Abstract
Kisspeptins, encoded by Kiss1 gene, play pivotal roles in the regulation of reproduction. Recently, several studies reported a sex difference in Kiss1 expression in the arcuate nucleus (ARC) during the neonatal period. In this study, we investigated the effect of gonadal steroid manipulation on the sex difference in Kiss1 expression in ARC of rats. At neonatal and prepubertal stages, females had a greater number of Kiss1 neurons than the males. Gonadectomy at those stages resulted in significant increases in the Kiss1 neuron number and the sex differences disappeared. We also confirmed the expression of estrogen receptor α in kisspeptin neurons in neonates. Altogether, our results indicate that ARC Kiss1 expression is negatively regulated by gonadal steroids from early postnatal stages, and that the sex difference in ARC Kiss1 expression is attributed to the difference in circulating gonadal steroid levels. We also found that neonatal estrogenization inhibits Kiss1 expression and impairs negative feedback system.
Keywords: Kisspeptin, Arcuate nucleus, Gonadal steroid, Negative feedback, Development
Introduction
Kisspeptin, a family of neuropeptides encoded by the Kiss1 gene, has been shown to play important roles in the development and regulation of reproductive functions. The lack of kisspeptin signaling caused by an inactivating mutation of the kisspeptin receptor gene (Kiss1r) is associated with hypogonadotropic hypogonadism and impairment of pubertal maturation in humans [1, 2] and mice [1, 3]. Central or peripheral administration of kisspeptin induces a robust release of LH in various experimental animals [4–9] and in humans [10]. Such an LH release induced by kisspeptin can be blocked by GnRH receptor antagonist [4, 5, 9]. Kisspeptin immunoreactive fibers have been found to be closely associated with somata and axons of GnRH neurons [11–13], indicating that kisspeptin regulates the hypothalamic–pituitary–gonadal (HPG) axis by directly acting on GnRH neurons.
In rodent hypothalamus, neuronal populations synthesizing kisspeptin are known to reside in two discrete regions, the anteroventral periventricular nucleus and the periventricular nucleus continuum (AVPV/PeN) and arcuate nucleus (ARC). The majority of kisspeptin neurons in AVPV/PeN and ARC of adult rodents express estrogen receptor (ER) α [14–16] and the expressions of kisspeptin in both hypothalamic regions are regulated by circulating gonadal steroids, but the means of regulation differ between the regions. In AVPV/PeN, estradiol and testosterone stimulate the expression of Kiss1 mRNA and kisspeptin protein, whereas in ARC, the same gonadal steroids inhibit their expression [15–18]. This indicates that kisspeptin neurons in AVPV/PeN and ARC are involved in positive and negative feedback regulation of gonadal steroids on GnRH release, respectively. In addition to such activational effects on the Kiss1 expression during adulthood, gonadal steroids also have organizational effects on kisspeptin neurons during the critical period of brain sexual differentiation. Kisspeptin neurons in AVPV/PeN of rodents are sexually dimorphic: females possess more kisspeptin neurons than males from prepubertal stages to adulthood [11, 17–20]. Although gonadectomy or steroid treatment in adulthood has no effect on this sex difference, neonatal exposure to estrogen or testosterone results in masculinization of Kiss1 expression in AVPV/PeN of female rats [18, 19], and conversely, neonatal gonadectomy results in an elevation in Kiss1 neurons in AVPV/PeN of male rats later in life [19]. These data indicate that the sex difference in AVPV/PeN kisspeptin neuron is attributed to the neonatal gonadal steroid milieu but not to the circulating gonadal steroid levels at the time of the experiments.
In contrast to AVPV/PeN, the expression of kisspeptin in rodent ARC has been reported to be comparable between both sexes at adulthood, regardless of the circulating gonadal steroid levels [18, 19]. However, in our previous study, we observed a clear sex difference in the number of kisspeptin neurons in ARC during the neonatal period [20], with female rats having a greater number of Kiss1 mRNA expressing neurons compared to males. Other researchers have also reported similar sex differences in postnatal Kiss1 mRNA expression by using autoradiographic in situ hybridization [21]. Moreover, Kauffman et al. [22] recently reported a sex difference in the response of ARC Kiss1 neuron to gonadectomy only during the prepubertal period in mice. All together, these observations suggest the possibility that Kiss1 expression and its regulation by gonadal steroids in ARC are also sexually dimorphic at early stages of postnatal development, but the details remain unclear.
In the present study, to clarify the effects of gonadal steroids on the sex difference in Kiss1 expression in ARC during postnatal development, we analyzed the number of kisspeptin neurons in ARC of neonates, prepubertal, and adult rats which underwent gonadal steroid manipulation at neonatal and/or prepubertal stages.
Materials and methods
Animals and tissue preparations
Male and female Wistar rats were purchased from Saitama Experimental Animal Supply (Saitama, Japan) and bred in the vivarium of Nippon Medical School. All rats were kept under controlled condition of light (14 h light/day; lights on from 0600 hours) and temperature (24 ± 2 °C) with free access to standard rodent chow and water. The day of parturition was designated as postnatal day (PND) 0. All rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused transcardially with 0.9 % NaCl followed by 4 % paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4 (PB). Brains were immediately removed and immersed in the same fixative at 4 °C overnight, and then transferred to 20 % sucrose for cryoprotection. Serial coronal sections of the hypothalamus (from the organum vasculosum of the lamina terminalis to the mammillary body) were cut at a thickness of 30 μm on a cryostat (Leica CM3050, Heidelberg, Germany), thaw-mounted onto RNase-free APS-coated glass slides (Matsunami Glass, Osaka, Japan), air-dried and kept at −80 °C until used for in situ hybridization. All experimental procedures were conducted in accordance with the guidance on animal bio-ethics of Nippon Medical School, based on the guidelines issued by the US National Institute of Health for the humane treatment of experimental animals.
Experimental design; neonatal treatment and gonadectomy
Experiment 1: effects of neonatal gonadal steroid manipulation on the number of Kiss1 mRNA expressing neurons at PND7
Male rats were bilaterally GDX under hypothermic anesthesia at PND0 or left intact (n = 5 each group). Newborn female rats received a single subcutaneous injection of either estradiol benzoate (EB, 25 μg/50 μl sesame oil; Sigma-Aldrich, St. Louis, MO, USA) or 50 μl sesame oil (n = 5 each group). At PND7, all rats were euthanized and brains were collected and processed for in situ hybridization and double labeling fluorescent in situ hybridization and immunohistochemistry.
Experiment 2: effects of neonatal estrogenization and prepubertal gonadectomy on the number of Kiss1 mRNA expressing neurons at PND18
Newborn female rats received a single subcutaneous injection of either EB or vehicle, as in Experiment 1. At PND14, male and female rats were GDX under isoflurane anesthesia or left intact (n = 6 each group). All rats were euthanized at PND18 and brains were collected.
Experiment 3: effects of neonatal estrogenization on the number of Kiss1 mRNA expressing neurons in adulthood
Newborn female rats received a single injection of either EB or vehicle (n = 5 each group), as described above. The animals were weaned at PND21. Neonatal estrogenization has been known to impair normal estrus cyclicity [23, 24]. To remove the influence of the difference in endogenous gonadal steroid levels, all animals were GDX under isoflurane anesthesia at the age of 8 weeks, and brains were collected at 9 weeks.
In situ hybridization
Kiss1 mRNA expressing neurons were visualized by in situ hybridization as previously described [20]. Briefly, digoxigenin (DIG)-labeled antisense and sense RNA probes were synthesized from template cDNA for full-length rat Kiss1 (GeneBank accession #AY196983) [25] by using DIG RNA labeling kit (Roche Diagnostics, Mannheim, Germany). Slides were washed in DEPC-treated 0.1 M phosphate-buffered saline (PBS) and incubated with Proteinase K (Invitrogen, Carlsbad, CA, USA) at 37 °C. After fixing again with 4 %PFA in PB and several washes in PBS, slides were incubated in 0.25 % acetic anhydride in 0.1 M triethanolamine for 10 min at room temperature (RT). Slides were incubated with 1× prehybridization solution (Sigma-Aldrich, Tokyo, Japan) containing 50 % formamide at RT, and then hybridized with DIG-labeled antisense or sense RNA probes diluted in 1× hybridization solution (Sigma-Aldrich) containing 50 % formamide and 10 % dextran sulfate for 16 h at 60 °C. After hybridization, slides were treated with RNase A (20 μg/ml; Roche Diagnostics) for 45 min at 37 °C and washed under conditions of increasing stringency. Visualization of the DIG labeling was achieved using anti-DIG fragments conjugated to alkaline phosphatase (AP) (1:500; Roche Diagnostics) with 4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate solution (Roche Diagnostics). DIG-labeled sense RNA probe was used as the negative control.
Every second section through AVPV/PeN and every fourth section through ARC from each animal were photographed with an AX80 microscope equipped with DP-70 (Olympus, Tokyo, Japan) and the images were exported as tiff format. Kiss1 mRNA expressing cell bodies in AVPV/PeN and ARC were counted on a computer display and the sum of the cell number for each area was calculated. The analyzer was blind to the experimental groups.
Double labeling fluorescent in situ hybridization and immunohistochemistry
A series of slides of brain sections from oil-treated PND7 females (n = 4) and neonatally gonadectomized PND7 males (n = 3) were processed as described above and hybridized with DIG-labeled antisense RNA probe for Kiss1 without Proteinase K treatment. After hybridization, slides were incubated with blocking buffer [4 % normal donkey serum and 1%BSA in 0.1 M Tris-buffered saline (TBS)] for 1 h at RT. Slides were incubated with a cocktail of anti-DIG fragments conjugated to AP (1:500; Roche Diagnostics) and rabbit anti-ERα antibody (1:200, sc-542; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted with blocking buffer overnight at 4 °C. After several washes in TBS, slides were incubated with Alexa Fluor 488 conjugate donkey anti-rabbit IgG antibody (1:200; Molecular Probes, USA) diluted with blocking buffer for 2 h at RT. Finally DIG-labeling was detected using 2-hydroxy-3-naphthoic acid-2′-phenylanilide phosphate (HNPP) and fast red TR (Roche Diagnostics). Confocal images were obtained using a confocal laser scanning microscope (LSM710; Carl Zeiss, Oberkochen, Germany). Scanning at each wavelength was performed individually. Pinhole diameter was optimized to 1.0 Airy disk. Kiss1 mRNA expressing cells exhibiting ERα immunoreactivity were counted on a computer display and the amount of double labeled cells was calculated as a percentage of the number of Kiss1 mRNA expressing neurons.
Statistical analysis
The mean number of Kiss1 mRNA expressing cells and standard of error of mean in AVPV/PeN and ARC were calculated for the experimental group. Data were analyzed for statistically significant differences using t test, one-way ANOVA, and multiple comparisons with either Bonferroni or Games–Howell. Differences were considered statistically significant if the p value was <0.05.
Results
Experiment 1: effects of neonatal gonadal steroid manipulation on the number of Kiss1 mRNA expressing neurons at PND7
In the hypothalamus of PND7 rats, Kiss1 mRNA expressing neurons were found only in ARC and not in AVPV/PeN. In accord with our initial observation [20], the female rats that received a single oil injection at PND0 possessed a significantly greater number of ARC Kiss1 neurons than intact males at PND7 (Fig. 1b). The number of ARC Kiss1 neurons of neonatally oil-treated females was approximately fivefold greater than that of intact males (Fig. 1c). In males, gonadectomy at PND0 resulted in a significant increase in the number of Kiss1 mRNA expressing neurons at PND7, which was comparable to that of oil-treated females. In females, a single bolus of EB at PND0 significantly decreased the number of Kiss1 neurons at PND7. The number of ARC Kiss1 neurons in EB-treated females was not significantly different from that in intact males (Fig. 1c). To confirm the expression of ERα in Kiss1 neurons in ARC of neonates, we performed dual fluorescent in situ hybridization for Kiss1 mRNA and immunohistochemistry for ERα. We observed clear ERα immunoreactivities in nuclei of neuronal cells in ARC of PND7 rats and 93 ± 0.9 and 94 ± 1.2 % of Kiss1 mRNA expressing neurons showed ERα immunoreactivity in ARC of neonatally gonadectomized males and oil-treated females, respectively (Fig. 1d).
Fig. 1.

Effects of neonatal gonadal steroid manipulation on Kiss1 mRNA expression in arcuate nucleus (ARC) of neonatal rats. a Schematic representation of the experimental schedule of the experiment; EB estradiol benzoate, GDX gonadectomized, PND postnatal day. b Representative photographs of Kiss1 mRNA expressing neurons in ARC of postnatal day (PND) 7 males that were gonadectomized (GDX) or left intact on PND0 and females that received oil or estradiol benzoate (EB) injection on PND0. 3V third ventricle. Scale bar 100 μm. c Mean number of Kiss1 mRNA expressing neurons in ARC of each experimental group. Error bars SEM. Bars labeled with different letters are significantly different from each other (p < 0.05). d Representative single optical sections showing Kiss1 mRNA expressing neurons (red, arrows) which coexpressed ERα (green) in ARC of male rats that were GDX on PND0 and female that received oil injection at PND0. Scale bar 25 μm
Experiment 2: effects of neonatal estrogenization and prepubertal gonadectomy on the number of Kiss1 mRNA expressing neurons at PND18
In ARC of PND18 rats, as in PND7, neonatally oil-treated females possessed a significantly greater number of Kiss1 neurons than males and EB-treated females. The number of Kiss1 neurons in EB-treated females was significantly lower than in males and oil-treated females. Gonadectomy at PND14 significantly increased the number of Kiss1 neurons in ARC of males and oil-treated females, but no significant change was found in EB-treated females. In GDX animals, although oil-treated females possessed larger number of Kiss1 neurons than males, there was no significant difference between males and oil-treated females. EB-treated GDX females had a significantly lower number of Kiss1 neurons than GDX males and oil-treated GDX females (Fig. 2b, c).
Fig. 2.

Effects of neonatal estrogenization and prepubertal gonadectomy on Kiss1 mRNA expression in ARC of prepubertal rats. a Schematic representation of the experimental schedule of the experiment; EB estradiol benzoate, GDX gonadectomized, PND postnatal day. b Representative photographs of Kiss1 mRNA expressing neurons in ARC of PND18 males that were GDX or left intact on PND14 and females that received oil or EB injection on PND0 and were GDX or left intact on PND14. 3V Third ventricle. Scale bar 150 μm. c Mean number of Kiss1 mRNA expressing neurons in ARC of each experimental group. Error bars SEM. Bars labeled with different letters are significantly different from each other (p < 0.05). Asterisks indicate significant differences from corresponding intact group (p < 0.05)
In AVPV/PeN of intact PND18 rats, oil-treated females possessed a significantly greater number of Kiss1 neurons, compared to males. The number of Kiss1 neurons in EB-treated females was significantly lower than those in males and in oil-treated females. Gonadectomy at PND14 resulted in significant decreases in the numbers of Kiss1 neurons in AVPV/PeN of males and oil-treated females, but had no significant effects on EB-treated females. In GDX animals, oil-treated females possessed a significantly greater number of Kiss1 neurons than males and EB-treated females (Fig. 3).
Fig. 3.
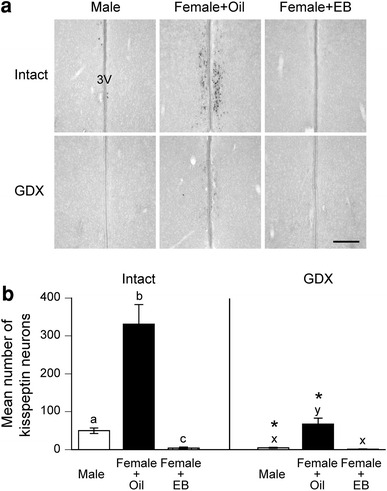
Effects of neonatal estrogenization and prepubertal gonadectomy on Kiss1 mRNA expression in anteroventral periventricular nucleus and periventricular nucleus continuum (AVPV/PeN) of prepubertal rats. a Representative photographs of Kiss1 mRNA expressing neurons in AVPV/PeN of PND18 males that were GDX or left intact on PND14 and females that received oil or EB injection on PND0 and were GDX or left intact on PND14. 3V Third ventricle. Scale bar 200 μm. b Mean number of Kiss1 mRNA expressing neurons in AVPV/PeN of each experimental group. Error bars SEM. Bars labeled with different letters are significantly different from each other (p < 0.05). Asterisks indicate significant differences between intact and GDX groups (p < 0.05)
Experiment 3: effects of neonatal estrogenization on the number of Kiss1 mRNA expressing neurons in adulthood
To determine whether neonatal estrogenization affects the expression of Kiss1 mRNA in adulthood, we analyzed the number of Kiss1 neurons of 9W females which were GDX at 8W to remove endogenous gonadal steroids. The neonatally EB-treated females possessed a significantly lower number of Kiss1 neurons in ARC compared to oil-treated females (Fig. 4b, c). Similarly, the number of Kiss1 mRNA expressing neurons in AVPV/PeN of EB-treated females was significantly lower than that of oil-treated females (Fig. 4d, e). Oil-treated females possessed 4- and 15-fold more Kiss1 neurons than EB-treated females in ARC and AVPV/PeN, respectively.
Fig. 4.
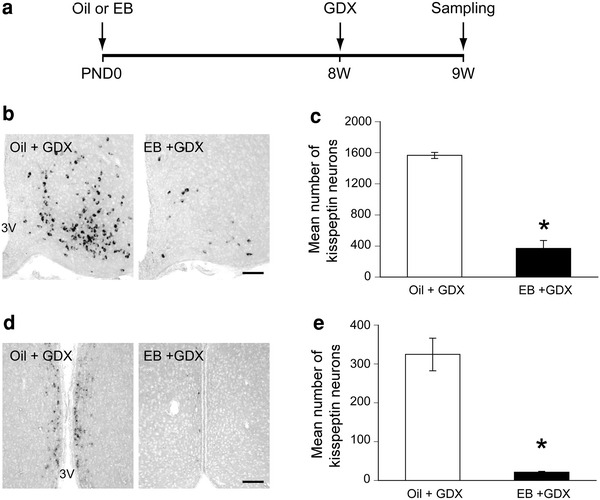
Effects of neonatal estrogenization on Kiss1 mRNA expression in adult female rats. a Schematic representation of the experimental schedule of the experiment; EB estradiol benzoate, GDX gonadectomized, PND postnatal day, 8W 8 weeks, 9W 9 weeks. b Representative photographs of Kiss1 mRNA expressing neurons in ARC of 9W female rats that received oil or EB injection on PND0 and were GDX at 8W. 3V third ventricle. Scale bar 100 μm. c Mean number of Kiss1 mRNA expressing neurons in ARC. d Representative photographs of Kiss1 mRNA expressing neurons in AVPV/PeN. 3V third ventricle. Scale bar 100 μm. e Mean number of Kiss1 mRNA expressing neurons in AVPV/PeN. Asterisks indicate significant differences between oil and EB groups (p < 0.05)
Discussion
Kisspeptin neurons in the hypothalamus have been shown to be an important regulator of HPG axis in mammals. Despite detailed descriptions of the sex difference in kisspeptin expressions in AVPV/PeN and the role of gonadal steroids on its development, there are still few studies focused on the sex difference in ARC. Here, we examined the effect of the neonatal gonadal steroid milieu and gonadectomy on the sex difference in expressions of Kiss1 mRNA in ARC during postnatal development.
We observed a clear sex difference in the number of Kiss1 neurons in ARC at neonatal stage; control females possessed more Kiss1 neurons than intact males at PND7. This is consistent with our previous reports and that of others [20, 21, 26]. During the neonatal period, the serum gonadotropin levels are known to be higher in female rats than in male rats [27, 28]. The sex difference in Kiss1 expression in ARC of neonates might be responsible for this difference of circulating gonadotropin levels. It is well established that kisspeptin neurons in adult rodents express ERα and the Kiss1 expression in ARC of adult rodents are inhibited by circulating gonadal steroids [15, 16, 18, 22]. In this study, we found that gonadectomy of newborn males resulted in a significant increase in Kiss1 neurons in ARC at PND7; conversely, neonatal EB injection caused a significant decrease in female rats. In addition, we also confirmed the majority of kisspeptin neurons in ARC of both sexes coexpressed ERα during neonatal period. To our knowledge, this is the first study to show that neonate kisspeptin neurons in ARC coexpress ERα and are negatively regulated by gonadal steroids as in adulthood. We also found a sex difference in the number of Kiss1 neurons in ARC of intact prepubertal rats. However, in GDX prepubertal animals, there is no significant difference between males and oil-treated females. All these results indicate that the sex difference in the number of Kiss1 neurons in ARC of intact neonates and prepubertal rats can be attributed to an activational effect of gonadal steroids: the difference in the levels of circulating gonadal steroids between the sexes at the time of development, rather than the organizational effect of neonatal gonadal steroid milieu. This is in good accordance with the fact that the serum androgen level is higher in males than in females throughout postnatal development, whereas the estradiol level is comparable between sexes [28].
In prepubertal male mouse, Kiss1 expression in ARC does not increase after gonadectomy, suggesting the presence of a gonadal steroid-independent inhibitory factor [22]. However, we demonstrated that Kiss1 expression in ARC of prepubertal male rats was significantly increased after gonadectomy as in females. This is consistent with the changes in serum LH [29, 30] and Kiss1 expression in the whole hypothalamus [31] after gonadectomy, indicating that, unlike in mice, gonadal steroid is a dominant inhibitory factor on Kiss1 expression in ARC during prepubertal period in rats.
Neonatal estrogenization of females resulted in a significant decrease in Kiss1 expression later in life. In this study, we found that prepubertal female rats which received a single bolus of EB at the day of birth had a significantly lower number of Kiss1 neurons in both of ARC and AVPV/PeN. This is in good agreement with a previous report [31] which showed that neonatal estrogenization decreased Kiss1 expression in the whole hypothalamus of both sexes at the prepubertal period. Moreover, we found that Kiss1 neurons in ARC of EB-treated prepubertal females did not respond to the changes in gonadal steroid levels caused by gonadectomy, suggesting that negative feedback regulation on Kiss1 expression is impaired in those rats. The effect of neonatal estrogenization was also present in adult animals: neonatally EB-treated females possessed a lower number of Kiss1 neurons in both of ARC and AVPV/PeN than oil-treated females at 9 weeks. The decrease in Kiss1 expression in neonatally estrogenized adult female rats are consistent with the data from neonatally estrogenized male rats [7], but inconsistent with a report in which female rats were estrogenized to mimic the testosterone surge in males and showed a similar number of ARC Kiss1 neurons as intact males in adulthood [19]. The doses of EB were the same but the time points of administration were different between this study (PND0) and the previous report (PND5), indicating that a relatively higher dose was administered in this study when it is standardized by body weight. However, it is intriguing that the effects of estrogenization in ARC is different between the two studies but not in AVPV/PeN, suggesting the possibility that Kiss1 neurons in AVPV/PeN might be more vulnerable to gonadal steroids than those in ARC, and that the critical time window for gonadal steroids effect on ARC is set earlier than for AVPV/PeN.
The persistent inhibition of Kiss1 in ARC by neonatal estrogenization also suggests the possibility that gonadal steroids have organizational effects on Kiss1 neurons not only in AVPV/PeN but also in ARC of males, where testosterone surge occurs during the perinatal period. Some of the sexual dimorphisms in the brain have been shown to be established by apoptosis mediated by gonadal steroids during perinatal period [32]. Recently, Semaan et al. [33] reported that BAX, a pro-apoptotic protein, knockout mice had more Kiss1 neurons in ARC than wild-type animals. Furthermore, within the BAX knockout mice, males possessed a greater number of ARC Kiss1 neurons than females, suggesting that BAX-dependent apoptosis regulates the final number of Kiss1 neurons in ARC and that a larger number of the programmed cell death occurs in ARC of male mice than females. It is possible that the decrease in Kiss1 neurons in ARC of neonatally estrogenized female rats is the result of apoptosis caused by higher levels of estradiol in this study. More detailed studies are necessary to determine what mechanism underlies the decrease in Kiss1 expression in ARC after estrogenization.
The sex difference in Kiss1 expression in AVPV/PeN in the intact prepubertal rats and a significant decrease in the number of AVPV/PeN Kiss1 neurons in neonatally estrogenized females are in good agreement with previous reports [19–21]. In prepubertal males and control females, Kiss1 expression was significantly decreased after gonadectomy as in adult animals. This is consistent with the similar study performed in mouse [22] and indicates that Kiss1 neurons in AVPV/PeN of rats are already capable of responding to the change in the gonadal steroid levels in the prepubertal period. As in ARC, there was no change in Kiss1 expression in AVPV/PeN of estrogenized females after gonadectomy, but this seems to be a floor effect because the number of Kiss1 neurons in intact EB-treated females was too small.
In summary, we demonstrated the sex-specific expression patterns of Kiss1 in ARC of neonates and prepubertal rats and that they are regulated by circulating gonadal steroids, suggesting that the basis of negative feedback regulation of gonadal steroids on Kiss1 expression in ARC is established early in development. We also showed that neonatal estrogenization inhibits Kiss1 expression in both of ARC and AVPV/PeN of females, and it affects the negative feedback regulation on Kiss1 gene expression in ARC.
Acknowledgments
We are grateful to Takeda Pharmaceutical Company Ltd. for kindly providing rat Kiss1 cDNA. This work was supported by the Grants-in-Aid from Ministry of Education, Science, Sports and Culture, Japan (#S0801035, 22590230 to H.O. and 23659125 to N.I.) and MEXT-Supported Program for the Strategic Research Foundation at Private Universities.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 2.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 5.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 6.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 7.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 8.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic–pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus Interactions with GnRH neuronal system. J Chem Neuroanat. 2008;36:131–137. doi: 10.1016/j.jchemneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 16.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 17.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 18.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 19.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81:1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 20.Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci. 2011;43:138–145. doi: 10.1007/s12031-010-9430-1. [DOI] [PubMed] [Google Scholar]
- 21.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297:E1212–E1221. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorski RA. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am J Physiol. 1963;205:842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- 24.Aihara M, Hayashi S. Induction of persistent diestrus followed by persistent estrus is indicative of delayed maturation of tonic gonadotropin-releasing systems in rats. Biol Reprod. 1989;40:96–101. doi: 10.1095/biolreprod40.1.96. [DOI] [PubMed] [Google Scholar]
- 25.Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678:102–110. doi: 10.1016/j.bbaexp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153:782–793. doi: 10.1210/en.2011-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dohler KD, Wuttke W. Serum LH, FSH, prolactin and progesterone from birth to puberty in female and male rats. Endocrinology. 1974;94:1003–1008. doi: 10.1210/endo-94-4-1003. [DOI] [PubMed] [Google Scholar]
- 28.Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 29.Gupta D, Rager K, Zarzycki J, Eichner M. Levels of luteinizing hormone, follicle-stimulating hormone, testosterone and dihydrotestosterone in the circulation of sexually maturing intact male rats and after orchidectomy and experimental bilateral cryptorchidism. J Endocrinol. 1975;66:183–193. doi: 10.1677/joe.0.0660183. [DOI] [PubMed] [Google Scholar]
- 30.Swerdloff RS, Walsh PC, Jacobs HS, Odell WD. Serum LH and FSH during sexual maturation in the male rat: effect of castration and cryptorchidism. Endocrinology. 1971;88:120–128. doi: 10.1210/endo-88-1-120. [DOI] [PubMed] [Google Scholar]
- 31.Navarro VM, Sanchez-Garrido MA, Castellano JM, Roa J, Garcia-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology. 2009;150:2359–2367. doi: 10.1210/en.2008-0580. [DOI] [PubMed] [Google Scholar]
- 32.Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21:370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- 33.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151:5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]


