Abstract
Hypoxia and light illumination can both decrease oxygen consumption in the photoreceptor layers. The purpose of the present study was to investigate whether the mutual effects of hypoxia and intense illumination to the photoreceptors are additive. The a-wave of flash electroretinogram (fERG) was recorded to indirectly measure the photoreceptors function under given conditions. Six normal healthy subjects, mean age 34.0 ± 3.8 years, all of whom had high-altitude (>3,000 m) mountain hiking experience, were recruited for the study. Flash a-wave electroretinography was examined under four conditions: (1) normal (D/N); (2) systemic hypoxia induced by inhaling a mixture of O2 and N2 gases, which caused oxyhemoglobin saturation (SaO2) ≈80% (D/H); (3) intense light illumination, which resulted in photoreceptor bleaching (B/N); and (4) a combination of conditions b and c (B/H). Thirty light stimuli, each with a 20-ms ON and 1,980-ms OFF cycle, were given and ERG performed to probe the photoreceptor function. The results showed that a-wave at the various conditions did not respond to all stimuli. The average a-wave amplitudes were 91.4 ± 46.5, 22.8 ± 42.5, 15.5 ± 28.9, and 35.2 ± 41.1 μV for D/N, D/H, B/N, and B/H, respectively. Nonparametric Friedman test for a-wave amplitude indicated that significant differences occurred in D/N–D/H, D/N–B/N, D/N–B/H, D/H–B/H, and B/N–B/H (all p values were <0.001, but D/H–B/N was 0.264). Thus, systemic hypoxia or strong illumination to the retina can cause an absence of the ERG a-wave or change its response, although individual differences were observed. In this study, systemic hypoxia appeared to reduce photoreceptor bleaching, an interesting finding in itself. The mechanisms underlying the disappearance of the ERG a-wave following hypoxia or intense illumination to the photoreceptors seem to differ.
Keywords: ERG, a-wave, Hypoxia, Photoreceptor, Photo-bleaching
Introduction
Flash electroretinography (fERG) is a noninvasive, extremely valuable technique for studying outer retinal function in vivo [1]. Sources of ERG a-, b-, and c-waves are photoreceptors [2, 3], Müller cells [4–7], and retinal pigment epithelial (RPE) cells [8–11], respectively.
Electrophysiologically, photoreceptors are depolarized while in darkness, a state in which their oxygen and glucose consumptions are greater than when they are illuminated [12]. The source of approximately one-third of photoreceptor nutrition is the retinal circulation, which auto-regulates delivery of nutrients, while the remaining two-thirds is furnished by the choroidal circulation, which is not auto-regulated. All materials transported to the photoreceptors are delivered via diffusion. The oxygen tension in the outer retina (photoreceptors and RPE) is also the highest of all the retinal layers [13–15]. These features suggest that a sufficient oxygen supply is required to maintain normal functioning of the outer retinal layers. Therefore, the outer retina is considered to be one of the body’s most metabolically active regions [16, 17], and thus visual function can be affected by even a slight drop in the oxygen gradient between the choroidal capillaries and the outer retina [18, 19]. From a physiological point of view, mild hypoxia is able to slow down the metabolic activities of the outer retina. ERG studies have shown reduced a-wave amplitudes in humans [20, 21] and cats [22] under conditions of mild systemic hypoxia. However, severe systemic hypoxia affects b-wave amplitude (inner retina) more than a-wave (outer retina) in cats [22], indicating that auto-regulation in the retinal circulation is disturbed under conditions of severe hypoxia.
Since photoreceptors consume more oxygen in darkness than they do in light, stimulation with intense light should result in decreased oxygen consumption and bleaching. Therefore, there should be no immediate ERG response by bleached photopigments, unless they are at least partially regenerated. Light illumination to the cat eye causes a diminution of the photoreceptor’s metabolic activity as well as the effects of hypoxia [18]. Two events are known to cause such a slow-down of photoreceptor metabolic activity. One is illumination, resulting in decreased concentrations of intracellular Na+. The other is hypoxia, resulting in decreased concentrations of ATP. In a study of ATP consumption in mammalian rods, the total ATP expenditure under steady light, either in the outer or inner segments, declines with respect to increasing light intensity [23]. Animal studies have shown that cones are barely saturated in bright light, when they consume more of the ATP necessary for activation of transduction and pigment phosphorylation [24–27]. The question arises: is there a lesser ERG response from photoreceptors (a-wave) that are stimulated by light following adaptation to intense light under pre-hypoxic conditions? To answer this, we examined photoreceptor function by using fERG under a variety of conditions associated with systemic hypoxia and intense light adaptation.
Methods
Subjects
Six healthy subjects (four men, two women, average age = 34 ± 2.1 years) were included in the study. None had any evidence of ocular disease. All subjects had mountain-hiking experiences at high altitude (>3,000 m above sea level), and all appeared to tolerate low-oxygen environments well. Blood was drawn to determine hemoglobin, red blood cell count, and hematocrit to ensure that the existing oxygen parameters did not influence experimental results. The study conformed to The Declaration of Helsinki for human subjects, and prior written informed consents were obtained.
Apparatus
Traditional Ganzfeld fERG, with a background illumination of ~2.5 log trolands (1 troland ≈ 0.0036 lumen/m2) white light to desensitize rods and isolate cone ERG, was used [28]. To keep a stable systemic hypoxic condition for the subject being tested, inhaled air contained a mixture of O2 and N2 gases, both controlled by individual flow meters. During hypoxia-associated tests, arterial oxyhemoglobin saturation (SaO2) was kept at 80%. A three-way mouthpiece was employed to separate inhaled and exhaled air mixtures. A nose clip was used to ensure that breathing was from the mouth, not the nose, and to prevent room air from leaking into the subject’s airway. ERGs were measured with a Burian-Allen ERG electrode, and amplified (P511; Grass Technologies, USA) in two stages, with gains of 1,000 and 50, respectively. Amplifier bandwidth-corner frequencies were set at 3 Hz (high-pass) and 100 Hz (low-pass). The signal was sampled at 1,000 Hz and digitized with 12-bit resolution.
Procedure
ERG responses were obtained under four different test conditions: normal (D/N), systemic hypoxia (D/H), photopigment bleaching (B/N), and photobleaching during hypoxia (B/H), where D denotes a 30-min dark adaptation period prior to the test, B denotes an 8-min white-light adaptation period with an intensity of 7.7 log trolands (to cause photoreceptor bleaching), N indicates normal room air breathing, and H is systemic hypoxia resulting from inhaling mixtures of O2 and N2 (which would result in the subject’s SaO2 ≈ 80%). SaO2 was continuously monitored using a pulse oximeter (Novametrix System, Wallingford, CT, USA) [29].
To obtain large-field ERG responses, the subject’s pupil was dilated to ≥4 mm in diameter by repeated applications 1% of mydriacyl (tropicamide) prior to testing. Alcaine (0.5%; Alcon) was used to anesthetize the cornea to make the subject feel comfortable while wearing the ERG electrode. During the test, a total of 30 successive stimuli, with 20 ms ON and 1,980 ms OFF for each single period, were applied for each test run. This resulted in a total of 60-s data collections. The maximum flash intensity of white light was ~4,500 trolands. The schematic protocol for test conditions is shown in Fig. 1. Only ERG a-wave amplitude was measured in this study.
Fig. 1.
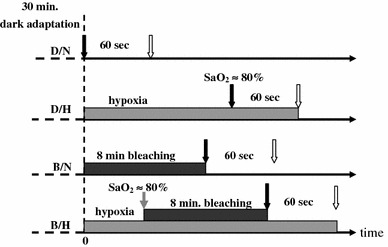
The protocol for ERG measurement under various conditions of D/N, D/H, B/N, and B/H. Black/white arrows indicate the start/end of ERG measurement. The gray one denotes the start of light stress for photoreceptors bleaching 8 min long
Results
Subjects’ blood hemoglobin, red blood cell count, and hematocrit were all within normal ranges (Table 1).
Table 1.
Examination of blood hemoglobin (Hb), red blood cell count (RBC) and hematocrit (Hct) of the six subjects
| Subjects | Sex | Age (years) | Hb (g/dl) | RBC (×106/Ul) | Hct (%) |
|---|---|---|---|---|---|
| 1 | M | 30 | 15.8 | 5.26 | 46.2 |
| 2 | F | 34 | 12.6 | 4.31 | 37.3 |
| 3 | F | 33 | 13.9 | 4.65 | 39.8 |
| 4 | M | 30 | 14.4 | 4.55 | 43.6 |
| 5 | M | 38 | 14.5 | 4.52 | 43.1 |
| 6 | M | 39 | 14.7 | 4.61 | 42.8 |
The normal range of Hb is 14–17 g/dl for man and 12–16 g/dl for woman. The normal range of RBC is 4.2–6.0 × 106/Ul for man and 3.6–5.6 × 106/Ul for woman. The normal range of Hct is 40–52% for man and 37–47% for woman
ERG a-wave amplitude
A typical fERG obtained under condition D/N is shown in Fig. 2. In which the amplitudes of a-, b- and c-waves are indicated. Figure 3 illustrates ERG amplitude responses under the various test conditions. As shown, not every stimulus elicited a response. For conditions D/N, D/H, B/N, and B/H, the average a-wave amplitudes (data due to eye blink was uncounted) for all subjects were 91.4 ± 46.5, 22.8 ± 42.5, 15.5 ± 28.9, and 35.2 ± 41.1 μV, respectively. Statistics with nonparametric Friedman test showed that p values (p < 0.05 indicates significant difference) were <0.001, <0.001, <0.001, 0.264, <0.001, and <0.001 for D/N–D/H, D/N–B/N, D/N–B/H, D/H–B/N, D/H–B/H, and B/N–B/H, respectively. These results unequivocally demonstrate that a-wave amplitudes change when photoreceptors are under conditions of systemic hypoxia, intensive light illumination, or both. It also clearly demonstrates that both systemic hypoxia and intensive light illumination can cause similar results which match our expectation. However, a more interesting phenomenon is that the appearance of a-wave time in B/H occurred much earlier than in B/N and the appearing frequency is also higher. Thus, the combined effects on photoreceptors of hypoxia and bleaching appear to be antagonistic.
Fig. 2.
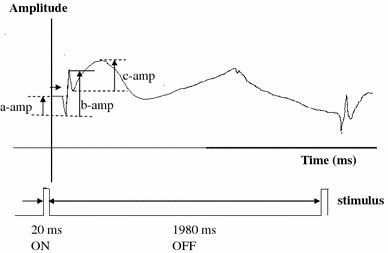
A typical fERG obtained under condition D/N
Fig. 3.
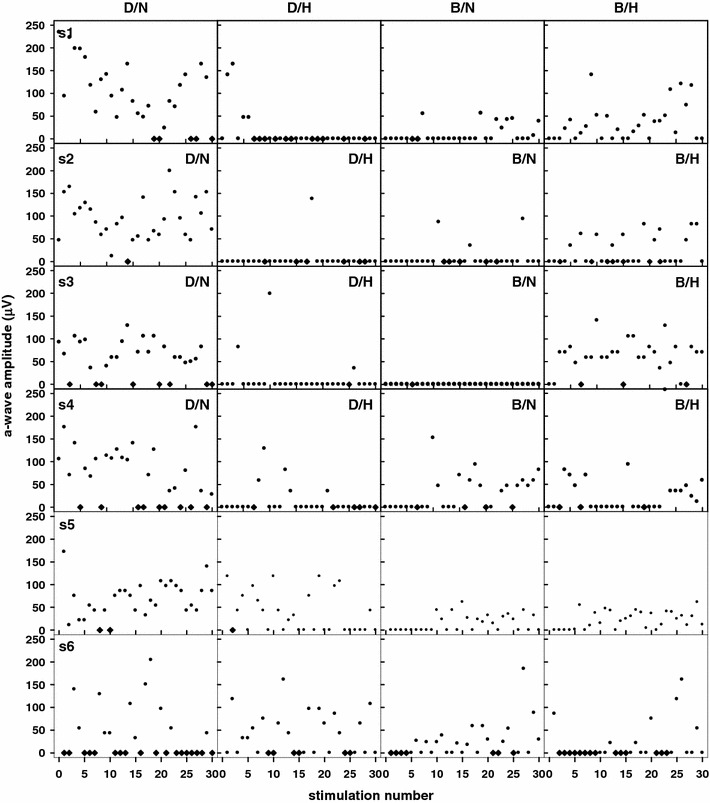
The ERG a-wave amplitudes obtained from the 6 subjects under the four conditions of D/N, D/H, B/N, and B/H. We use absolute value for displaying a-wave amplitude instead of actual corneal negative value. Filled circles regular data; filled diamonds the signal is not counted due to eye blinking or movement. The abscissa shows the timing sequence presented by the in-ordered number of stimuli
Discussion
a-Wave amplitude
Condition D/H
It is well known that systemic hypoxia can reduce oxygen tension in the outer retina. In cats, hypoxia caused a ~9% average reduction in ERG a-wave amplitude while the PaO2 was 50–60 mmHg [27]. At such levels, the b-wave showed no change until the PaO2 had decreased to 20–30 mmHg, which resulted in an average drop of ~35%. Meanwhile, the a-wave amplitude was reduced to a maximum of 14.7%. It is thus concluded that the ERG a-wave is resistant to hypoxemia. The frequency of the a-wave diminishes greatly under this condition. However, when compared to D/N (91.4 ± 46.5 μV), the average amplitude (22.8 ± 42.5 μV) diminished by ~75%. This result was quite different from that obtained in cats. It should be noted that our protocol for ERG a-wave measurement differed from that used by Derwent and Linsenmeier [22]. In cats, the eye was allowed to have a dark adaptation period of at least 3 min after each flash.
It is also known that both light and hypoxia can decrease oxygen consumption in photoreceptors via a variety of mechanisms [18, 30–32]. In darkness, according to the mechanism of phototransduction, Na+–Ca2+–K+ exchange can have exchange rates of Na+(influx):Ca2+(efflux):K+(efflux) = 4:1:1 at the outer segments, whereas the exchange rates of Na+(outward)–K+(inward) is 3:2 for the ATP pump in the plasma membrane of the inner segments [33, 34]. If the photoreceptor’s resting membrane potential is constant in darkness, due to ion homeostasis (if only the principal cations are involved), then the speed of ion flow at the pump (active system) must be higher than that of ion flux (passive system) at the outer segment. Otherwise, the accumulation of cations inside the photoreceptor will monotonically cause the membrane potential to incline, and this does not happen. Thus, when the hypoxic condition is applied to the dark-adapted photoreceptor, the ATP pump is slowed down, although the passive system might not be affected. The consequence, therefore, is that the membrane potential of the photoreceptor becomes more depolarized than when under conditions of normoxia. Animal studies on the influence of hypoxia on pulmonary artery smooth muscle or carotid body glomus cells demonstrated that hypoxia can cause membrane potential depolarization [35–38]. However, a study of mouse dorsal root ganglion neurons indicated that hypoxia inhibits hypoxia-sensitive neurons by shifting the activation potential to a more hyperpolarized level [39].
Although some evidences indirectly implicate the photoreceptor as being a kind of hypoxia-insensitive neuron [22, 40], the hypothesis of how hypoxia causes depolarization of the photoreceptor needs verification. Even if this hypothesis is true, it is still difficult to explain the high rate of disappearance of the a-wave in D/H trials. In the normal situation, the ratio of total ATP expenditure for rods in darkness to saturated illumination is ~4:1 [41]. However, when the photoreceptor is exposed to light following dark-adaptation, its membrane potential is hyperpolarized due to the rapid change of the illumination level, and its ATP expenditure is relatively higher than in cGMP cyclase and Ca2+ channels [23]. This change is like an ‘energy surge’ in the amount of ATP required to ignite the ‘bio-engine’ to shift the photoreceptor’s membrane potential from ‘depolarization’ to ‘hyperpolarization.’ Thus, the hypoxic photoreceptor is analogous to a car whose battery is low, where one cranks (analogous to light stimulation) the engine several times to get ignition, with energy being consumed each time. Similarly, although stimulations do not generate measurable a-waves at first, they may have lowered the ‘more depolarized’ membrane potential to some extent, so that a measurable a-wave can be detected in the following stimulations. To continue with the analogy, in order to successfully start a car engine, a sufficient threshold current (provided by the battery) is required. Frequent cranking of the engine (light stimulation) depletes the energy supply (ATP pump) in the battery. In the case of photoreceptors, this would lead to no response of the a-wave. The cone’s ATP expenditure is similar to that of a rod in darkness [42–44]. In light, however, a cone uses much more ATP than does a rod [45–48]. Thus, in our study, the ATP expenditure of photoreceptors (cones) in the ERG study (in human) should be greater than that which occurred in cats.
Condition B/N
It is apparent that the appearance of a-waves was delayed in the stimulation sequence as result of the time required for photopigment re-synthesis. The stronger the adaptation light, the smaller the overall ERG amplitude [49, 50]. Rushton and Henry’s model [51] is commonly used to estimate the recovery rate for cone pigments. According to the pigment regeneration curve, if ~2–3% of the total pigment is regenerated, then it would respond to stimulation within 16 s after an 8-min exposure to 7.7 log trolands of white light. In our study, the time-constant required to fit the Rushton–Henry model is ~525 s. In other words, it will take ~25 min to recover ~95% pigments after complete bleaching of the photopigments. Individual differences do exist, and are shown in Fig. 3. For example, subject S3, who did not respond at all to light stimulation following bleaching, differed from the others in pigment regeneration rate. This difference did not seem to be related to gender or the biochemical parameters shown in Table 1. Extreme hyperpolarization of the membrane potential resulting from intense illumination should cause a large depletion of photopigment, resulting in no electrophysiological response to the next stimulus. Therefore, there is a difference in the causes of lack of response to light stimulation on photoreceptor in conditions D/H and B/N.
Condition B/H
According to the plots shown in Fig. 3, we cannot determine whether disappearance of the first few a-waves resulted from hypoxia or bleaching or both. However, this result implies that pre-hypoxic conditions inhibit photoreceptor bleaching. The average a-wave amplitude under this condition diminished by ~61% compared to that in D/N. It is interesting that the first a-wave in S3 appeared earlier (~11 s) than most of the other subjects (except S6) in B/H, but showed no response in B/N. However, subjects who had earlier a-wave responses in D/H also had vigorous a-wave responses during subsequent stimulations in B/H. This implies that hypoxia-tolerable subjects have a greater ability to resist photo-bleaching, and supports the hypothesis that hypoxia causes greater depolarization of the photoreceptor membrane potential than in normoxia. Therefore, the feature of membrane-depolarization at hypoxia appears to be able to shift (activate) the hyperpolarized (saturation) condition under light illumination so that there might have a response to the next stimulus. Figure 4 illustrates the dynamic range of the photoreceptor membrane potential in response to light stimulation. It identifies two unusual (hypoxia and saturation) regions. In the region of hypoxia, the photoreceptor is in a more depolarized state than in normoxia. In the region of intensive light illumination, the photoreceptor is in an extremely hyperpolarized (saturated) state and is unable to respond to the light stimulus. In other words, while in saturation, the membrane potential has no room for further lowering. Thus, the maximal a-wave amplitude is the entire dynamic range from the level of ‘the normal resting membrane potential’ to the level of ‘saturated potential.’
Fig. 4.
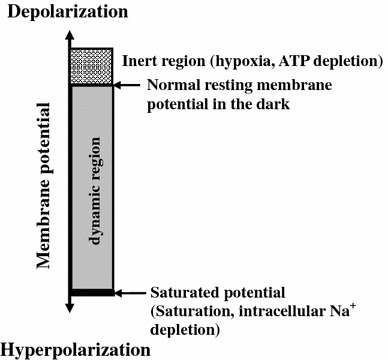
The dynamic range of the a-wave amplitude is from the level of ‘the normal resting membrane potential’ to the ‘saturated potential’, which should be the maximum of the a-wave in response to an intensive light stimulation. Hypoxia could pull photoreceptor’s ‘saturated potential’ back to the dynamic region but with limited room (from that level to the level of ‘saturated potential’) for light response
Conclusion
Systemic hypoxia or strong photobleaching of the retina can cause absence or change in response of the ERG a-wave under most conditions. However, individual differences are observed. Interestingly, systemic hypoxia appeared to reduce the bleaching effect of photoreceptors, a notion supported by the finding that the appearance of a-wave under condition B/H occurred much earlier than in B/N. This indicates that hypoxia reduces the effect of prolonged, intense light stimulation to the photoreceptor. The mechanisms behind the disappearance of the ERG a-wave caused by hypoxia or intense illumination could be different and need to have further study.
Acknowledgments
This work was supported in part by the Grants NSC98-2627-B-010-001 and NSC99-2218-E-010-003 of the Taiwan National Science Council and in part by the UST-UCSD International Center of Excellence in Advanced Bio-engineering sponsored by the Taiwan National Science Council I-RiCE Program under Grants NSC-99-2911-I-009-101 and NSC-100-2911-I-009-101.
Contributor Information
Jorn-Hon Liu, Email: ch9043@chgh.org.tw.
Yin Chang, FAX: +886-2-28210847, Email: yichang@ym.edu.tw.
References
- 1.Carr RW, Seigel IM. Visual electrodiagnostic testing : a practice guide for the clinician. Baltimore: Williams and Wilkins; 1982. [Google Scholar]
- 2.Gouras P, Carr RE. Light-induced DC responses of monkey retina before and after central retinal artery interruption. Invest Ophthalmol. 1965;4:310–317. [PubMed] [Google Scholar]
- 3.Henkes HE. Electroretinography in circulatory disturbance of the retina: II. The electroretinogram in cases of occlusion of the central retinal artery or of one of its branches. Arch Ophthalmol. 1954;51:42–53. doi: 10.1001/archopht.1954.00920040044006. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto Y, Murakami M, Tomita T. Localization of the ERG by aid of histological method. Jpn J Physiol. 1961;11:62–69. doi: 10.2170/jjphysiol.11.62. [DOI] [PubMed] [Google Scholar]
- 5.Miller RF, Dowling JE. Intracellular responses of the Muller (glial) cells of the mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970;33:323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- 6.Newman EA. B-wave currents in the frog retina. Vision Res. 1979;19:227–234. doi: 10.1016/0042-6989(79)90167-6. [DOI] [PubMed] [Google Scholar]
- 7.Newman EA. Current source-density analysis of the b-wave of frog retina. J Neurophysiol. 1980;43:1355–1366. doi: 10.1152/jn.1980.43.5.1355. [DOI] [PubMed] [Google Scholar]
- 8.Noell WK. The origin of the electroretinogram. Am J Ophthalmol. 1954;38:78–90. doi: 10.1016/0002-9394(54)90012-4. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg RH, Schmidt R, Brown KT. Intracellular responses to light from cat pigment epithelium: origin of the electroretinogram c-wave. Nature. 1970;227:728–730. doi: 10.1038/227728a0. [DOI] [PubMed] [Google Scholar]
- 10.Dowling JE, Ripps H. Adaptation in skate photoreceptor. J Gen Physiol. 1972;60:698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granit R, Munsterhjelm A. The electrical response of dark-adapted frog’s eyes to monochromatic stimuli. J Physiol (Lond) 1937;88:436–458. doi: 10.1113/jphysiol.1937.sp003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alm A, Bill A, Young FA. The effects of pilocarpine and neostigmine on the blood flow through the anterior uvea in monkeys: a study with radioactively labeled microspheres. Exp Eye Res. 1973;15:31–36. doi: 10.1016/0014-4835(73)90186-3. [DOI] [PubMed] [Google Scholar]
- 14.Folkow B, Neil E. Circulation. New York: Oxford University Press; 1971. [Google Scholar]
- 15.Alder VA, Cringle SJ, Constable IJ. The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci. 1983;24:30–36. [PubMed] [Google Scholar]
- 16.Futterman S. Metabolism of the retina, III. The role of reduced triphosphopyridine nucleotide in the visual cycle. J Biol Chem. 1963;238:1145–1150. [PubMed] [Google Scholar]
- 17.Futterman S, Hendrickson A, Bishop PE, Rollins MH, Vacano E. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J Neurochem. 1970;17:149–156. doi: 10.1111/j.1471-4159.1970.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg RH. Monitoring communications between photoreceptors and pigment epithelial cells: effects of mild systemic hypoxia. Invest Ophthalmol Vis Sci. 1987;28:1888–1904. [PubMed] [Google Scholar]
- 19.Yancey CM, Linsenmeier RA. Oxygen distribution and consumption in the cat retina at increased intraocular pressure. Invest Ophthalmol Vis Sci. 1989;30:600–611. [PubMed] [Google Scholar]
- 20.Brown JL, Hill JH, Burke BE. The effect of hypoxia on the human electroretinogram. Am J Ophthalmol. 1957;44:57–67. doi: 10.1016/0002-9394(57)91955-4. [DOI] [PubMed] [Google Scholar]
- 21.Klemp K, Lund-Andersen H, Sander B, Larsen M. The effect of acute hypoxia and hyperoxia on the slow multifocal electroretinogram in healthy subjects. Invest Ophthalmol Vis Sci. 2007;48:3405–3412. doi: 10.1167/iovs.06-0471. [DOI] [PubMed] [Google Scholar]
- 22.Derwent JK, Linsenmeier RA. Effects of hypoxemia on the a- and b-waves of the electroretinogram in the cat retina. Invest Ophthalmol Vis Sci. 2000;41:3634–3642. [PubMed] [Google Scholar]
- 23.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews HR, Fain GL, Murphy RL, Lamb TD. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol. 1990;420:447–469. doi: 10.1113/jphysiol.1990.sp017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neuroscience. 1994;14(3 Pt 1):1091–1105. doi: 10.1523/JNEUROSCI.14-03-01091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Jr, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamura S, Tachibanaki S. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- 28.Boynton RM. Stray light and human electroretinogram. J Opt Soc Am. 1953;43:442–449. doi: 10.1364/JOSA.43.000442. [DOI] [PubMed] [Google Scholar]
- 29.Tremper KK, Barker SJ (1986) Pulse oximetry and oxygen transport. In: Payne JP, Severinghaus JW (eds) Pulse Oximetry. Springer, Berlin, pp 19–30
- 30.Torre V. The contribution of the electrogenic sodium–potassium pump to the electrical activity of toad rods. J Physiol. 1982;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimble EA, Svoboda RA, Ostroy SE. Oxygen consumption and ATP changes of the vertebrate photoreceptor. Exp Eye Res. 1980;31:271–288. doi: 10.1016/S0014-4835(80)80037-6. [DOI] [PubMed] [Google Scholar]
- 32.Biernbaum MS, Bownds MD. Light-induced changes in GTP and ATP in frog rod photoreceptors. Comparison with recovery of dark current and light sensitivity during dark adaptation. J Gen Physiol. 1985;85:107–121. doi: 10.1085/jgp.85.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alder VA, Cringle SJ, Constable IJ. The retinal oxygen profile in cats. Invest Ophthalmol Vis Sci. 1983;24:30–36. [PubMed] [Google Scholar]
- 34.Schnetkamp PPM. Sodium–calcium exchange in the outer segment of bovine rod photoreceptors. J Physiol. 1986;373:25–45. doi: 10.1113/jphysiol.1986.sp016033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasicko MJ, Breitwieser GE, Kim I, Carroll JL. Postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2006;154:356–371. doi: 10.1016/j.resp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Manoury B, Etheridge SL, Reid J, Gurney AM. Organ culture mimics the effects of hypoxia on membrane potential, K(+) channels and vessel tone in pulmonary artery. Br J Pharmacol. 2009;158:848–861. doi: 10.1111/j.1476-5381.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priest RM, Robertson TP, Leach RM, Ward JP. Membrane potential-dependent and -independent vasodilation in small pulmonary arteries from chronically hypoxic rats. J Pharmacol Exp Ther. 1998;285:975–982. [PubMed] [Google Scholar]
- 38.Hong Z, Weir EK, Varghese A, Olschewski A. Effect of normoxia and hypoxia on K(+) current and resting membrane potential of fetal rabbit pulmonary artery smooth muscle. Physiol Res. 2005;54:175–184. [PubMed] [Google Scholar]
- 39.Gao LL, Song YL, Tang M, Liu CJ, Hu XW, Luo HY, Hescheler J. Effect of hypoxia on hyperpolarization-activated current in mouse dorsal root ganglion neurons. Brain Res. 2006;1078:49–59. doi: 10.1016/j.brainres.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 40.Linsenmeier RA, Steinberg RH. Effects of hypoxia on potassium homeostasis and pigment epithelial cell in the cat retina. J Gen Physiol. 1984;84:945–970. doi: 10.1085/jgp.84.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yau KW. Phototransduction mechanism in retinal rods and cones. Invest Ophthal Vis Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- 42.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagi T, MacLeish PR. Ionic conductances of monkey solitary cone inner segments. J. Neurophysiol. 1994;71:656–665. doi: 10.1152/jn.1994.71.2.656. [DOI] [PubMed] [Google Scholar]
- 44.Thoreson WB, Tranchina D, Witkovsky P. Kinetics of synaptic transfer from rods and cones to horizontal cells in the salamander retina. Neuroscience. 2003;122:785–798. doi: 10.1016/j.neuroscience.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Matthews HR, Fain GL, Murphy RL, Lamb TD. Light adaptation in cone photoreceptors of the salamander: a role for cytoplasmic calcium. J Physiol. 1990;420:447–469. doi: 10.1113/jphysiol.1990.sp017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci. 1994;14:1091–1105. doi: 10.1523/JNEUROSCI.14-03-01091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Jr, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawamura S, Tachibanaki S. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- 49.Dowling JE. Neural and photochemical mechanisms of visual adaptation in the rat. J Gen Physiol. 1963;46:1287–1301. doi: 10.1085/jgp.46.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowling JE, Ripps H. Adaptation in Skate Photoreceptors. J Gen Physiol. 1972;60:689–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rushton WAH, Henry GH. Bleaching and regeneration of cone pigments in man. Vision Res. 1968;8:617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]


