Abstract
Obesity is well known to be associated with a wide variety of illnesses, and is an increasing problem not only in developed countries but also in developing countries. It is well known that large bite size contributes to excess energy intake and obesity, whereas an increased number of chews before swallowing the food bolus is associated with suppression of obesity. However, the effect of food diameter on bite size per mouthful and on chewing behavior remains poorly understood. Here, we examined the effects of food diameter on bite size and chewing behavior using a masticatory counter during the mastication of stick-type biscuits having the same length (10 cm) and ingredients, but with four different diameters (3.0, 3.5, 4.0, and 8.0 mm). Bite length and bite weight per mouthful were similar among the 3.0, 3.5, and 4.0 mm groups. However, bite length in the 8.0 mm group was significantly smaller, whereas bite weight was significantly greater than in the 3.0/3.5 mm groups. Further, the number of chews gradually increased, whereas the number of chews per bite weight gradually decreased, with an increase of biscuit diameter. These results indicate that a smaller biscuit diameter is associated with a smaller bite weight per mouthful and a greater number of chews per bite weight. This is the first report to quantity the effect of food diameter on bite weight per mouthful and on chewing behavior; these results should be helpful in the design of effective, safe, and low-cost behavioral modification therapy to combat obesity.
Keywords: Food diameter, Bite length, Bite weight, Number of chews
Introduction
Obesity is a significant public health concern, being linked to a wide array of illnesses and disabilities, including type 2 diabetes, cardiovascular diseases, chronic kidney disease, sleep apnea and its resultant fatigue and poor attention, arthritis, lung disease, and several forms of cancer (e.g., breast, prostate, and tongue) [1–8]. Thus, obese individuals are at increased risk of premature death. The problem is not limited to developed (high-income) countries but is also found in developing (low- and middle-income) countries [9]. Thus, simple, safe, and effective treatments, in place of surgical treatment or drugs, are needed to combat obesity [10].
Obese people often take larger bites of food and do not chew it intensity, leading to decreased oral processing time and thus increased food intake [11–13]. Importantly, increasing the number of chews before swallowing was reported to reduce food intake not only in normal-weight adults but also in obese adults [14, 15]. Therefore, modification of chewing behavior might be a simple and effective therapy for obesity.
Major oral physiological factors that regulate bite size and chewing include the cycle of jaw and tongue movements [16, 17] and the activity of masticatory muscles [18–21] during feeding, because these influence not only mouth opening before the breakage of food but also the intra-oral transport of the food. Muscle activity predominantly depends upon the physiological characteristics of the food, i.e., oral sensations from somatic sensory receptors relating to weight, diameter, hardness, crispness, flavor, and viscosity of foods [17, 22, 23]. However, the effects of food diameter on bite size and chewing behavior have not yet been clearly explored in well-controlled and laboratory-based studies. The present study was designed to address this issue.
Therefore, in this study, we examined the effect of food diameter on bite size per mouthful and on chewing behavior (number of chews and number of chews per bite weight) using an in-house-developed masticatory counter [24] and stick-type biscuits with four different diameters (3.0, 3.5, 4.0, and 8.0 mm), but with the same length (10 cm) and the same ingredients.
Materials and methods
Subjects
Twelve adult subjects (seven males, five females, mean age, 32.4 ± 7.4 years) participated in this study. The subjects had no history of major medical problems and had normal dentition without any stomatognathic problems. The subjects were asked to keep their evening meal and their activity level as normal as possible on the day before the experimental day and to refrain from eating or drinking (except water) after 10 pm. The subjects were also asked to refrain from drinking alcohol on the day before and throughout the experimental day and to eat a normal breakfast and lunch during the experimental day, as usual. During the experimental day, the subjects were instructed not to consume any food or energy-containing beverages for 2 h and not to drink water for 1 h before the study. Each subject was seated on a chair in a magnetically shielded room and the experiment was performed for approximately 1 h.
This study was conducted with the approval of the Ethics Committee of Tsurumi University School of Dental Medicine (approval No. 1020), and written informed consent was obtained from each subject after a full explanation of the experimental protocol.
Test foods and experimental procedure
Stick-type biscuits with four different diameters (3.0, 3.5, 4.0, and 8.0 mm) were used in this study (Fig. 1). The length of all the biscuits was 10 cm and the ingredients were the same. Subjects were asked to take one bite of each sample with a single occlusion and to chew as usual before swallowing. This task was repeated more than three times for each test food, and the foods were served in a random order.
Fig. 1.
Stick-type biscuits with four different diameters (3.0, 3.5, 4.0, and 8.0 mm) were used in this study. The length of all biscuits was 10 cm and the ingredients were the same
Food length (mm) and weight (gram: g) per mouthful for each test food were evaluated by subtracting the length/weight of the remaining portion after the first bite from the original length/weight. The number of chews was counted with an in-house-developed masticatory counter (Fig. 2a) [24].
Fig. 2.
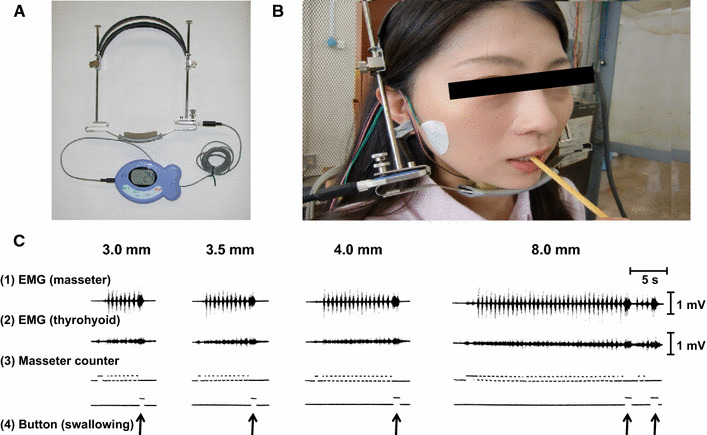
The masticatory counter used in this study. The masticatory counter (a) was applied to each subject during the experiments, as shown in (b). c Examples of the EMG of masseter (1) and thyrohyoid muscles (2), and output of the sensor of the masticatory counter (3). Subjects were asked to push a button at the commencement of swallowing (4). Modified from Ref. [24] with permission
The masticatory counter was applied to each subject during each experiment (Fig. 2b). The output signals from the sensor were recorded simultaneously with electromyographic (EMG) activity in the masseter muscle to confirm correct operation of the counter (Fig. 2c-1). We also recorded the EMG activity of the thyrohyoid muscle to confirm commencement of swallowing (Fig. 2c-2) [25]. Sensor outputs of the masticatory counter were recorded (Fig. 2c-3). In addition, subjects were asked to push a button at the commencement of swallowing (Fig. 2c-4). EMG activities were measured with bipolar surface electrodes, amplified, and recorded.
Statistical analysis
All data were reported as the mean ± standard deviation. Statistical comparisons were performed with the Kruskal–Wallis non-parametric test followed by the Dunn test (Figs. 3, 4). Differences were considered significant when p < 0.05.
Fig. 3.
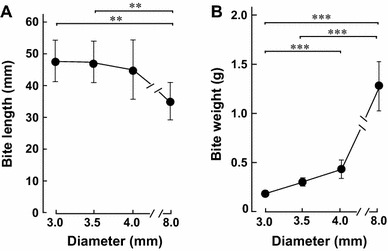
Effects of food diameter on bite length and bite weight. a The length (mm) of the first bite decreased significantly with increasing diameter of the biscuits. b On the other hand, the bite weight (g) increased significantly with increasing diameter of the biscuits. **p < 0.01, ***p < 0.001 (n = 12)
Fig. 4.
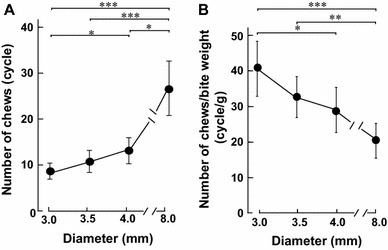
Effects of food diameter on number of chews and number of chews per bite weight. a The number of chews (cycles) increased significantly with increasing diameter of the biscuits. b On the other hand, the number of chews per bite weight (cycles/g) decreased significantly with increasing diameter of the biscuits. **p < 0.01, ***p < 0.001 (n = 12)
Results
Effects of food diameter on bite length and bite weight per mouthful
We measured the first bite length (mm) for the four different-diameter test foods (Fig. 1). Bite length was similar among the 3.0, 3.5, and 4.0 mm groups (45 ± 6.2, 44 ± 6.2, 42 ± 8.9 mm respectively, n = 12 each). However, it was significantly smaller in the 8.0 mm group (34 ± 6.1 mm, n = 12), compared to the 3.0 and 3.5 mm groups (each p < 0.01) (Fig. 3a).
We also examined the weight (g) of the first bite for each test food (Fig. 3b), and found that it increased gradually with increase of food diameter. The bite weight in the 8.0 mm group (1.3 ± 0.2 g, n = 12) was significantly greater than those in the 3.0 mm group (0.2 ± 0.03 g) and the 3.5 mm group (0.3 ± 0.04 g) (each p < 0.001). Bite weight in the 4.0 mm group (0.5 ± 0.01 g, n = 12) was also significantly greater than that in 3.0 mm group (0.2 ± 0.03 g) (p < 0.001).
Effects of food diameter on chewing properties
We next used the masticatory counter to examine the number of chews (cycles) on the bite bolus of each test food before swallowing. The number of chews tended to increase gradually with an increase of the food diameter (Fig. 4a), and the number of chews in the 8.0 mm group (27 ± 6.0 cycles) was significantly greater than those in 3.0 mm group (8 ± 1.8 cycles) and 3.5 mm group (11 ± 2.4 cycles) (each p < 0.001, n = 12). In addition, the number of chews in the 4.0 mm group (13 ± 2.9 cycles) was significantly greater than that in the 3.0 mm group (8 ± 1.8 cycles) (p < 0.05, n = 12) and significantly smaller than that in the 8.0 mm group (27 ± 6.0 cycles) (p < 0.05, n = 12).
We also examined the number of chews per weight (cycles/g) of the bolus obtained at the first bite for each test food. The number of chews per bite weight gradually decreased with an increase of biscuit diameter (Fig. 4b). The number of chews per bite weight in the 3.0 mm group (41 ± 7.7 cycles/g) was significantly greater than those in the 4.0 mm group (29 ± 6.3 cycles/g, p < 0.05) and 8.0 mm group (20 ± 4.9 cycles/g, p < 0.01). The number of chews per bite weight in the 3.5 mm group (32 ± 5.8 cycles/g) was also significantly greater than that in the 8.0 mm group (p < 0.01). Thus, the bite bolus of the smallest-diameter biscuit was the most extensively chewed before swallowing.
Discussion
In the present study, we first examined the effect of food diameter on bite length and bite weight per mouthful using test biscuits with four different diameters. We found that bite weight in the 4.0 mm group was slightly (by approximately 2.5-fold) but significantly greater than that in the 3.0 mm group, while that in the 8.0 mm group was much greater (by approximately 6.5-fold) than that in the 3.0 mm group; in other words, there appeared to be a tendency for bite weight to increase with increasing food diameter. This suggests that food diameter might be a conveniently modifiable factor to decrease bite size and thus control food intake. We also found that the number of chews increased gradually with increase of biscuit diameter, and was much greater in the 8.0 mm group, compared to the other groups. However, in contrast, the number of chews per bite weight decreased gradually with increase of biscuit diameter.
These findings are potentially important, because marketplace food portions have increased in size and now exceed federal standards in the United States (US) [26]. Portion sizes began to grow in the 1970s, rose sharply in the 1980s, and have continued to grow in parallel with increasing body weight in US [26]. In addition, household surveys have indicated that individuals are consuming larger portion sizes at home than they have in the past [27]. Importantly, the increase of portion size parallels the rising prevalence of obesity according to a WHO report in 1998. Nevertheless, the mechanisms underlying this relationship remain poorly understood, although it was recently reported that an increase of food intake in response to increased portion sizes was due to increased bite size in both children and adults [11, 28]. Although it has been suggested that treatments for obesity should focus on food selection and the stimulatory effects of palatability on intake, rather than factors such as bite size [29], our present data clearly show that bite size is a modifiable determinant of energy intake that should be addressed in connection with the prevention and treatment of obesity.
Several recent studies have examined how the way we eat food affects appetite and food intake. An increased number of chews is associated with suppression of appetite [30–33] and a low risk of weight gain [34]. In rats, it was shown that food intake is suppressed by mastication-induced activation of histamine neurons through H1-receptor in the hypothalamic paraventricular nucleus and the ventromedial hypothalamus [35].
These findings, together with our present data, may be significant in relation to obesity, because food diameter is an easily modifiable factor. These data suggest that educating people about the importance of bite size per mouthful and chewing behavior will be helpful for the design of widely available, effective, safe, and low-cost behavioral modification therapy to combat obesity [36, 37].
There are several possible limitations of our study. First, to avoid confounding effects of gastric distention and appetite sensation, fluid consumption was not allowed; this is probably atypical of mealtime behavior, and chewing behavior may have been different from that under unrestricted conditions. Second, the study group consisted of seven males and five females, and it is known that there is a significant gender effect on food intake [38]. Third, the health status of participants was self-reported in this study and they were not specifically screened for mental disorders [39]. Fourth, the present work was limited to stick-type foods with diameters of 3.0, 3.5, 4.0, and 8.0 mm, and it would be desirable to carry out further studies with foods having other specifications.
Despite these limitations, this study is the first to have evaluated the effect of food diameter on bite weight and chewing behavior before swallowing, and the data might be helpful as a guide to simple behavior modification as a means to control obesity.
Acknowledgments
We would like to thank Ezaki Glico Co., Ltd. (Osaka, Japan) for providing the test foods. This study was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (Drs. Shiozawa (15K12330) and Okumura (23591087)), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (Dr. Okumura (S1511018)), a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan (Dr. Hanada), a Grant-in-Aid for Scientific Research on Innovative Areas (22136009: Dr. Okumura), Yokohama Foundation for Advancement of Medical Science (Dr. Okumura), Yokohama Academic Foundation (Dr. Ohnuki), Research Foundation for Community Medicine (Dr. Okumura), and Suzuken Memorial Foundation (14-014: Dr. Okumura), Research Promotion Grant (27010) from the Society for Tsurumi University School of Dental Medicine (Dr. Ito), the Academic Contribution from Pfizer Japan (Dr. Okumura) and Naito foundation (Dr. Okumura).
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interests.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Diez WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Cupples LA, Ramaswami R, Stokes J, 3rd, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-B. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nahan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger NA. Obesity-associated gastrointestinal tract cancer: from beginning to end. Cancer. 2014;120:935–939. doi: 10.1002/cncr.28534. [DOI] [PubMed] [Google Scholar]
- 6.Fukumura H, Sato M, Kezuka K, Sato I, Feng X, Okumura S, Fujita T, Yokoyama U, Eguchi H, Ishikawa Y, Saito T. Effect of ascorbic acid on reactive oxygen species production in chemotherapy and hyperthermia in prostate cancer cells. J Physiol Sci. 2012;62:251–257. doi: 10.1007/s12576-012-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato I, Umemura M, Mitsudo K, Kioi M, Nakashima H, Iwai T, Feng X, Oda K, Miyajima A, Makino A, Iwai M, Fujita T, Yokoyama U, Okumura S, Sato M, Eguchi H, Tohnai I, Ishikawa Y. Hyperthermia generated with ferucarbotran (Resovist®) in an alternating magnetic field enhances cisplatin-induced apoptosis of cultured human oral cancer cells. J Physiol Sci. 2014;64:177–183. doi: 10.1007/s12576-014-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamide T, Okumura S, Ghosh S, Shinoda Y, Mototani Y, Ohnuki Y, Jin H, Cai W, Suita K, Sato I, Umemura M, Fujita T, Yokoyama U, Sato M, Furutani K, Kitano H, Ishikawa Y. Oscillation of cAMP and Ca2+ in cardiac myocytes: a systems biology approach. J Physiol Sci. 2015;65:195–200. doi: 10.1007/s12576-014-0354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteghamati A, Mazaheri T, Vahidi Rad M, Noshad S. Complementary and alternative medicine for the treatment of obesity: a critical review. Int J Endocrinol Metab. 2015;13:e19678. doi: 10.5812/ijem.19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhurosy T, Jeewon R. Overweight and obesity epidemic in developing countries: a problem with diet, physical activity, or socioeconomic status? Sci World J. 2014;2014:964236. doi: 10.1155/2014/964236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger KS, Fisher JO, Johnson SL. Mechanisms behind the portion size effect: visibility and bite size. Obesity (Silver Spring) 2011;19:546–551. doi: 10.1038/oby.2010.233. [DOI] [PubMed] [Google Scholar]
- 12.Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and overweight men and women. Am J Clin Nutr. 2002;76:1207–1213. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- 13.Fontijn-Tekamp FA, van der Bilt A, Abbink JH, Bosman F. Swallowing threshold and masticatory performance in dentate adults. Physiol Behav. 2004;83:431–436. doi: 10.1016/j.physbeh.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Hollis JH. Increasing the number of chews before swallowing reduces meal size in normal-weight, overweight, and obese adults. J Acad Nutr Diet. 2014;114:926–931. doi: 10.1016/j.jand.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Hollis JH. Chewing thoroughly reduces eating rate and postprandial food palatability but does not influence meal size in older adults. Physiol Behav. 2014;123:62–66. doi: 10.1016/j.physbeh.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Foster KD, Grigor JM, Cheong JN, Yoo MJ, Bronlund JE, Morgenstern MP. The role of oral processing in dynamic sensory perception. J Food Sci. 2011;76:R49–R61. doi: 10.1111/j.1750-3841.2010.02029.x. [DOI] [PubMed] [Google Scholar]
- 17.Thexton AJ. Mastication and swallowing: an overview. Br Dent J. 1992;173:197–206. doi: 10.1038/sj.bdj.4808002. [DOI] [PubMed] [Google Scholar]
- 18.Ohnuki Y, Umeki D, Cai W, Kawai N, Mototani Y, Shiozawa K, Jin HL, Fujita T, Tanaka E, Saeki Y, Okumura S. Role of masseter muscle β2-adrenergic signaling in regulation of muscle activity, myosin heavy chain transition, and hypertrophy. J Pharmacol Sci. 2013;123:36–46. doi: 10.1254/jphs.12271FP. [DOI] [PubMed] [Google Scholar]
- 19.Umeki D, Ohnuki Y, Mototani Y, Shiozawa K, Fujita T, Nakamura Y, Saeki Y, Okumura S. Effects of chronic Akt/mTOR inhibition by rapamycin on mechanical overload-induced hypertrophy and myosin heavy chain transition in masseter muscle. J Pharmacol Sci. 2013;122:278–288. doi: 10.1254/jphs.12195FP. [DOI] [PubMed] [Google Scholar]
- 20.Ohnuki Y, Umeki D, Mototani Y, Jin H, Cai W, Shiozawa K, Suita K, Saeki Y, Fujita T, Ishikawa Y, Okumura S. Role of cyclic AMP sensor Epac1 in masseter muscle hypertrophy and myosin heavy chain transition induced by β2-adrenoceptor stimulation. J Physiol. 2014;592:5461–5475. doi: 10.1113/jphysiol.2014.282996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umeki D, Ohnuki Y, Mototani Y, Shiozawa K, Suita K, Fujita T, Nakamura Y, Saeki Y, Okumura S. Protective effects of clenbuterol against dexamethasone-induced masseter muscle atrophy and myosin heavy chain transition. PLoS One. 2015;10:e0128263. doi: 10.1371/journal.pone.0128263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchings SC, Bronlund JE, Lentle RG, Foster KD, Jones JR, Morgenstern MP. Variation of bite size with different types of food bars and implications for serving methods in mastication studies. Food Qual Pref. 2009;20:456–460. doi: 10.1016/j.foodqual.2009.04.007. [DOI] [Google Scholar]
- 23.de Wijk RA, Zijlstra N, Mars M, de Graaf C, Prinz JF. The effects of food viscosity on bite size, bite effort and food intake. Physiol Behav. 2008;95:527–532. doi: 10.1016/j.physbeh.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Shiozawa K, Hamada N. Accuracy of the newly developed “masticatory counter”. J Masticat Health Soc. 2010;20:27–34. [Google Scholar]
- 25.Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol. 2006;115:334–339. doi: 10.1177/000348940611500503. [DOI] [PubMed] [Google Scholar]
- 26.Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92:246–249. doi: 10.2105/AJPH.92.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smiciklas-Wright H, Mitchell DC, Mickle SJ, Goldman JD, Cook A. Foods commonly eaten in the United States, 1989–1991 and 1994–1996: are portion sizes changing? J Am Diet Assoc. 2003;103:41–47. doi: 10.1053/jada.2003.50000. [DOI] [PubMed] [Google Scholar]
- 28.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children’s intake at a meal. Am J Clin Nutr. 2007;86:174–179. doi: 10.1093/ajcn/86.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegel TA. Rate of intake, bites, and chews-the interpretation of lean-obese differences. Neurosci Biobehav Rev. 2000;24:229–237. doi: 10.1016/S0149-7634(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 30.Higgs S, Jones A. Prolonged chewing at lunch decreases later snack intake. Appetite. 2013;62:91–95. doi: 10.1016/j.appet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhang N, Hu L, Li Z, Li R, Li C, Wang S. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men. Am J Clin Nutr. 2011;94:709–716. doi: 10.3945/ajcn.111.015164. [DOI] [PubMed] [Google Scholar]
- 32.Cassady BA, Hollis JH, Fulford AD, Considine RV, Mattes RD. Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. Am J Clin Nutr. 2009;89:794–800. doi: 10.3945/ajcn.2008.26669. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Hsu WH, Hollis JH. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br J Nutr. 2013;110:384–390. doi: 10.1017/S0007114512005053. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda H, Saito T, Mizuta M, Moromugi S, Ishimatsu T, Nishikado S, Takagi H, Konomi Y. Chewing number is related to incremental increases in body weight from 20 years of age in Japanese middle-aged adults. Gerodontology. 2013;30:214–219. doi: 10.1111/j.1741-2358.2012.00666.x. [DOI] [PubMed] [Google Scholar]
- 35.Sakata T, Yoshimatsu H, Masaki T, Tsuda K. Anti-obesity actions of mastication driven by histamine neurons in rats. Exp Biol Med (Maywood) 2003;228:1106–1110. doi: 10.1177/153537020322801002. [DOI] [PubMed] [Google Scholar]
- 36.Bellisle F, Le Magnen J. The structure of meals in humans: eating and drinking patterns in lean and obese subjects. Physiol Behav. 1981;27:649–658. doi: 10.1016/0031-9384(81)90237-7. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka R, Tamakoshi K, Yatsuya H, Murata C, Sekiya A, Wada K, Zhang HM, Matsuoka K, Sugiura K, Takefumi S, OuYang P, Nagasawa N, Kondo T, Sasaki S, Toyoshima H. Eating fast leads to obesity: findings based on self-administered questionnaires among middle-aged Japanese men and women. J Epidemiol. 2006;16:117–124. doi: 10.2188/jea.16.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin CK, Anton SD, Walden H, Arnett C, Greenway FL, Williamson DA. Slower eating rate reduces the food intake of men, but not women: implications for behavioral weight control. Behav Res Ther. 2007;45:2349–2359. doi: 10.1016/j.brat.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Latner JD, Mond JM, Vallance JK, Gleaves DH, Buckett G. Quality of life impairment and the attitudinal and behavioral features of eating disorders. J Nerv Ment Dis. 2013;201:592–597. doi: 10.1097/NMD.0b013e3182982bbe. [DOI] [PubMed] [Google Scholar]


