in the preoptic region synchronized with the estrus cycle and drastically increased from the proestrus morning to afternoon [18]. Therefore, it is suggested that low concentration of acetylcholine suppresses GnRH secretion via mAChRs and locally rapid increase of acetylcholine concentration functions via nAChRs as a trigger of GnRH release. The synchronization of acetylcholine concentration with the estrous cycle may change the activation status of acetylcholine receptors and the mode of GnRH secretion in GnRH neurons.
GT1-7 cells express several types of functional receptors for neurotransmitters [46–48]. The GT1-7 cells used in this study exhibited the predominant expression of functional α7 nAChRs. The α7 nAChRs are therapeutic targets to treat cognitive impairment in Alzheimer’s disease and schizophrenia [49], which prompted the generation of selective ligands for the α7 nAChRs. Therefore, GT1-7 cells can serve as a good cell model to screen specific compounds that interact with α7 nAChRs.
In conclusion, we determined the mRNA expression of α4, α5, α7, β1, β2, β4, and δ for nAChR subunits, and m2 and m4 for mAChR subtypes in GT1-7 cells using conventional RT-PCR, and then quantified expression levels using real-time RT-PCR. The functional nAChRs were shown to be comprised predominantly of α7 subunits. The functional expression of Gi/o-coupled mAChRs and the absence of Gq/11-coupled mAChRs were confirmed. Furthermore, the GnRH assays revealed an increase of GnRH release through activation of nAChRs and suppression of GnRH secretion through mAChRs. Therefore, we have comprehensively demonstrated the molecular and functional expression profiles of acetylcholine receptors and the direct contribution of acetylcholine receptors to GnRH release in GT1-7 cells. We believe that these findings will shed light on the potential modulatory effects of acetylcholine on the GnRH neuronal system.
Acknowledgments
We are grateful to Dr. Richard Weiner for donating GT1-7 cells. This work was supported, in part, by JSPS KAKENHI Grants-in-Aid for Scientific Research (Grant #s: 25460319 and 26460323).
Abbreviations
- BSA
Bovine serum albumin
- [Ca2+]i
Intracellular calcium concentration
- DMEM
Dulbecco’s modified Eagle’s medium
- EIA
Enzyme immunoassay
- GnRH
Gonadotropin-releasing hormone
- IBMX
Isobutylmethylxanthine
- mAChR
Muscarinic acetylcholine receptor
- MLA
Methyllycaconitine
- nAChR
Nicotinic acetylcholine receptor
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants and/or animals
This study does not contain any studies with human participants performed by any of the authors. All procedures performed in studies involving animals were in accordance with the ethical standards of Nippon Medical School.
Informed consent
Not applicable.
References
- 1.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haga T. Molecular properties of muscarinic acetylcholine receptors. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:226–256. doi: 10.2183/pjab.89.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- 4.Zouridakis M, Zisimopoulou P, Poulas K, Tzartos SJ. Recent advances in understanding the structure of nicotinic acetylcholine receptors. IUBMB Life. 2009;61:407–423. doi: 10.1002/iub.170. [DOI] [PubMed] [Google Scholar]
- 5.Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caulfield MP, Birdsall NJ. International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 7.Kizer JS, Palkovits M, Tappaz M, Kebabian J, Brownstein MJ. Distribution of releasing factors, biogenic amines, and related enzymes in the bovine median eminence. Endocrinology. 1976;98:685–695. doi: 10.1210/endo-98-3-685. [DOI] [PubMed] [Google Scholar]
- 8.Everett JW, Sawyer CH, Merkee JE. A neurogenesis timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology. 1949;44:234–250. doi: 10.1210/endo-44-3-234. [DOI] [PubMed] [Google Scholar]
- 9.Libertun C, McCann SM. Blockage of the release of gonadotropins and prolactin by subcutaneous or intraventricular injection of atropine in male and female rat. Endocrinology. 1973;92:1714–1724. doi: 10.1210/endo-92-6-1714. [DOI] [PubMed] [Google Scholar]
- 10.Libertun C, McCann SM. Blockage of the postorchidectomy increase in gonadotropins by implants of atropine into the hypothalamus. Proc Soc Exp Biol Med. 1976;152:143–146. doi: 10.3181/00379727-152-39347. [DOI] [PubMed] [Google Scholar]
- 11.Simonovic I, Motta M, Martini L. Acetylcholine and the release of the follicle-stimulating hormone-releasing factor. Endocrinology. 1974;95:1373–1379. doi: 10.1210/endo-95-5-1373. [DOI] [PubMed] [Google Scholar]
- 12.Richardson SB, Prasad JA, Hollander CS. Acetylcholine, melatonin, and potassium depolarization stimulate release of luteinizing hormone-releasing hormone from rat hypothalamus in vitro. Proc Natl Acad Sci USA. 1982;79:2686–2689. doi: 10.1073/pnas.79.8.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turi GF, Liposits Z, Hrabovszky E. Cholinergic afferents to gonadotropin-releasing hormone neurons of the rat. Neurochem Int. 2008;52:723–728. doi: 10.1016/j.neuint.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Merchenthaler I, Görcs T, Sétáló G, Petrusz P, Flerkó B. Gonadotropin-releasing hormone (GnRH) neurons and pathway in the rat brain. Cell Tissue Res. 1984;237:15–29. doi: 10.1007/BF00229195. [DOI] [PubMed] [Google Scholar]
- 16.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-E. [DOI] [PubMed] [Google Scholar]
- 17.Krsmanovic LZ, Mores N, Navarro CE, Saeed SA, Arora KK, Catt KJ. Muscarinic regulation of intracellular signaling and neuro-secretion in gonadotropin-releasing hormone neurons. Endocrinology. 1998;139:4037–4043. doi: 10.1210/endo.139.10.6267. [DOI] [PubMed] [Google Scholar]
- 18.Egozi Y, Kloog Y, Sokolovsky M. Acetylcholine rhythm in the preoptic area of the rat hypothalamus is synchronized with estrous cycle. Brain Res. 1986;383:310–313. doi: 10.1016/0006-8993(86)90030-2. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa T, Ajiki K, Matsuura J, Misawa H. Localization of two cholinergic markers, choline acetyltransferase and vesicular acetylcholine transporter in the central nervous system of the rat: in situ hybridization histochemistry and immunohistochemistry. J Chem Neuroanat. 1997;13:23–39. doi: 10.1016/S0891-0618(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 20.Iramain CA, Owasoyo JO, Egbunike GN. Influence of estradiol on acetylcholinesterase activity in several female rat brain areas and adenohypophysis. Neurosci Lett. 1980;16:81–84. doi: 10.1016/0304-3940(80)90105-6. [DOI] [PubMed] [Google Scholar]
- 21.Lapchak PA, Araujo DM, Quirion R, Beaudet A. Chronic estradiol treatment alters central cholinergic function in the female rat: effect on choline acetyltransferase activity, acetylcholine content, and nicotinic autoreceptor function. Brain Res. 1990;525:249–255. doi: 10.1016/0006-8993(90)90871-8. [DOI] [PubMed] [Google Scholar]
- 22.Sano A, Funabashi T, Kawaguchi M, Shinohara K, Kimura F. Intravenous injections of nicotine decrease the pulsatile secretion of LH by inhibiting the gonadotropin-releasing hormone (GnRH) pulse generator activity in female rats. Psychoneuroendocrinology. 1999;24:397–407. doi: 10.1016/S0306-4530(98)00089-4. [DOI] [PubMed] [Google Scholar]
- 23.Kimura F, Shinohara K, Funabashi T, Daikoku S, Suyama K, Mitsushima D, Sano A. Nicotine inhibition of pulsatile GnRH secretion is mediated by GABAA receptor system in the cultured rat embryonic olfactory placode. Psychoneuroendocrinology. 2004;29:749–756. doi: 10.1016/S0306-4530(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 24.Yin C, Ishi H, Tanaka N, Sakuma Y, Kato M. Activation of A-type gamma-amino acid receptors excites gonadotropin-releasing hormone neurons isolated from adult rats. J Neuroendocrinol. 2008;20:566–575. doi: 10.1111/j.1365-2826.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Sakuma Y, Kato M. GABAA receptors mediate excitation in adult rat GnRH neurons. Biol Reprod. 2009;81:327–332. doi: 10.1095/biolreprod.108.074583. [DOI] [PubMed] [Google Scholar]
- 26.Moenter SM, DeFazio RA. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- 27.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Awanson LW. Distribution of alpha2, alpha3, alpha4 and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 28.Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horrne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morley BJ, Rodriguez-Sierra JF. A phenotype for the alpha 7 nicotinic receptor null mutant. Brain Res. 2004;1023:41–47. doi: 10.1016/j.brainres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Charles AC, Hales TG. Mechanism of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. J Neurophysiol. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Martínez de la Escalera G, Choi AL, Weiner RI. Singling pathways involved in GnRH secretion in GT1 cells. Neuroendocrinology. 1995;61:310–317. doi: 10.1159/000126853. [DOI] [PubMed] [Google Scholar]
- 33.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescence protein in rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka N, Ishi H, Yin C, Koyama M, Sakuma Y, Kato M. Voltage-gated Ca2+ channel mRNAs and T-type Ca2+ currents in rat gonadotropin-releasing hormone neurons. J Physiol Sci. 2010;60:195–204. doi: 10.1007/s12576-010-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe M, Sakuma Y, Kato M. High expression of the R-type voltage-gated Ca2+ channel and its involvement in Ca2+-dependent gonadotropin-releasing hormone release in GT1-7 cells. Endocrinology. 2004;145:2375–2383. doi: 10.1210/en.2003-1257. [DOI] [PubMed] [Google Scholar]
- 36.Koren D, Egozi Y, Sokolovsky M. Muscarinic involvement in the regulation of gonadotropin-releasing hormone in the cyclic rat. Mol Cel Endocrinol. 1992;90:87–93. doi: 10.1016/0303-7207(92)90105-F. [DOI] [PubMed] [Google Scholar]
- 37.Frattarelli JL, Krsmanovic LZ, Catt KJ. The relationship between pulsatile GnRH secretion and cAMP production in immortalized GnRH neurons. Am J Physiol Endocrinol Metab. 2011;300:E1022–E1030. doi: 10.1152/ajpendo.00081.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales A, Diaz M, Ropero AB, Nadal A, Alonso R. Estradiol modulates acetylcholine-induced Ca2+ signals in LHRH-releasing GT1-7 cells through a membrane binding site. Eur J Neurosci. 2003;18:2505–2514. doi: 10.1046/j.1460-9568.2003.02997.x. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Abe H, Oka Y. Burst generation mediated by cholinergic input in terminal nerve-gonadotrophin releasing hormone neurons of the goldfish. J Physiol. 2013;591:5509–5523. doi: 10.1113/jphysiol.2013.258343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 41.Lazareno S, Birdsall NJ. Pharmacological characterization of acetylcholine-stimulated [35S]-GTP gamma S binding mediated by human muscarinic m1-m4 receptors: antagonist studies. Br J Pharmacol. 1993;109:1120–1127. doi: 10.1111/j.1476-5381.1993.tb13738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuffler SW, Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975;251:465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz B, Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977;196:59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- 44.Matthews-Bellinger JA, Salpeter MM. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Land BR, Saltpeter EE, Saltpeter MM. Acetylcholine receptor side density affects the rising phase of miniature endplate currents. Proc Natl Acad Sci USA. 1980;77:3736–3740. doi: 10.1073/pnas.77.6.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hales TG, Kim H, Longoni B, Olsen RW, Tobin AJ. Immortalized hypothalamic GT1-7 neurons express functional gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1992;42:197–202. [PubMed] [Google Scholar]
- 47.El-Etr M, Akwa Y, Baulieu EE, Schumacher M. The neuroactive steroid pregnenolone sulfate stimulates the release of gonadotropin-releasing hormone from GT1-7 hypothalamic neurons, through N-methyl-D-aspartate receptors. Endocrinology. 2006;147:2737–2743. doi: 10.1210/en.2005-1191. [DOI] [PubMed] [Google Scholar]
- 48.Martínez de la Escalera G, Gallo F, Choi AL, Weiner RI. Dopaminergic regulation of the GT1 gonadotropin-releasing hormone (GnRH) neuronal cell lines: stimulation of GnRH release via D1-receptors positively coupled to adenylate cyclase. Endocrinology. 1992;131:2965–2971. doi: 10.1210/endo.131.6.1280208. [DOI] [PubMed] [Google Scholar]
- 49.Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signaling: roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]


