Abstract
We aimed to determine whether acupuncture to the auricular region increases cortical regional cerebral blood flow (rCBF). The rCBF was measured using laser speckle contrast imaging in urethane-anesthetized rats. Acupuncture stimulation was performed manually at the auricular concha or abdomen. The former’s stimulation significantly increased the rCBF of the bilateral cerebral cortex in the frontal, parietal, and occipital lobes without altering the systemic arterial pressure. In contrast, abdominal stimulation affected neither rCBF nor systemic arterial pressure. The increase in the rCBF was completely abolished by the severance of the somatic nerves that innervated the auricular region, comprising the trigeminal nerve, facial nerve, auricular branch of the vagal nerve, glossopharyngeal nerve, and great auricular nerve. Thus, application of acupuncture to the auricular region increases the rCBF without increasing arterial pressure.
Keywords: Manual acupuncture, Auricular region, Cortical cerebral blood flow, Somatic afferent nerves, Rats
Introduction
Our previous study demonstrated that acupuncture stimulation of a forepaw in anesthetized rats increased regional cortical cerebral blood flow (rCBF) and raised the systemic arterial pressure in similar temporal manner [1, 2]. The increase in rCBF was also observed in spinalized animals (T1–T2) without a pressor response [1–4], suggesting that the response was blood pressure independent.
In rats with an intact central nervous system (CNS), manual acupuncture stimulation of the auricular regions does not elevate the systemic arterial pressure [5]. Somatosensory information from the external ear is transmitted to the brainstem and the upper cervical spinal cord [6–8]. These data suggest that, in CNS-intact animals, stimulation of the auricular region may increase rCBF without elevating the systemic arterial pressure. The first aim of this study was to examine whether manual acupuncture stimulation of the auricular region increased the rCBF independent of systemic blood pressure alterations. Second, if there was an increase in the rCBF, then the involvement of the somatic afferent nerves was examined by severing the somatic nerves innervating the auricular region. Acupuncture stimulation of the abdominal region is also known to exert limited influence on systemic arterial pressure [9, 10]. Finally, to clarify the specificity of stimulation site, the effect of acupuncture stimulation of the abdominal region on the rCBF was compared with that of the auricular region.
Materials and methods
The experiments were performed on 9 male adult Wistar rats (body weight 350–420 g; age 4–8 months) bred at the Tokyo Metropolitan Institute of Gerontology. The study was conducted after obtaining approval from and in accordance with the guidelines for animal experimentation of the Animal Care and Use Committee of Tokyo Metropolitan Institute of Gerontology.
All nine rats were used for measuring rCBF response to the acupuncture stimulation of the auricular region. The somatic nerves innervating the auricular region were severed in 4 rats. The rCBF response to the stimulation of the auricular region was compared with that of the abdomen in the remaining 5 rats.
General surgery and anesthesia
The rats were anesthetized subcutaneously with urethane (1.2 g/kg). Our preliminary study revealed that nicotinic cholinergic vasodilation in the cerebral cortex was reduced in inhalation anesthetic agents such as isoflurane but preserved in urethane anesthesia. Nicotinic cholinergic vasodilation is involved in rCBF increase response induced by acupuncture stimulation of the forepaw [1]. Therefore, urethane was used in the present study. Respiration was maintained with an artificial respirator (model 683, Harvard, Massachusetts, USA) through a tracheal cannula. The end-tidal CO2 concentration was maintained at 3.0–4.0% by monitoring using a respiratory gas monitor (Microcap, Oridion Medical, Jerusalem, Israel). The arterial blood pressure was measured via a catheter inserted into a femoral artery with a pressure transducer (TP-400T, Nihon Kohden, Tokyo, Japan). The body temperature was continuously measured rectally using a thermistor and maintained at approximately 37.5 °C with a body temperature control system (ATB-1100, Nihon Kohden). The anesthesia depth was monitored using body movement, blood pressure stability, and respiratory movement. Additional urethane doses (approximately 0.11 g/kg) were administered intravenously via a femoral vein catheter as necessary.
Measurement of regional blood flow in the cerebral cortex
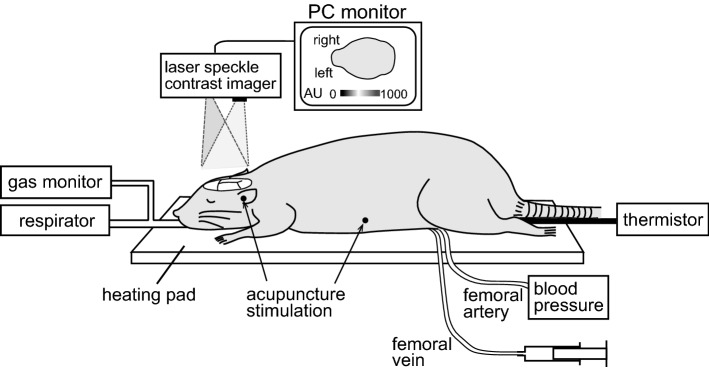
The animals were placed in the prone position, and their heads were fixed with ear bars in a stereotaxic instrument (SR-5R-HT, Narishige, Tokyo, Japan). The skull was made transparent with a dental drill, allowing the visualization of the rCBF through the bone, leaving the meninges and the cerebrospinal fluid intact. The skull surface was covered with mineral oil. Then, the rat’s head was fixed using a metal bar set on the caudal part of the occipital bone using dental cement and screws instead of ear bars to access the auricular concha for acupuncture stimulation. The laser speckle contrast imaging device was then fixed. The zoom was adjusted to cover the dorsal surface of the brain from the most anterior part of the olfactory bulbs to the most posterior aspect of the occipital cortex, with the polarizer lens carefully adjusted to minimize speckle reflection (Fig. 1), as previously described [11]. Laser speckle contrast imaging was performed with a Moor full-field perfusion-imaging device that comprised of an infrared laser diode (785 nm wavelength) and a CCD camera (Moor Instruments, Devon, UK). The viewing field covered approximately 300 mm2 (20 × 15 mm) with a matrix of 760 × 568 pixels, providing an approximate resolution of 26.4 µm/pixel. The images were sampled at 1 Hz with exposure time of 4 ms. On-line averaging generated one mean image every 5 s. These images were acquired continuously throughout the trial, providing a total of 60 images over 5 min.
Fig. 1.
Schematic diagram of the experimental procedures
For analyzing spatial changes in the blood flow, the acquired images were further averaged over 1-min time bins. The baseline image immediately before acupuncture stimulation was then subtracted from the image during stimulation to assess the relative blood flow changes. To quantify the temporal blood flow changes (in arbitrary units: AU), time courses were extracted from six regions of interest depicted by a 1.5-mm-diameter circle positioned bilaterally to avoid the visible blood vessels in the area of the frontal (AP = 1.0–4.0 mm, L = 1.0–4.0 mm), parietal (AP = 0 to − 3.0 mm, L = 2.0–5.0 mm), and occipital cortices (AP = − 5.0 to − 8.0 mm, L = 2.0–5.0 mm) [12, 13]. These time courses were the averaged signal over 10-s or 60-s time bins. The rCBF changes induced by acupuncture stimulation were expressed relative (%) to the baseline signal immediately before the stimulation. The biological zero rCBF level at the end of the experiment (after euthanasia) was less than 1% of the levels when alive and, therefore, was not subtracted from the images [14, 15].
Acupuncture stimulation
A stainless steel needle (160 μm diameter) (Seirin, Shizuoka, Japan) was inserted to a depth of approximately 5 mm in the skin and underlying tissues, and it was kept there. Manual stimulation was performed by rotating the needle to the right and left at a frequency of about 2 Hz for 1 min. The needle was inserted in the auricular region (cavum concha) or the abdomen (approximately 4 cm lateral to the midline and 3 cm caudal to the processus xiphoideus), either on the left or the right side.
Surgical severance of the somatic nerves innervating the auricular region
The somatic nerves that innervate the auricular region [6], including the trigeminal nerve, facial nerve, auricular branch of the vagal nerve, glossopharyngeal nerve, and great auricular nerve, were separated from the surrounding tissues and cut at around the cartilaginous portion of the external ear canal.
Statistical analyses
All values are presented as means ± standard errors of the mean (SEM). The effect of acupuncture stimulation on rCBF and mean arterial pressure (MAP) was tested using a one-way repeated-measures ANOVA followed by Dunnett’s multiple comparison test (Fig. 2), or a paired t-test (Figs. 3 and 4). The comparison of percent change in rCBF among the wide cortical areas (bilateral frontal, parietal, and occipital cortices) were conducted using a one-way repeated-measures ANOVA followed by Tukey’s multiple comparisons test. A P value < 0.05 was considered statistically significant.
Fig. 2.
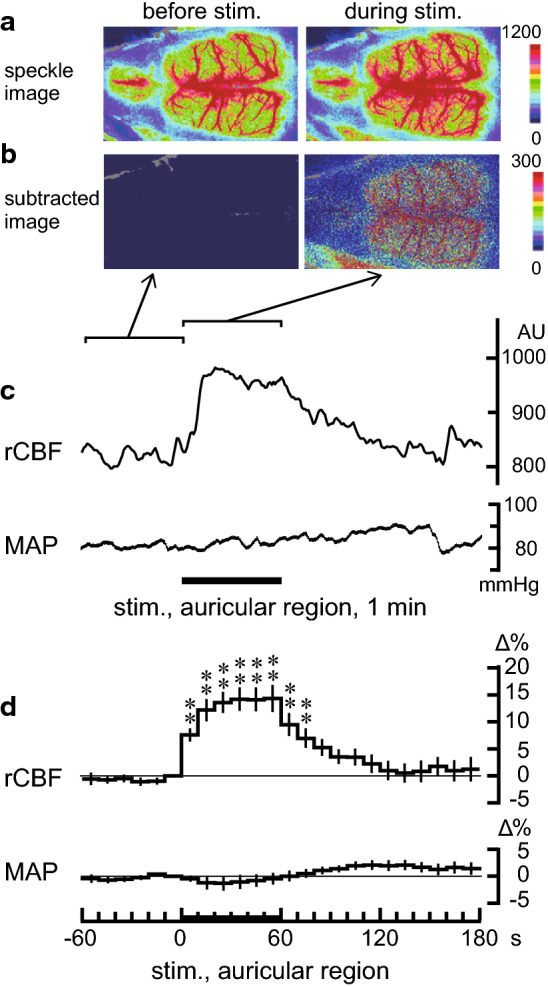
Spatiotemporal changes in the regional cerebral blood flow (rCBF) and mean arterial pressure (MAP) induced by manual acupuncture stimulation to the auricular region. a–c Sample recordings of the rCBF and MAP in one rat. a Averaged signal over a selected period of 1 min. b Differential signal changes obtained by subtracting the baseline (−60 to 0 s) signal from the subsequent image (0–60 s). c The rCBF signal from the right frontal cortex and MAP. d Percentage changes in the signal in the contralateral frontal cortex and MAP averaged at every 10-s period when the unilateral auricular region was stimulated. Each column and vertical bar represents the mean ± standard error of the mean (SEM) (n = 18, obtained from 9 rats). In each rat, 2 trials were conducted. **p < 0.01; significantly different from the pre-stimulus control values (−10 to 0 s) using one-way repeated-measures ANOVA followed by Dunnett’s multiple comparison test
Fig. 3.
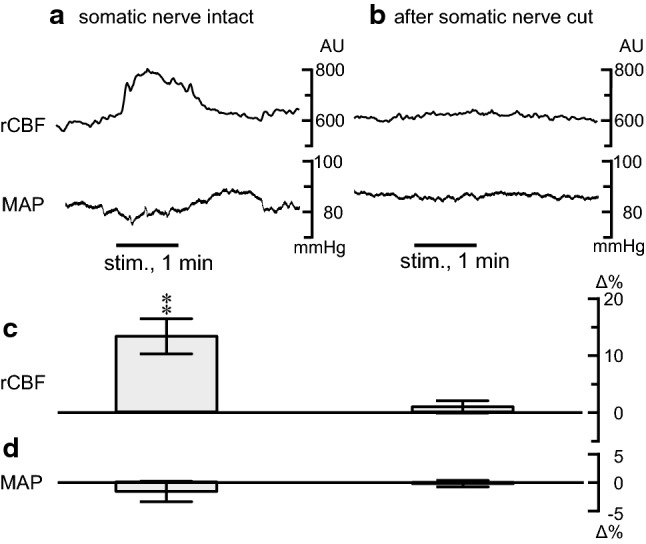
Effect of the severance of somatic nerves that innervate the auricular region on the rCBF and MAP responses following acupuncture stimulation of the auricular region. a, b Sample recordings of the rCBF in the contralateral frontal cortex and MAP. c, d Summary of changes in the rCBF (in the contralateral frontal cortex) and MAP during the 60-s stimulation (n = 8, obtained from 4 rats). **p < 0.01; significantly different from the pre-stimulus control values using a paired t-test. Other details are presented in Fig. 2
Fig. 4.
Comparison of the effect of manual acupuncture stimulation of the auricular region and the abdomen. a Site of stimulation. b, c, e, f Sample recordings of the rCBF in the contralateral frontal cortex (b, e) and MAP (c, f). d, g Summary of changes in the rCBF (in the contralateral frontal cortex) and MAP during the 60-s stimulation (n = 10, obtained from 5 rats). **p < 0.01; significantly different from the pre-stimulus control values using a paired t-test. Other details are included in Fig. 2
Results
Changes of rCBF in response to the manual acupuncture stimulation of the auricular region
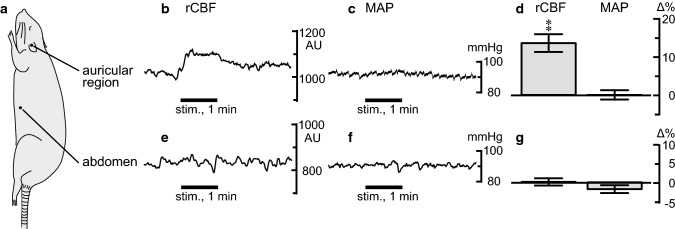
Changes in the rCBF and MAP induced by manual acupuncture stimulation to the auricular region were examined. As shown in Fig. 2c, rCBF was increased during the auricular stimulation and decreased gradually after stimulation ended. In contrast, MAP remained unchanged during the stimulation. Changes were observed bilaterally in the frontal, parietal, and occipital areas, following unilateral auricular stimulation (Fig. 2a, b). Figure 2d summarizes the time courses of the responses of rCBF in the frontal cortex contralateral to the stimulating site and MAP measured every 10 s in all nine rats. The rCBF in the frontal cortex was significantly increased following auricular stimulation (p < 0.01, assessed using one-way repeated-measures ANOVA). The rCBF started increasing within the first 10 s of initiating stimulation; it remained elevated during the stimulation, and gradually returned to the pre-stimulus basal level after the cessation of stimulation. The peak rCBF change was 15% ± 3% (at 50–60 s). MAP was not significantly affected by the auricular stimulation. There were no significant differences among the bilateral cortices (frontal, parietal, and occipital cortices) regarding the rCBF response induced by manual acupuncture stimulation of the unilateral auricular region.
Effect of cutting the somatic afferent nerves on the rCBF response
We explored the afferent pathway of rCBF elevation by transecting the somatic nerves innervating the auricular region. As shown in the sample recordings and summarized graphs of Fig. 3, the increase in the rCBF following manual acupuncture stimulation of the auricular region was abolished after the somatic nerves innervating the auricular region were severed.
Comparison with abdominal stimulation
The rCBF and MAP responses induced by auricular stimulation were compared with the responses to abdominal stimulation. In contrast to the elevation of rCBF following auricular stimulation, manual acupuncture stimulation of the abdomen did not affect rCBF (Fig. 4). MAP remained unchanged in both the auricular and abdominal stimulation.
Discussion
To our knowledge, this is the first study to demonstrate that manual acupuncture stimulation, by twisting a needle into the auricular region, increases rCBF in wide cortical areas, including the bilateral frontal, parietal, and occipital cortices, without affecting the systemic arterial pressure in anesthetized rats. The afferent neural pathway of this responsive change in the rCBF was found to be the somatic nerves innervating the auricular region. The rCBF increase response was specific to the auricular stimulation but not abdominal stimulation.
Our previous study demonstrated that manual acupuncture stimulation of a forepaw in anesthetized rats produced increased rCBF along with increased systemic arterial pressure in a similar temporal manner [1, 2]. This increased rCBF was observed in spinalized animals (T1–T2) without pressor response by the forepaw stimulation [1–4]. In this study, we demonstrated in CNS-intact rats, that manual acupuncture stimulation of a unilateral auricular region increased rCBF without changing the systemic arterial pressure.
The external ear is innervated by somatic afferent fibers in the trigeminal nerve, auricular branch of the vagal nerve, glossopharyngeal nerve, facial nerve, and great auricular nerve [6, 8]. A retrograde tracing study in rats revealed that the outer cartilaginous portion of the external ear canal is innervated by neurons from the trigeminal, glossopharyngeal, vagal, geniculate, dorsal root (C2–C3), and superior cervical ganglia [6]. The present study showed that the increased rCBF response elicited by manual acupuncture stimulation applied to the auricular region was completely abolished when the somatic nerves innervating the auricular region were severed. This result indicates that the afferent nerve pathway for the rCBF response is composed of auricular somatic afferent nerves. Activation of trigeminal afferents by masseter muscle stimulation induces a pressor response in urethane-anesthetized rats [16]. Further studies are required to investigate which somatic afferent nerves innervating the auricular region are involved in the auricular stimulation-induced rCBF elevation without MAP changes. Although the surgically severed nerves innervating the auricular region included both somatic (trigeminal nerve, auricular branch of the vagal nerve, glossopharyngeal nerve, facial nerve, and great auricular nerve) and sympathetic nerves, the sympathetic nerves might not be involved in somatic afferent transmission from the auricular region.
Concerning the efferent neural pathway for the rCBF response, we did not examine in this study. The rCBF is regulated by several types of perivascular nerves [17–21]. In future experiments, we will investigate the efferent neural pathway for the rCBF response.
Our results showed that acupuncture administration to the auricular region increases the rCBF via the somatic afferent nerves without influencing systemic arterial pressure. These findings may provide a scientific basis for the application of acupuncture to the auricular region in patients with rCBF disturbances, such as those with cerebrovascular disease [22, 23] and dementia [24]. Acupuncture to the auricular region may also be advantageous for older patients with hypertension because stimulation of the auricular region does not elevate systemic arterial pressure.
Author contributions
SU and FK conceived and designed the research; all authors performed experiments, analyzed data, and interpreted results of experiments; SU drafted the manuscript; SU and FK edited and revised the manuscript; and all authors approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant number 15K08225 to S.U.).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Uchida S, Kagitani F, Suzuki A, Aikawa Y. Effect of acupuncture-like stimulation on cortical cerebral blood flow in anesthetized rats. Jpn J Physiol. 2000;50:495–507. doi: 10.2170/jjphysiol.50.495. [DOI] [PubMed] [Google Scholar]
- 2.Uchida S, Kagitani F. Effect of acupuncture-like stimulation on cortical cerebral blood flow in aged rats. J Physiol Sci. 2015;65:67–75. doi: 10.1007/s12576-014-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi T, Meguro K, Sato A, Sato Y. Cutaneous stimulation regulates blood flow in cerebral cortex in anesthetized rats. NeuroReport. 1990;1:41–44. doi: 10.1097/00001756-199009000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Sato A, Sato Y, Schmidt RF. The impact of somatosensory input on autonomic functions. Rev Physiol Biochem Pharmacol. 1997;130:328. [PubMed] [Google Scholar]
- 5.Gao XY, Zhang SP, Zhu B, Zhang HQ. Investigation of specificity of auricular acupuncture points in regulation of autonomic function in anesthetized rats. Auton Neurosci. 2008;138:50–56. doi: 10.1016/j.autneu.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Folan-Curran J, Hickey K, Monkhouse WS. Innervation of the rat external auditory meatus: a retrograde tracing study. Somatosens Mot Res. 1994;11:65–68. doi: 10.3109/08990229409028858. [DOI] [PubMed] [Google Scholar]
- 7.He W, Jing XH, Zhu B, Zhu XL, Li L, Bai WZ, Ben H. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci. 2013 doi: 10.1186/1471-2202-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 9.Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18:53–62. doi: 10.1016/0168-0102(93)90105-Y. [DOI] [PubMed] [Google Scholar]
- 10.Sato A, Sato Y, Suzuki A, Uchida S. Reflex modulation of catecholamine secretion and adrenal sympathetic nerve activity by acupuncture-like stimulation in anesthetized rat. Jpn J Physiol. 1996;46:411–421. doi: 10.2170/jjphysiol.46.411. [DOI] [PubMed] [Google Scholar]
- 11.Piché M, Uchida S, Hara S, Aikawa Y, Hotta H. Modulation of somatosensory-evoked cortical blood flow changes by GABAergic inhibition of the nucleus basalis of Meynert in urethane-anaesthetized rats. J Physiol. 2010;588:2163–2171. doi: 10.1113/jphysiol.2010.187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The rat brain in stereotaxic coordinates (compact edition) 6. Amsterdam: Academic Press; 2009. [Google Scholar]
- 13.Zilles K. The cortex of the rat. Berlin: Springer; 1985. [Google Scholar]
- 14.Ayata C, Dunn AK, Gursoy-Özdemir Y, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- 15.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Shoji I, Kemuriyama T, Tandai-Hiruma M, Maruyama S, Tashiro A, Yokoe H, Nishida Y. Reflex arc of the teeth clenching-induced pressor response in rats. J Physiol Sci. 2018;68:89–100. doi: 10.1007/s12576-016-0513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- 18.Kurosawa M, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases acetylcholine release in the cerebral cortex in rats. Neurosci Lett. 1989;98:45–50. doi: 10.1016/0304-3940(89)90371-6. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, Sato Y. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neurosci Res. 1992;14:242–274. doi: 10.1016/0168-0102(92)90071-J. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Hardebo JE, Kåhrström J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab. 1990;10:383–391. doi: 10.1038/jcbfm.1990.68. [DOI] [PubMed] [Google Scholar]
- 21.Morita-Tsuzuki Y, Hardebo JE, Bouskela E. Inhibition of nitric oxide synthase attenuates the cerebral blood flow response to stimulation of postganglionic parasympathetic nerves in the rat. J Cereb Blood Flow Metab. 1993;13:993–997. doi: 10.1038/jcbfm.1993.124. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C. Treatment of acute cerebro-vascular diseases and sequelae with acupuncture. J Tradit Chin Med. 1990;10:70–73. [PubMed] [Google Scholar]
- 23.Jia CS, Ma XS, Li XF, Shi J, Li CF, Liu EJ, Zheng LY, Qin L, Xu XK. Clinical study on auricular acupoint penetration needling along the skin for treatment of a variety of pain syndrome and dysfunction. J Tradit Chin Med. 2011;31:169–172. doi: 10.1016/S0254-6272(11)60034-4. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Mansilla J, González-López-Arza MV, Varela-Donoso E, Montanero-Fernández J, Jiménez-Palomares M, Garrido-Ardila EM. Ear therapy and massage therapy in the elderly with dementia: a pilot study. J Tradit Chin Med. 2013;33:461–467. doi: 10.1016/S0254-6272(13)60149-1. [DOI] [PubMed] [Google Scholar]