Abstract
Mitochondrial dysfunction and decreased mitochondrial content are hallmarks of aging that leads to decreased physical endurance. Our aim was to explore the anti-aging effect of resveratrol (RSVT) supplementation, a polyphenol, and/or exercise training, started at an older age, on improving physical activity, therefore, help in frailty avoidance and promotion of healthy aging in elderly. Eighteen-month-old aged mice received RSVT (15 mg/kg/day) and/or exercise trained for 4 weeks showed significant longer time to exhaustion with decreased blood lactate and free fatty acids levels associated with improved oxidative stress evidenced by decreased gastrocnemius muscle lipid peroxidation and increased antioxidant enzymes activities, catalase and superoxide dismutase, when compared to aged mice control group. These changes were accompanied by over-expression of skeletal muscle peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) mRNA, the master regulator of mitochondrial biogenesis, and increased muscle citrate synthase activity, a marker for mitochondrial function. These findings may provide evidence for improved physical endurance by RSVT supplementation or exercise training with better results of their combination, even at an older age, through increasing mitochondrial biogenesis and function. Increased muscle PGC-1α mRNA expression and citrate synthase enzyme activity in addition to improved aging-associated oxidative damage were among the mechanisms involved in this protection.
Keywords: Aging, Mitochondrial dysfunction, Resveratrol, PGC-1α, Citrate synthase
Introduction
Aging is an inevitable part of life that is characterized by a general decrease in cellular function that eventually will affect body homeostasis and reduce health-span [1]. The majority of sub-cellular structures, including mitochondria, necessitate continuous recycling and regeneration throughout the lifespan. Mitochondria are exposed to high risk of damage throughout life, being they are the major bioenergetics machinery and a major source of reactive oxygen species (ROS) in cells during electron transport chain. Consequently, efficient control of mitochondrial biogenesis and turnover becomes vital for the preservation of energy production, the prevention of endogenous oxidative stress, and the promotion of healthy aging [2].
Mitochondria play a pivotal role in muscle cell physiology, largely by ATP supply via oxidative phosphorylation. The decline in muscle mitochondrial content is observed throughout the lifetime [3]. The aging process causes a decrease in mitochondrial content, which is thought to play a crucial role in aging-associated sarcopenia, loss of muscle mass, strength, and subsequently physical endurance [4]. The decreased physical endurance appears to be one of the hallmarks of aging, an important health dilemma among the elderly, and an unavoidable consequence of aging [5]. So, maintenance of skeletal muscle function is important to improve the life quality during aging [6].
Peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) is a transcriptional co-activator that is known to be the master regulator of mitochondrial biogenesis and oxidative metabolism [7, 8]. Many natural agents of biological activity affecting skeletal muscle performance act through affecting PGC-1α [6]. The best-known of these is resveratrol (RSVT). It is a polyphenol abundant in berries, grapes, walnuts, and peanuts. RSVT has been verified as a modulator of osteopenia in the postmenopausal period [9], a helpful agent in motor behavior, and neuronal function during aging [10], in addition to cardio-protective effect against hypertrophy progression [11] among many other different health effects.
Many epidemiologic and experimental studies have highlighted the importance of physical activity and exercise training in fighting aging-associated health problems [12]. Indeed, the World Health Organization stated that physical inactivity as the fourth leading risk factor for worldwide mortality [13]. Exercise is an excellent therapeutic intervention for many conditions such as obesity, type 2 diabetes, neuro-degeneration, and osteoporosis [14]. So, an exercise training protocol that begins in older age may have an effect on the aging-associated decline in mitochondrial function and skeletal muscle performance.
Consequently, healthy aging promotion and prolonged health span in elderly became an essential issue. So, this study aimed to explore the anti-aging effect of RSVT supplementation and/or exercise training on improving physical endurance by targeting mitochondrial biogenesis and function in skeletal muscles through PGC-1α expression and citrate synthase activity.
Materials and methods
Animals used
Male mice 6 and 12 months of age were purchased from Animal House, Faculty of Veterinary Medicine, Benha University (Egypt), and then they were raised at our animal center for 6 months until 12 and 18 months of age. They were housed in metallic cages (three per cage) and maintained on prevailing atmospheric conditions and room temperature. They were fed with standard pellet diets and drinking water ad libitum. The experimental procedures, animal handling, sampling, and scarification were done according to the Guide for the Care and Use of Laboratory Animals, Eighth Edition (2011) [15]. The protocol was revised and approved by the Ethical Committee of Animal Experiments, Faculty of Medicine, Benha University, Egypt.
Drugs and chemicals
Resveratrol, NC9382296, was purchased from (Cayman Chemicals, MI, USA). It was supplied as a crystalline solid and dissolved in ethanol.
Experimental groups and design
Thirty mice were used in this experiment divided into five groups of mice (n = 6), as follows: Group I (Mature control); 12 months old, were administrated ethanol by oral gavage as a vehicle and served as mature control. Group II (Aged control); 18 months old aged mice received ethanol by oral gavage as a vehicle. Group III (RSVT group); 18 months old mice were administrated resveratrol at a dose of (15 mg/kg/day) by oral gavage. Group IV (Exercise group); 18 months old aged mice + exercise training; exercise training protocol consisted of swimming for 30 min daily in a tank (30 × 30 × 40 cm) filled with warm water and to a depth of 25 cm, so that mice could not support themselves by touching the bottom with their tails, for 4 weeks. Group V (RSVT + Exercise group); 18 months old aged mice with both same swimming exercise protocol and resveratrol treatment (15 mg/kg/day) for 4 weeks [16].
At the end of the 4 weeks, swimming-until-exhaustion exercise test was carried out for all groups to evaluate the anti-fatigue effects. The mice were made to swim to exhaustion with a load corresponding to 5% of their body weight in the form of steel rings attached to the tail root. Each mouse was considered to reach exhaustion when it failed to raise its face to the water surface within 5 s. The time to exhaustion was recorded by stopwatch immediately. The endurance performance for each mouse was measured as the swimming times recorded from the beginning to exhaustion [17]. Blood lactate level was measured just after exercise using Lactate Pro 2 (Arkray, Japan) in a blood sample from the tail vein. The mice were then subjected to overnight fasting. After that, they were sacrificed under general anesthesia using an intra-peritoneal injection of pentobarbital sodium (50 mg/kg). Blood samples were collected by cardiac puncture in dry clean test tubes and left at room temperature to be clotted. Serum was separated by centrifugation at 3000 rpm for 15 min and was stored at − 80 °C to be analyzed. The gastrocnemius muscle was quickly removed, washed with PBS, immediately frozen in liquid N2 and stored at − 80 °C until processing and analysis.
Tissue homogenate preparation
For real-time PCR analysis, citrate synthase and antioxidants enzymatic activity; frozen gastrocnemius muscles were homogenized in nine volumes of ice-cold, tissue-lysis buffer with protease inhibitors (Roche, Spain). Homogenates were centrifuged at 1000×g for 10 min at 4 °C. Pellets containing unlysed cells and cellular debris were discarded.
Real-time polymerase chain reaction for PGC-1α mRNA
To study the alterations in the PGC-1α gene expression, real-time reverse transcription polymerase chain reaction (RT-PCR) analysis was performed. Total RNA was extracted using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. A two-step RT-PCR method was used to synthesize single-stranded complementary DNA (Prime Script RT Reagent Kit; Perfect Real Time; TaKaRa Code RR037A, Otsu, Japan). PGC-1α gene was analyzed by real-time PCR using Applied Biosystems (Foster City, CA, USA) 7500 Fast Real-Time PCR System with SYBR Green I dye (catalog no. 4309155). Temperature cycles were as follows: 95 °C for 30 s followed by 42 cycles of 95 °C for 5 s and 60 °C for 30 s. The SYBR Green fluorescence was detected at the end of each cycle to monitor the amount of PCR product formed during that cycle. Because the relative quantities of the PGC-1α gene are normalized against the relative quantities of the endogenous control β-actin gene fold expression changes are calculated using the equation 2−ΔΔct [18]. Oligonucleotide sequences of the sense and antisense primers as follow:
PGC-1α F: GAGAACAAGACTATTGAGCGAAC and R: GTGGAGTGGCTGCCTTGGGT.
β-actin F: CACCCGCGAGTACAACCTT and R: CCCATACCCACCATCACACC.
Citrate synthase (CS) activity assessment
CS activity was determined spectrophotometrically according to the method of Srere [19], using Citrate Synthase Enzyme Activity Assay kits (BioVision Inc, CA, USA) and the obtained results reported in as nmoles/minute/mg protein.
Antioxidant enzyme activities assessment
Gastrocnemius muscle tissue homogenate was used for colorimetric enzymatic activity assessment for catalase (CAT) [20] and superoxide dismutase (SOD) [21]. Assay kits were provided from (Cayman Chemical Co., Ann Arbor, MI, USA).
Lipid peroxidation assay
Lipid peroxidation assay kits (BML-AK171, Enzo Life Science, NY, USA) were used for determination of malondialdehyde (MDA) in frozen muscular tissue homogenate. Peroxidized lipids were expressed as μmol MDA equivalents/mg protein. The assay was as mentioned by Gérard-Monnier et al. [22].
Measurement of biochemical parameters
Estimation of free fatty acids (FFA) was by enzymatic colorimetric methods using commercially available kits obtained from Diamond Diagnostics Company (Egypt). The procedure was as described by Foster and Dunn [23].
Statistical analysis
All data were analyzed using the program Statistical Package for Social Sciences (SPSS) version 19 (SPSS Inc., Chicago, IL, USA). They are presented as the mean ± standard deviation (SD). Comparisons of all parameters among the study groups were analyzed by using one-way analysis of variance (ANOVA) test and post hoc multiple comparisons (LSD test). The probability of chance (P value) < 0.05 was considered statistically significant.
Results
Effect of RSVT and/or exercise training on endurance performance in experimental groups (Fig. 1)
Fig. 1.
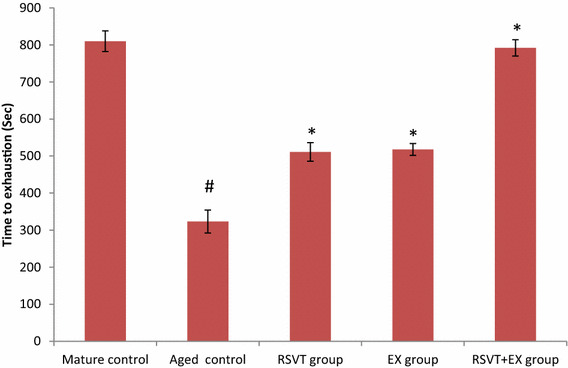
Effect of resveratrol administration and/or exercise training on time to exhaustion (seconds) in aged mice. Data are expressed as mean ± standard deviation SD; n = 6; P value = probability of chance, *P < 0.05 significant difference compared with aged control group while, # P < 0.05 significant difference compared with mature control group. RSVT resveratrol, EX exercise
Time to exhaustion, as a measure of physical endurance, was significantly decreased in the aged control group versus mature control mice (P < 0.05). When we considered the effect of RSVT and/or EX in the aged groups, there was a significant increase in time to exhaustion among these groups, with RSVT + EX group showed the longest time to exhaustion and exhibited non-significant change when compared with mature control group (P > 0.05) (Fig. 1).
Effect of RSVT and/or exercise training on skeletal muscle PGC-1α mRNA expression in experimental groups (Fig. 2)
Fig. 2.
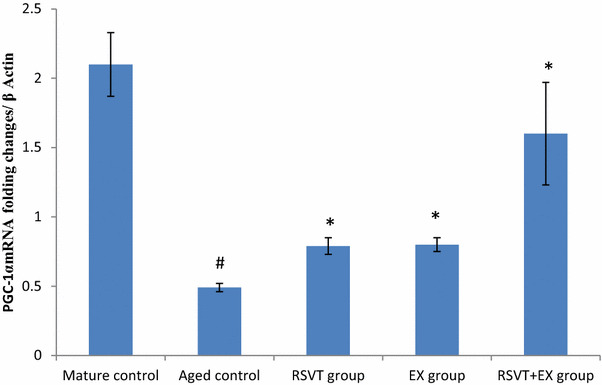
Effect of resveratrol administration and/or exercise training on the muscular expression of mRNA PGC-1α in aged mice. Data are expressed as mean ± standard deviation SD; n = 6; P value = probability of chance, *P < 0.05 significant difference compared with aged control group while, # P < 0.05 significant difference compared with mature control group. RSVT resveratrol, EX exercise
Skeletal muscle PGC-1α mRNA expression in aged mice control group was greatly suppressed when compared to mature control group (P < 0.05). With respect to the aged control group, RSVT, EX, and RSVT + EX groups exhibited significant increases in the expression of PGC-1α mRNA level with the highest expression in RSVT + EX group (P < 0.05). Additionally, we found non-significant changes among RSVT and exercise groups (P > 0.05) (Fig. 2).
Effect of RSVT and/or exercise training on skeletal muscle citrate synthase activity in experimental groups (Table 1)
Table 1.
Enzyme activities and biochemical parameters in experimental groups
| Mature control | Aged control | RSVT group | Exercise group | RSVT + EX group | |
|---|---|---|---|---|---|
| Citrate synthase (nmol/min/mg) | 375 ± 33 | 231 ± 18# | 462 ± 20* | 454 ± 15* | 523 ± 13* |
| Blood lactate (mmol/l) | 9.01 ± 0.55 | 7.6 ± 0.35# | 6.2 ± 0.52* | 6.3 ± 0.37* | 5.3 ± 0.38* |
| Serum FFA (mEq/l) | 0.92 ± 0.06 | 2.24 ± 0.04# | 1.69 ± 0.05* | 1.68 ± 0.06* | 1.41 ± 0.05* |
| Muscle MDA (µmol/mg protein) | 0.38 ± 0.05 | 1.31 ± 0.07# | 0.69 ± 0.03* | 1.49 ± 0.06* | 0.8 ± 0.23* |
|
Muscle CAT (nmol/mg) |
40.8 ± 3.6 | 39.9 ± 2.5 | 55.1 ± 3.7* | 59.1 ± 4.5* | 57.1 ± 2.1* |
|
Muscle SOD (nmol/mg) |
21.5 ± 3.1 | 17.2 ± 2.3# | 30.1 ± 3* | 28.1 ± 3.1* | 38.2 ± 2.6* |
RSVT resveratrol, EX exercise, FFA free fatty acids, MDA malondialdehyde, CAT catalase, SOD superoxide dismutase
Data are expressed as mean ± standard deviation SD; n = 6; P value = probability of chance, *P < 0.05 significant difference compared with aged control group while, # P < 0.05 significant difference compared with mature control group
Citrate synthase enzyme activity (CS) was significantly decreased in the aged mice control group versus the mature control group (P < 0.05). While in RSVT or EX or RSVT + EX groups, there was a significant increase in CS activity among these groups when compared to aged control group. There was a non-significant change in CS activity between RSVT and EX groups and more increase in the RSVT + EX group (P < 0.05) Table 1.
Effect of RSVT and/or exercise training on blood lactate and free fatty acids levels in experimental groups (Table 1)
Blood lactate level in the aged control group was significantly decreased when compared to the mature control group (P < 0.05). By administration of RSVT or exercise training, the blood lactate level was decreased when compared to the aged control group with a non-significant change between the two groups. Furthermore, the combination of both RSVT and exercise produced the more significant decrease in blood lactate level when compared to the aged control group (P < 0.05) (Table 1).
Free fatty acid (FFA) levels were significantly increased in the aged control group when compared to the mature control group (P < 0.05). By RSVT supplementation or EX-training or combination of both, there was a significant decrease in their levels in the corresponding groups when compared to the aged control group (P < 0.05). FFA lowering effect of either RSVT or EX was more or less similar due to non-significant changes between the corresponding groups (P > 0.05).
Effect of RSVT and exercise training on gastrocnemius muscle lipid peroxidation in experimental groups (Table 1)
In the aged mice group, there was a significant increase in muscle MDA levels when compared to mature control group (P < 0.05). Furthermore, exercise training tended to increase lipid peroxidation levels while RSVT group exhibited the lowest peroxidation levels in the aged mice compared to aged control (P < 0.05).
Effect of RSVT and exercise training on antioxidant enzyme activities in experimental groups (Table 1)
Interestingly, in aged mice, ROS scavenging antioxidants CAT and SOD showed a dissimilar pattern; CAT activity was non-significantly changed (P > 0.05) while, SOD activity was significantly decreased (P < 0.05) when compared to mature control group.
RSVT administration and/or exercise training cause a significant increase (P < 0.05) in CAT activity when compared to the aged control group but a non-significant change among RSVT group and RSVT + EX group (P > 0.05) was present. The SOD activity was significantly higher (P < 0.05) in RSVT + EX group when compared to other groups. There was a non-significant change between RSVT group and EX group (P > 0.05).
Discussion
In our research to evaluate age-associated decline in physical performance, swimming-until-exhaustion exercise test was used to the measure anti-fatigue effects. A longer time to exhaustion is interpreted as a reduced susceptibility to fatigue and improved physical activity. In the aged control group, time to exhaustion was significantly lower than in mature control group. This denotes aging-associated easy fatigability and decreased physical endurance in aged mice. Fortunately, either intervention by RSVT supplementation and/or exercise training, time to exhaustion was significantly increased when compared to aged control group. Moreover, RSVT + EX group achieved longer time to exhaustion among aged groups and was non-significantly when compared to the mature control group. This revealed the better anti-aging effect of combined RSVT administration during exercise training on physical performance.
The mitochondrial theory of aging has anticipated mitochondrial dysfunction as an important participant in the aging process [24]. Among the three different species, human, mice, and flies, the expression level of mitochondrial electron transport chain genes was lower in aging [25]. Notably, existing anti-aging strategies suggest an important role of the key regulator of mitochondrial biogenesis, PGC-1α, in the delay or even inhibition of aging process, onset and progression of aging-associated diseases [26]. Hence, to explore resveratrol and/or exercise effects on the aging-associated mitochondrial dysfunction and decline in physical performance, we analyzed expression of PGC-1α mRNA levels and citrate enzyme activity in aged gastrocnemius skeletal muscle. CS is a mitochondria matrix enzyme. It acts as a rate-limiting enzyme in the first step of the Krebs cycle. It has been extensively used as a metabolic marker for evaluating mitochondrial oxidative capacity [27].
Our data revealed that aged mice exhibited significant lower gastrocnemius muscle expression of PGC-1α mRNA and decreased CS activity when compared to mature control group. These findings coincide with that of muscle-specific PGC-1α knockout mice studies [28] and were in agreement with the mitochondrial theory of aging [24]. This gives thoughtfulness to the worth of PGC-1α as a regulator of physical endurance and exercises adaptation during the aging process.
Conversely, RSVT supplementation or exercise training significantly increased PGC-1α mRNA expression and CS activity in skeletal muscle when compared to the aged control group. Furthermore, RSVT + EX group exhibited the higher PGC1-α expression thus indicating the better effect of RSVT supplementation during exercise training of aged mice. This is in agreement with data observed by Murase et al. in senescence-accelerated prone mice those given resveratrol with exercise training had increased PGC-1α and improved physical activity when compared to senescence-accelerated resistant mice, the control strain that ages normally [29].
As regards molecular mechanisms; RSVT activates PGC-1α through Sirt-1 of sirtuin family or might be through the energy sensor adenosine monophosphate-activated protein kinase (AMPK). Meanwhile, exercise modulates the PGC-1α activity through the energy sensor AMPK that is linked to muscle contraction [30].
For the sequel of up-regulated skeletal muscle PGC-1α mRNA and increased CS activity on fuel handling during aging, we investigated blood lactate and free fatty acids levels. Blood lactate is an anaerobic metabolite produced by skeletal muscles during exercise when carbohydrates are used as a source of energy [31]. It reflects the ratio between lactate formation and clearance [32]. It is taken as a biochemical parameter related to fatigue because it produces H+ ions that inhibit enzymes involved in muscle contraction, leading to fatigue [33].
In our study after swimming-until-exhaustion exercise test, RSVT and/or EX-treated groups exhibited lower blood lactate level versus the aged control group. Increased skeletal muscle PGC-1α expression regulates blood lactate level through different mechanisms in addition to increased mitochondrial biogenesis and function. It increases expression of lactate dehydrogenase B that catalyzes lactate to pyruvate reaction. Furthermore, it up-regulates mono-carboxylate transporter-1 that enhances lactate uptake by the muscle [31]. Moreover, the increased PGC-1α expression is a mechanism for promoting angiogenesis, thus improving oxygen supply to the muscle [34]. So, we can believe that RSVT and/or EX supporting a switch from glycolytic to oxidative metabolism.
Unexpectedly, the aged control group exhibited lower blood lactate levels with respect to the mature control group. This is in agreement with Kan et al. [35]. It may unveil another contributing factor that associate mitochondrial dysfunction as oxidative damage, to the decline in physical endurance.
Concerning free fatty acids, in the aged control group, the FFA levels were significantly increased with respect to the mature control group while intervention by RSVT and/or exercise, produced a significant decrease. PGC-1α affects FFA oxidative capacity of skeletal muscle cells by increasing the expression of carnitine palmitoyl-transferase-1β present in the outer membrane of mitochondria. This enzyme is concerned with increased fatty acid uptake by the mitochondria to be metabolized through β oxidation [36]. Also, PGC-1α activates the expression of vascular endothelial growth factor-B. It enhances endothelial uptake of fatty acids in addition to angiogenesis that might contribute to increased fatty acid oxidation through increased substrate delivery into the skeletal muscle [37]. Therefore, the improved fatty acid oxidative capacity buffered against the decline in oxidative phosphorylation that associates the aging process and thereby maintained ATP supply to muscles [38].
Oxidative stress is believed to be a primary mechanism potentiating many aging-related disorders. During aging, a greater number of generated electrons can escape from the mitochondria, creating a long trail of ROS that peroxides mitochondrial membranes, leading to further mitochondrial dysfunction and a vicious cycle of oxidative damage [39]. Also, the antioxidant capacity to buffer oxidants is overwhelmed by ROS, resulting in oxidative damage and reductions in muscle function [40]. This goes ahead with our findings regards increased muscle MDA content and decreased antioxidant enzymes activities CAT and SOD in the aged control group in comparison with mature control group.
In our study, RSVT increased the activity of CAT and SOD and decreased MDA content in gastrocnemius muscle. This is in agreement with Rodríguez-Bies et al. [41]. RSVT can directly scavenge superoxide anion, hydrogen peroxide, and hydroxyl radical. Thus, it inhibits lipid peroxidation and damage within the mitochondrial membrane preserving its integrity and preventing “leaky” mitochondria. It also may act as an indirect antioxidant; by increasing PGC-1α expression, promoting mitochondrial biogenesis and thus, potentiating the possibility to replace the damaged mitochondria in aged animals [42, 43].
Exercise and regular physical activity could counteract the deleterious effects of aging not only by combating the mitochondrial dysfunction, the major trigger of oxidative stress in aging, but also by exerting additional antioxidant effect [39]. Herein, exercise training causes a significant increase in CAT and SOD activity when compared to the aged control group. The improved antioxidant activities in skeletal muscles were in agreement with Hammeren et al. [44] and Radák et al. [45] findings.
Surprisingly, we found that exercise was associated with increased muscular MDA content. There is growing evidence that exercise increases the whole body and tissue oxygen consumption with enhancement of mitochondrial activity [46] that was already malfunctioning with aging thus, promoting a free radical generation. As a result, accumulated ROS leads to lipid peroxidation. However, the concomitant increase in antioxidant enzymes activities, PGC-1α expression, and CS activity let exercise partially prevent oxidative muscular damage that reflected by significant longer time to exhaustion with respect to the aged mice.
Ongoing studies required to elucidate the contribution of downstream mediators of PGC-1α and its target genes as cytochrome C and myostatin, regards to the aging-associated decline of physical endurance. Additional mechanisms involved in the improved FFA in aging by RSVT and EX need to be further elucidated.
Conclusions
The data obtained from this study suggest that the anti-aging effect of RSVT and exercise training was obtained even at an older age. Increased PGC-1α mRNA expression, CS activity, and antioxidant enzymes were partly among the mechanisms. The improved age-related decline in mitochondrial biogenesis and its dysfunction with the associated oxidative damage were about the protective effects offered by RSVT. Exercise can ameliorate aging-related decline in physical performance in spite of associated-increase in lipid peroxidation. RSVT supplementation during exercise training offered better anti-aging effect beyond each one alone.
Acknowledgements
This research was officially supported by the Physiology Department, Faculty of Medicine, Benha University, Egypt.
Abbreviations
- RSVT
Resveratrol
- EX
Exercise
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator-1α
- CS
Citrate synthase
- FFA
Free fatty acids
- AMPK
Adenosine monophosphate-activated protein kinase
- ROS
Reactive oxygen species
- CAT
Catalase
- SOD
Superoxide dismutase
- MDA
Malondialdehyde
Compliance with ethical standards
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and the use of animals were followed and approved by the Ethical Committee of Animal Experiments, Faculty of Medicine, Benha University, Egypt.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Auwerx J. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43(9):813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyes CD, Mathieu-Costello OA, Tsuchiya N, Filburn C, Hansford RG. Mitochondrial biogenesis during cellular differentiation. Am J Physiol. 1997;272:C1345–C1351. doi: 10.1152/ajpcell.1997.272.4.C1345. [DOI] [PubMed] [Google Scholar]
- 4.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 5.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Russo A, Bernabei R, Onder G. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 6.Sanchis-Gomar F, Pareja-Galeano H, Mayero S, Perez-Quilis C, Lucia A. New molecular targets and lifestyle interventions to delay aging sarcopenia. Front Aging Neurosci. 2014;6:156. doi: 10.3389/fnagi.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsukawa T, Motojima H, Sato Y, Takahashi S, Villareal MO, Isoda H. Up-regulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci Rep. 2017 doi: 10.1038/srep44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Li X, Li N, Liu T, Liu J, Li Z, Xiao H, Li J. Long-term resveratrol treatment prevents ovariectomy-induced osteopenia in rats without hyperplastic effects on the uterus. Br J Nutr. 2014;111(5):836. doi: 10.1017/S0007114513003115. [DOI] [PubMed] [Google Scholar]
- 10.Shukitt-Hale B, Bielinski DF, Lau FC, Willis LM, Carey AN, Joseph JA. The beneficial effects of berries on cognition, motor behavior and neuronal function in aging. Br J Nutr. 2015;114:1542–1549. doi: 10.1017/S0007114515003451. [DOI] [PubMed] [Google Scholar]
- 11.Mashhadi FD, Reza JZ, Jamhiri M, Hafizi Z, Mehrjardi FZ, Safari F. The effect of resveratrol on angiotensin II levels and the rate of transcription of its receptors in the rat cardiac hypertrophy model. J Physiol Sci. 2017;67(2):303–309. doi: 10.1007/s12576-016-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99(7):963–972. doi: 10.1161/01.CIR.99.7.963. [DOI] [PubMed] [Google Scholar]
- 13.WHO (2009) Global health risks: mortality and burden of disease attributable to selected major risks, World Health Organization, Geneva, Switzerland, 2009. https://www.cabdirect.org/cabdirect/abstract/20093343418
- 14.Dela F, Kjaer M. Resistance training, insulin sensitivity and muscle function in the elderly. Essays Biochem. 2006;42:75–88. doi: 10.1042/bse0420075. [DOI] [PubMed] [Google Scholar]
- 15.Academies NRCoN (2011) Guide for the care and use of laboratory animals. Eighth edition. Washington: The National Academies Press, Washington, DC, 220 p. https://www.ncbi.nlm.nih.gov/books/NBK54050/
- 16.Lin CH, Lin CC, Ting WJ, Pai PY, Kuo CH, Ho TJ, Kuo WW, Chang CH, Huang CY, Lin WT. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age. 2014;36:9705. doi: 10.1007/s11357-014-9705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, Machida M, Kohara A, Omi N, Takemasa T. Effect of citrulline supplementation on fatigue and exercise performance. J Nutr Sci Vitaminol (Tokyo) 2011;57:246–250. doi: 10.3177/jnsv.57.246. [DOI] [PubMed] [Google Scholar]
- 18.Alhusseini NF, Odaa MM, Mohamed MA, Abd El Wahab WB, Hasan AA. Expression of adiponectin receptors in human placenta and its possible implication in gestational diabetes. Am J Biochem Biotechnol. 2010;2010(6):136–140. doi: 10.3844/ajbbsp.2010.136.140. [DOI] [Google Scholar]
- 19.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. doi: 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- 20.Aebi H. Catalase in vitro. In: Lester P, editor. Methods enzymol. San Diego: Academic Press; 1984. pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 21.Marklund S. Distribution of CuZn superoxide dismutase and Mn-superoxide dismutase in human tissues and extracellular fluids. Acta Physiol Scand Suppl. 1980;492:19–23. [PubMed] [Google Scholar]
- 22.Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11:1176–1183. doi: 10.1021/tx9701790. [DOI] [PubMed] [Google Scholar]
- 23.Foster LB, Dunn RT (1973) Stable reagents for determination of serum triglycerides by colorimetric condensation method. Clin Chem Acta 19: 338–340. http://www.sciepub.com/reference/141214 [PubMed]
- 24.Dillon LM, Rebelo AP, Moraes CT. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life. 2012;64:231–241. doi: 10.1002/iub.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;2(7):e115. doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenz T. Mitochondria and PGC-1α in aging and age-associated diseases. J Aging Res. 2011 doi: 10.4061/2011/810619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spina RJ, Chi MMY, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- 28.Chan MC, Arany Z. The many roles of PGC-1α in muscle-recent developments. Metabolism. 2014;63:441–451. doi: 10.1016/j.metabol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murase T, Haramizu S, Ota N, Hase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10(4):423–434. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- 30.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB Journal. 2002;16(14):1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 31.Summermatter S, Santos G, Pérez-Schindler J, Handschin C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA. 2013;110:8738–8743. doi: 10.1073/pnas.1212976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldeam F, Irving R, Dilworth L. Overview of lactate metabolism and the implications for athletes. AJSSM. 2013;1:42–46. doi: 10.12691/ajssm-1-3-3. [DOI] [Google Scholar]
- 33.Li M, Donglian C, Huaixing L, Bende T, Lihua S, Ying W. Anti-fatigue effects of salidroside in mice. J Med Coll PLA. 2008;23:88–93. doi: 10.1016/S1000-1948(08)60028-3. [DOI] [Google Scholar]
- 34.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, et al. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kan NW, Ho CS, Chiu YS, Huang WC, Chen PY, Tung YT, Huang CC. Effects of resveratrol supplementation and exercise training on exercise performance in middle-aged mice. Molecules. 2016;21(5):661. doi: 10.3390/molecules21050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson K, Albert V, Cardel B, Salatino S, Handschin C. Skeletal muscle PGC1α modulates systemic ketone body homeostasis and ameliorates diabetic hyperketonemia in mice. FASEB J. 2016;30:1976–1986. doi: 10.1096/fj.201500128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PloS one. 2011;6(12):e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA. 2010;106(48):20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxidat Med Cell Longev. 2015 doi: 10.1155/2016/7239639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Bies E, Tung BT, Navas P, López-Lluch G. Resveratrol primes the effects of physical activity in old mice. Br J Nutr. 2016;116(06):979–988. doi: 10.1017/S0007114516002920. [DOI] [PubMed] [Google Scholar]
- 42.Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M, Maciag A, Puca AA, Vecchione C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Guha P, Dey A, Dhyani V, Sen R, Chatterjee M, Chattopadhyay S, Bandyopadhyay SK. Calpain and caspase orchestrated death signal to accomplish apoptosis induced by resveratrol and its novel analog hydroxstilbene-1 in cancer cells. J Pharmacol Exp Ther. 2010;334(2):381–394. doi: 10.1124/jpet.110.167668. [DOI] [PubMed] [Google Scholar]
- 44.Hammeren J, Powers SK, Lawler J, Criswell D, Martin D, Lowenthal D, Pollock M. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med. 1992;13(5):412–416. doi: 10.1055/s-2007-1021290. [DOI] [PubMed] [Google Scholar]
- 45.Radák Z, Naito H, Kaneko T, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445(2):273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Zhao J, He Y, Huangfu X. Screening for characteristic genes in osteoarthritis induced by destabilization of the medial meniscus utilizing bioinformatics approach. J Musculoskelet Neuronal Interact. 2014;14:343–348. [PubMed] [Google Scholar]


