Abstract
The present study was designed to evaluate the antioxidant effects of oleuropein against oxidative stress in the hippocampal area of rats. We used seven experimental groups as follows: Control, Propofol, Propofol-Ketamine (Pro.-Ket.), Xylazine-Ketamine (Xyl.-Ket.), and three oleuropein-pretreated groups (Ole.-Pro., Ole.-Pro.-Ket. and Ole.-Xyl.-Ket.). The oleuropein-pretreated groups received oleuropein (15 mg/kg body weight as orally) for 10 consecutive days. Propofol 100 mg/kg, xylazine 3 mg/kg, and ketamine 75 mg/kg once as ip was used on the 11th day of treatment. Spatial memory impairment and antioxidant status of hippocampus were measured via Morris water maze, lipid peroxidation marker, and antioxidant enzyme activities. Spatial memory impairment and lipid peroxidation significantly increased in Xyl.-Ket.-treated rats in comparison to the control, propofol, Ole.-Pro. and Ole.-Pro.-Ket. groups. Oleuropein pretreatment significantly reversed spatial memory impairment and lipid peroxidation in the Ole.-Xyl.-Ket. group as compared to the Xyl.-Ket.-treated rats. There was no significant difference between the control and the propofol group in lipid peroxidation and spatial memory status. Superoxide dismutase and catalase activities both significantly decreased in Xyl.-Ket.-treated rats when compared to the control, propofol, Ole.-Pro., Ole.-Pro.-Ket., and Ole.-Xyl.-Ket. groups. In contrast, glutathione peroxidase activity in Xyl.-Ket.-treated rats significantly increased as compared to the control, propofol, Pro.-Ket., Ole.-Pro., and Ole.-Pro.-Ket. groups. We concluded that xylazine in combination with ketamine is an oxidative anesthetic drug and oleuropein pretreatment attenuates cognitive dysfunction and oxidative stress induced by anesthesia in the hippocampal area of rats. We also confirmed the antioxidant properties of propofol as a promising antioxidant anesthetic agent.
Keywords: Anesthesia, Cognitive dysfunction, Oleuropein, Oxidative stress
Introduction
It is well known that appropriate anesthesia is necessary for surgery, neuroradiology, and other intensive care in humans, but the side effects cannot be ignored [1, 2]. Anesthetic drugs are a diverse group of compounds with the capability of producing many simultaneous extracellular and intracellular side effects [2, 3]. The production of free radicals derived from oxygen is one of the major mechanisms of tissue damage in processes of ischemia–reperfusion during anesthesia [4]. The increase in oxidative damage is usually accompanied by a decrease in antioxidant defenses and impairment of spatial memory and cognitive dysfunction [2, 4].
A wide variety of different injectable anesthetic agents have been used for induction of anesthesia in the rat, including; pentobarbital, thiopental, ketamine, and tiletamine [1, 3]. Ketamine alone does not provide sufficient analgesia and muscle relaxation but in combination with sedative agents like xylazine is widely used in rodents [1]. Recent reports have established the concept that propofol (2,6-diisopropylphenol) contributes to neuroprotection against ischemic injury and oxidative stress by mechanisms such as decreasing body temperature, cerebral metabolism, and antioxidant activity [2, 4]. In this regard, propofol introduced as a candidate that it is able to prevent or attenuate focal cerebral ischemia [2]. In the present study, ketamine was added to propofol in order to improve analgesic effects. Due to poorly accessible peripheral vessels for intravenous injection, the intraperitoneally route of drug administration is probably the most popular parenteral method of drug delivery in rodents [1, 5]. Propofol and oleuropein both contain a phenolic hydroxyl group, which confers their antioxidant activities by scavenging free radicals [2, 6]. Hence, propofol plus oleuropein may be beneficial in the prevention of oxidative stress and spatial memory impairment during anesthesia in the hippocampal area of rats. In this sense, oleuropein demonstrated a protective effect against oxidative stress mediated by ethanol in testes, liver, intestine, and stomach of rats [7–10].
Therefore, the present study was designed to evaluate oleuropein pretreatment effects against oxidative damage and impairment of spatial memory induced by some anesthetic drugs such as ketamine or xylazine-ketamine in rats. Indeed, the aim of this study was to evaluate pretreatment effects of oleuropein on oxidative stress and cognitive dysfunction induced by some anesthetic drugs in hippocampal area of rats.
Materials and methods
Materials
Propofol (Lipuro 1 %-Propofol 10 mg/ml, B. Braun, Melsungen, Germany), xylazine (20 mg/ml, Alfasan, Woerden, Holland), and ketamine (100 mg/ml, Aesculaap, Boxtel, Holland) were prepared. Oleuropein was purified form olive leaf extract in our laboratory (94 % purity) as described previously [8, 10]. The glutathione peroxidase (GPx) and superoxide dismutase (SOD) kits were obtained via Randox® Company (Antrim, UK). Thiobarbituric acid (TBA) was supplied from Merck Chemical Company (KGaA, Darmstadt, Germany). Other chemicals used were of analytical grade.
Experimental design
A total of 56 adult male Sprague–Dawley rats (weighing 220 ± 20 g) were housed in temperature-controlled conditions under a 12:12-h light/dark photocycle with food and tap water supplied ad libitum. All rats were treated humanely and in compliance with the recommendations of the Animal Care Committee for Lorestan University of Medical Sciences (Khorramabad, Iran) with approval number 91/17. All of the experimental procedures were carried out between 8:00 am and 4:00 pm for prevention of circadian rhythm changes among days and weight gain and food consumptions were determined at 5-day intervals. The rats were divided into a control and six anesthetic groups. The control group received 1 ml of distilled water orally by gavage for 10 consecutive days. The anesthetic group (Propofol, Propofol-Ketamine (Pro.-Ket.), Xylazine-Ketamine (Xyl.-Ket.), Oleuropein-Propofol (Ole.-Pro.), Oleuropein-Propofol-Ketamine (Ole.-Pro.-Ket.), and Oleuropein-Xylazine-Ketamine (Ole.-Xyl.-Ket.) were treated as ip in the following order: the propofol group anesthetized with propofol (100 mg/kg, once), the Pro.-Ket. group received propofol (100 mg/kg, once) and ketamine (40 mg/kg, once), the Xyl.-Ket group anesthetized by xylazine (3 mg/kg, once) plus ketamine (75 mg/kg, once), the Ole.-Pro. group received oleuropein (15 mg/kg for 10 consecutive days, as orally and anesthetized by propofol (100 mg/kg, once) at the 11th day of the treatment, the Ole.-Pro.-Ket. group received oleuropein (15 mg/kg for 10 consecutive days as orally, and anesthetized by propofol (100 mg/kg, once), and ketamine (40 mg/kg, once) at the 11th day of the treatment, and the Ole.-Xyl.-Ket. group received oleuropein (15 mg/kg as orally for 10 consecutive days, and anesthetized by xylazine (3 mg/kg, once), and ketamine (75 mg/kg, once) at the 11th day of the treatment. Doses of the anesthetic drugs were determined according to our previous report in which several drug combinations for intraperitoneal anesthesia were examined in the adult male rats [1], and oleuropein dosage was based on previous works [7–10]. Blood pressure, heart rate, and respiratory rate were measured during anesthesia (Data in press). Herein, intraperitoneal injection of drugs caused neither death nor gross or microscopic lesions in the peritoneal cavity and all of the anesthetic drugs provide sleep time between 30 and 50 min in agreement with our previous report in rats [1].
Twenty-four hours after the full recovery, the anesthetic groups, compared to the control group, were prepared for assessing the spatial memory by the Morris water maze (MWM) apparatus [11, 12]. After measurement of spatial memory for 5 days, all of the rats were killed upon light diethyl ether (Dagenham, UK) anesthesia. The cerebellum and the brain were carefully cleaned by phosphate buffer and dissected by a scalpel. The brain and cerebellum samples were stored at −70 °C for later biochemical analysis up to 2 months.
Spatial memory assessment
The Morris water maze (MWM) apparatus was used to assess hippocampal-dependent spatial learning and memory. The MWM consists of a circular black tank 140 cm in diameter and 80 cm in height that is placed in center room with several extra maze-clues on the wall rounded (environment) the maze [11, 12]. The rats performed four trials per day for four consecutive days. In the swimming trials, each individual rat was released gently into the water at a randomly chosen quadrant. The rat swam and learned how to find the hidden platform within 60 s. After reaching the platform, the rat was allowed to stay on the platform for 10 s and was then taken back into the cage. The rats were placed on the platform by hand for 10 s if they could not escape to the platform within 60 s by themselves, and their escape latency was accepted as 60 s. During the inter-trial intervals, animals were kept in a dry home cage for 60 s. The time to reach the platform (latency), the length of swim path, and the swim speed were recorded semi-automatically with a video tracking system. Twenty-four hours after the last day of training, subjects were tested on a probe trial, during which the escape platform was removed and the time spent in the correct quadrant was measured for a 60-s trial. The percentage of time spent in the target quadrant zone was recorded as time in the target zone.
Tissue preparation for biochemical analysis
Approximately <1 g of the hippocampal area of rats was taken for determination of antioxidant status in rats [13]. The samples were thawed and manually homogenized in cold phosphate buffer (0.1 M, pH 7.4) containing 5 mM EDTA on liquid nitrogen and debris were removed by centrifugation at 2000 RPM for 10 min [14]. Supernatants were recovered and used for GPx, SOD, and catalase (CAT) assay, thiobarbituric acid reactive substances (TBARS) concentration and protein measurement. Protein content of tissue homogenates was determined using a colorimetric method of Lowry with bovine serum albumin as a standard [15].
Measurement of lipid peroxidation
The amount of lipid peroxidation was indicated by the content of TBARS in the hippocampal area homogenates. Tissue TBARS was determined by following the production of thiobarbituric acid reactive substances as described previously [16], which was reported in our laboratory [8–10]. In short, 40 µl of homogenate was added to 40 µl of 0.9 % NaCl and 40 µl of deionized H2O, resulting in a total reaction volume of 120 µl. The reaction was incubated at 37º C for 20 min and stopped by the addition of 600 µl of cold 0.8 M hydrochloride acid, containing 12.5 % trichloroacetic acid. Following the addition of 780 µl of 1 % TBA, the reaction was boiled for 20 min and then cooled at 4º C for 1 h. In order to measure the amount of TBARS produced by the homogenate, the cooled reaction was spun at 1500 × g in a microcentrifuge for 20 min and the absorbance of the supernatant was spectrophotometrically (S2000 UV model; WPA, Cambridge, UK) read at 532 nm, using and extinction coefficient of 1.56 × 105/M cm. The blanks for all of the TBARS assays contained an additional 40 µl of 0.9 % NaCl instead of homogenate as just described. TBARS results were expressed as nanomoles per milligram of tissue protein (nmol/mg protein).
Measurement of GPx activity
The activity of glutathione peroxidase (GPx) was evaluated with the Randox® GPx detection kit according to the manufacturer’s instructions, which was reported in our laboratory [8–10]. GPx catalyze the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of glutathione reductase (GR) and NADPH, the oxidized glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance was measured spectrophotometrically against blank at 340 nm. One unit (U) of GPx was defined as l µmol of oxidized NADPH per min per milligram of tissue protein. The GPx activity was expressed as unit per milligram of tissue protein (U/mg protein).
Measurement of SOD activity
The activity of superoxide dismutase (SOD) was evaluated with the Randox® SOD detection kit according to the manufacturer’s instructions, which was reported in our laboratory [8–10]. The role of SOD is to accelerate the dismutation of the toxic superoxide (O2 −) produced during oxidative energy processes to hydrogen peroxide and molecular oxygen. This method employs xanthine and xanthine oxidase to generate superoxide radicals that react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (I.N.T.) to form a red formazan dye. The SOD activity is then measured by degree of inhibition of this reaction. One unit of SOD is that which causes 50 % inhibition of the rate of reduction of INT under the conditions of the assay. SOD levels were recorded at 505 nm and through a standard curve and expressed as unit per milligram of tissue protein (U/mg protein).
Measurement of CAT activity
Tissue catalase activity was assayed using the method as described previously [17], which was reported in our laboratory [8–10]. The reaction mixture (1 ml) consisted of 50 mM potassium phosphate (pH 7.0), 19 mM H2O2, and a 20–50-μl sample. The reaction was initiated by the addition of H2O2 and absorbance changes were measured at 240 nm (25 °C) for 30 s. The molar extinction coefficient for H2O2 is 43.6/M cm. The CAT activity was expressed as the unit that is defined as μ mol of H2O2 consumed per min per milligram of tissue protein (U/mg protein).
Statistical analysis
Statistical analysis was performed using the statistical package GraphPad PRISM version 5 (GraphPad Software Inc., San Diego, CA, USA). All variables were tested for normal and homogeneous variances by Leven’s statistic test. All results are presented as mean ± (SEM). The statistical differences were applied among the all groups by one-way analysis of variance (ANOVA) with Tukey’s post hoc analysis. Independent t test was also used for demonstration comparison between two groups, for example: Pro.-Ket. versus Xyl.-Ket., Xyl.-Ket. versus Ole.-Xyl.-Ket., Pro.-Ket. versus Ole.-Pro.-Ket., propofol versus Ole.-propofol, and control versus propofol. We used asterisk (*, **, and #) signs for differences among the groups in one-way ANOVA analysis and alphabetic letters (a, b, c, d, e, f) for differences between groups regarding independent t test results.
Results
Mean ± SEM of latency times to locate the hidden platform gradually measured in the control and anesthetic groups over the 4 days of MWM training and latency times on the 4th day of training trails in anesthetic-treated rats were compared with the control group.
Anesthesia effects on spatial memory and cognitive dysfunction
The latency time significantly increased in the xylazine-ketamine group when compared to the controls (p < 0.001). Propofol-ketamine-treated rats indicated significantly lower latency time when compared to the Xyl.-Ket. group (p < 0.05). Moreover, there was no significant difference between control and propofol group for latency time, indicating antioxidant ability of propofol (Fig. 1). The time spent in the target zone significantly decreased in the xylazine-ketamine group as compared to the controls (p < 0.001). There was also no significant difference between the control and propofol group, indicating antioxidant effects of propofol (Fig. 2).
Fig. 1.
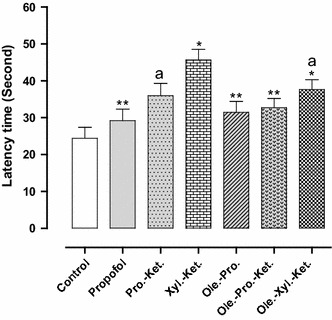
Effects of anesthesia drugs on latency times in control and anesthetic groups. Values represent mean ± SEM of latency times (latency times of Morris water maze training). *p < 0.001 versus control group. **p < 0.001 versus Xyl.-Ket. group. a p < 0.05 versus Xyl.-Ket. group. Pro.-Ket. propofol-ketamine, Xyl.-Ket. xylazine-ketamine, Ole.-Pro. oleuropein-propofol, Ole.-Pro.-Ket. oleuropein-propofol-ketamine, Ole.-Xyl.-Ket. oleuropein-xylazine-ketamine (n = 8 rats per group)
Fig. 2.
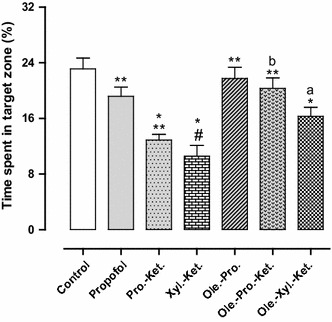
Effects of anesthesia drugs on time spent in target zone from control and anesthetic groups. Values represent mean ± SEM of time spent in target zone (percentage time spent in target zone from Morris water maze training). *p < 0.001 versus control group. **p < 0.001 versus Xyl.-Ket. group. # p < 0.01 versus propofol, Ole.-Pro. and Ole.-Pro.-Ket. groups. a p < 0.05 versus Xyl.-Ket. group. b p < 0.05 versus Pro.-Ket. group. Pro.-Ket. propofol-ketamine, Xyl.-Ket. xylazine-ketamine, Ole.-Pro. oleuropein-propofol, Ole.-Pro.-Ket. oleuropein-propofol-ketamine, Ole.-Xyl.-Ket. oleuropein-xylazine-ketamine (n = 8 rats per group)
Oleuropein effects on spatial memory and cognitive dysfunction
There was significantly higher latency time for Xyl.-Ket.-treated rats in comparison with propofol, Ole.-Pro. and Ole.-Pro.-Ket. groups (p < 0.001). The latency time significantly decreased in Ole.-Xyl.-Ket. group in comparison with Xyl.-Ket.-treated rats (p < 0.05) (Fig. 1). Xylazine-ketamine-treated rats indicated significantly lower time spent in the target zone in comparison to the Ole.-Xyl.-Ket. group (p < 0.05). The Pro.-Ket. group also demonstrated lower time spent in the target zone when compared to the Ole.-Pro.-Ket. group (p < 0.05). Therefore, while xylazine and ketamine acted as oxidative agents, oleuropein improved the acquisition and retention of memory in pretreated rats, principally in combination with propofol (Fig. 2).
Anesthesia effects on lipid peroxidation
Lipid peroxidation (TBARS concentration) significantly increased in Xyl.-Ket.-treated rats when compared to the controls (p < 0.01). There was also significantly higher TBARS concentration in Xyl.-Ket.-treated rats as compared to the propofol group (p < 0.01). There was also no significant difference between control and propofol group for lipid peroxidation marker, even it was very similar, once again indicating antioxidant abilities of propofol (Fig. 3).
Fig. 3.
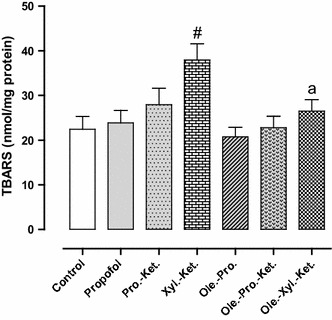
Effects of anesthesia drugs on thiobarbituric acid reactive substances (TBARS) concentration in control and anesthetic groups. Values represent mean ± SEM of TBARS (nanomoles per milligram protein of hippocampal area). # p < 0.01 versus control, propofol, Ole.-Pro. and Ole.-Pro.-Ket groups. a p < 0.05 versus Xyl.-Ket. group. Pro.-Ket. propofol-ketamine, Xyl.-Ket. xylazine-ketamine, Ole.-Pro. oleuropein-propofol, Ole.-Pro.-Ket. oleuropein-propofol-ketamine, Ole.-Xyl.-Ket. oleuropein-xylazine-ketamine (n = 8 rats per group)
Oleuropein effects on lipid peroxidation
Xylazine-ketamine-treated rats indicated significantly higher lipid peroxidation marker in comparison to Ole.-Xyl.-Ket. group (p < 0.05). Indeed, oleuropein pretreatment decreased lipid peroxidation marker in the Ole.-Xyl.-Ket. group (Fig. 3).
The mean values (±SEM) of the antioxidant enzyme activities (GPx, SOD, and CAT) in hippocampal homogenates are presented in Figs. 4, 5, 6.
Fig. 4.
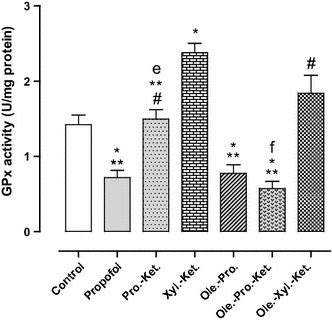
Effects of anesthesia drugs on GPx activity in control and anesthetic groups. Values represent mean ± SEM of enzyme activity (unit/mg protein of hippocampal area). *p < 0.001 versus control group. **p < 0.001 versus Xyl.-Ket. group. # p < 0.01 versus propofol, Ole.-Pro. and Ole.-Pro.-Ket. groups. e p = 0.000 versus Xyl.-Ket. group. f p = 0.000 versus Pro.-Ket. group. Pro.-Ket. propofol-ketamine, Xyl.-Ket. xylazine-ketamine, Ole.-Pro. oleuropein-propofol, Ole.-Pro.-Ket. oleuropein-propofol-ketamine, Ole.-Xyl.-Ket. oleuropein-xylazine-ketamine (n = 8 rats per group)
Fig. 5.
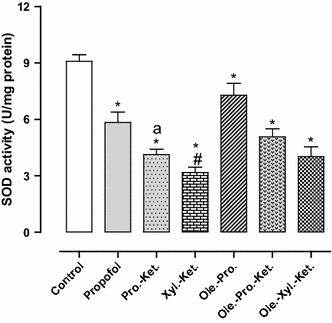
Effects of anesthesia drugs on SOD activity in control and anesthetic groups. Values represent mean ± SEM of enzyme activity (unit/mg protein of hippocampal area). *p < 0.001 versus control group. # p < 0.01 versus propofol and Ole.-Pro. groups. a p < 0.05 versus Xyl.-Ket. group. Pro.-Ket. propofol-ketamine, Xyl.-Ket. xylazine-ketamine, Ole.-Pro. oleuropein-propofol, Ole.-Pro.-Ket. oleuropein-propofol-ketamine, Ole.-Xyl.-Ket. oleuropein-xylazine-ketamine (n = 8 rats per group)
Fig. 6.
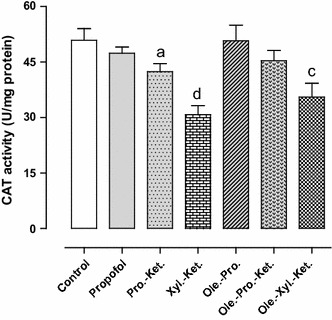
Effects of anesthesia drugs on CAT activity in control and anesthetic groups. Values represent mean ± SEM of enzyme activity (unit/mg protein of hippocampal area). a p < 0.05 versus Xyl.-Ket. group. c p < 0.05 versus control and Ole.-Pro. groups. d p < 0.05 versus control, propofol, Ole.-Pro. and Ole.-Pro.-Ket. groups. Pro.-Ket. propofol-ketamine, Xyl.-Ket. xylazine-ketamine, Ole.-Pro. oleuropein-propofol, Ole.-Pro.-Ket. oleuropein-propofol-ketamine, Ole.-Xyl.-Ket. oleuropein-xylazine-ketamine (n = 8 rats per group)
Anesthesia effects on antioxidant enzyme activities
The GPx activity in Xyl.-Ket.-treated rats was significantly higher (in order compensatory) when compared to the controls (p < 0.01). The Pro.-Ket. group demonstrated significantly lower GPx activity when compared to the Xyl.-Ket. group (p = 0.000) (Fig. 4). SOD activity (as an anti-peroxidative enzyme) was significantly lower in the Xyl.-Ket.-treated rats as compared to the controls (p < 0.01). Moreover, the Pro.-Ket. group demonstrated higher SOD activity when compared to the Xyl.-Ket. group (p < 0.05), and represents oxidative effects of xylazine and ketamine versus propofol antioxidant ability (Fig. 5). CAT activity (another antiperoxidative enzyme) was significantly lower in the Xyl.-Ket.-treated rats as compared to the controls (p < 0.05). Furthermore, the Pro.-Ket. group demonstrated higher CAT activity when compared to the Xyl.-Ket. group (p < 0.05), and demonstrates oxidative effects of xylazine and ketamine again versus propofol antioxidant ability (Fig. 6).
Oleuropein effects on antioxidant enzyme activities
There was also significantly higher GPx activity (in order compensatory) in Xyl.-Ket.-treated rats as compared to the propofol, Ole.-Pro. and Ole.-Pro.-Ket. groups (p < 0.01). Nevertheless, GPx activity significantly decreased in the Ole.-Pro.-Ket. group as compared to the Pro.-Ket.-treated rats (p = 0.000). These differences emphasize antioxidant abilities of oleuropein and propofol versus oxidative effects of xylazine and ketamine (Fig. 4). There was also significantly lower SOD activity in Xyl.-Ket.-treated rats when compared to the propofol, and Ole.-Pro. groups (p < 0.01) (Fig. 5). There was also significantly lower CAT activity in Xyl.-Ket.-treated rats as compared to the propofol, Ole.-Pro. and Ole.-Pro.-Ket groups (p < 0.05). In addition, the Ole.-Xyl.-Ket.-treated rats indicated significantly lower CAT activity when compared to the control and Ole.-Pro. groups (p < 0.05) (Fig. 6).
Discussion
The present study demonstrated the antioxidant effects of oleuropein against cognitive dysfunction and oxidative stress induced by some anesthetic drugs in the hippocampal area of rats. The neuroprotective effect of oleuropein is associated by enhanced SOD and CAT activities and decreasing GPx activity as well as lipid peroxidation content in the hippocampal area of rats. The oleuropein antioxidant abilities further emphasized by attenuation of spatial memory deficits in oleuropein-pretreated rats in comparison with the xylazine-ketamine group. Our data indicated that the injection of xylazine-ketamine resulted in significant memory deficits in rats and increased the latency time for Xyl.-Ket.-treated rats than propofol-treated groups. Although ketamine has been widely used in combination with sedative drugs in rats, the xylazine-ketamine compound induced oxidative stress in rats. The present study also demonstrated that propofol could increase antioxidant status and spatial memory in anesthetic rats.
As previously mentioned, oleuropein is a phenolic compound that has been shown to possess diverse healing properties for its vasodilatory, hypotensive, anti-inflammatory, anti-rheumatic, diuretic, anti-atherogenic, antipyretic, and antioxidant effects [18–22]. It seems that many of these pharmacologic features of oleuropein are due to its potent antioxidant actions [18]. In the present study, oleuropein exerted as a natural suppressor of oxidative stress which resulting in spatial memory improvement. Oleuropein is able to chelate metal ions, such as Cu 2+ and Fe 2+, which catalyze free radical generation reactions [10]. Oleuropein and its metabolite hydroxytyrosol both possess the structural requirement (a catechol group) needed for optimum antioxidant and scavenging activity [18]. Therefore, it is possible that oleuropein decreased anesthetic-induced oxidative stress and cognitive dysfunction in this study by two pathways; First, by rapid conversion of H2O2 to H2O and preventing of H2O2 accumulation and second, by quenching the hydroxyl radicals that, trapping of hydroxyl radicals leads to oxidative breakdown of the phenolic compounds [10]. Our results are in agreement with previous reports in the liver, intestine, and testes of rats [7, 9, 10]. Administration of oleuropein improved the cognitive dysfunction and attenuated oxidative stress, suggesting that oleuropein improves spatial memory impairment and exerts as an antioxidant agent. In this protocol, we pretreated Ole.-Pro., Ole.-Pro.-Ket., and Ole.-Xyl.-Ket. groups with oleuropein (15 mg/kg as orally) based on our previous reports [7–10], and there was a clear difference in antioxidant enzyme activities and lipid peroxidation content in rats pretreated by oleuropein versus the Xyl.-Ket. group. Recent studies have shown that oleuropein induces antioxidant enzyme activities in midbrain during aging [23]. In addition, it has been shown that olive leaf extract decreases age-induced oxidative stress in major organs of aged rats [24]. Oleuropein treatment after spinal cord injury also indicated a neuroprotective effect in the rat [25]. Herein, the neuroprotective effects of the oleuropein against oxidative stress were evaluated by measuring the TBARS concentration and antioxidant enzyme activities. The results showed that oleuropein pretreatment inhibited significantly lipid peroxidation and decreased TBARS content in the oleuropein-treated rats (Fig. 3). Furthermore, oleuropein improved anesthetic-mediated memory deficits, i.e., decreasing the latency time and increasing the time spent in target quadrant in the Ole.-Pro.-Ket. and Ole.-Xyl.-Ket. groups (Figs. 1, 2). The results of this work indicated that administration of oleuropein for 10 consecutive days elevated SOD and CAT activities in oleuropein-treated rats compared to the Xyl.-Ket.-treated group (Figs. 5, 6). The observed results revealed that oleuropein has antioxidant properties such as propofol to increase antiperoxidative enzyme activities, i.e., SOD and CAT. Interestingly, oral administration of oleuropein caused a significant decrease in the GPx activity in oleuropein-pretreated rats. Indeed, oleuropein could quench the free radicals in these groups and suppress oxidative stress. However, GPx activity increased in Xyl.-Ket. and even Ole.-Xyl.-Ket. groups. It seems that elevation of GPx activity in Xyl.-Ket.-treated rats is a compensatory mechanism. It is well known that oxidative agents can act both as a pro-oxidant and anti-oxidant molecule, depending on the circumstances. For instance, low concentrations of ethanol (as an oxidative agent) could induce an increase in GPx activity possibly because the drug acts as a minimal stressor that enhances the production of protective molecules [7, 8]. On the other hand, although GPx activity was elevated to suppress oxidative stress in Xyl.-Ket.-treated rats, it was unable to prevent lipid peroxidation in the hippocampal area of rats (Fig. 3).
Animal models have shown a number of antioxidants that prevent oxidative brain injury through a variety of cellular mechanisms in the CNS [14]. The glutathione antioxidant system plays a fundamental role in cellular defense against ROS. The cellular tripeptide GSH thwarts peroxidative damage by neutralizing the free radicals [14]. In our study, the increase in GPx activity in xylazine-ketamine-treated rats is evidence of an attempt by the brain tissue to defend itself from free radical attack; however the defense is not entirely successful. In contrast, propofol indicated some antioxidant properties that are accompanied by decreased GPx activity [4]. Another antioxidant system against free radicals is SOD. Investigations in animal models of cerebral ischemia suggest a particular role of SOD in the reperfusion injury, however, the reports of the effect of cerebral ischemia on SOD expression and activity are contradictory [4, 26, 27]. A small decrease in SOD activity has been reported in most of the studies, including human acute cerebral ischemia [28]. It is well known that GPx and CAT are two key antioxidant enzymes that can decompose hydrogen peroxide as a common substrate to water [8]. The increase in GPx activity in Xyl.-Ket.-treated rats correlates well with the decrease of CAT activity as a competitive enzyme for GPx activity. Therefore, CAT activity decreased in Xyl.-Ket.-treated rats (Fig. 6).
Regarding the antioxidant ability of propofol, there was no significant difference between control and propofol group in lipid peroxidation markers (Fig. 3). There were also no differences in latency time and time spent in the target zone between the control and propofol group (Figs. 1, 2). Based on these results, we found that propofol inhibits spatial memory impairment and lipid peroxidation in the hippocampal area during anesthesia. These results are concordant with earlier findings that the anesthetic reduced peroxidation in a dose-dependent manner in several different rat tissues [4, 29, 30]. Although we did not directly investigate the mechanisms by which propofol decreased oxidative stress and improved spatial memory impairment, a number of mechanisms have been proposed. Propofol, like most other general anesthetics, reduces the cerebral metabolic rate for oxygen consumption, probably reflecting γ-aminobutyric acid receptor type A potentiation [2]. Another mechanism is related to its antioxidant properties [2, 4]. In this regard, antioxidant properties of propofol against free radicals and reactive oxygen species (ROS) demonstrated by inhibition of lipid peroxidation in both in vitro and in vivo studies [2, 4]. Propofol also inhibits the mitochondrial permeability pores [2, 31] and causes suppression of sodium influx through voltage-dependent sodium channels [2, 32–35]. It has been demonstrated that mitochondrial function is altered in ischemic-reperfusion models, mainly by a dysfunction of its respiratory activity, caused by oxygen radicals, intracellular pH, calcium homeostasis, and degradation of mitochondrial phospholipids. Mitochondria have a high content of glutathione, and propofol inhibits lipid peroxidation [4], which supports a possible effect of propofol in this subcellular level. Overall, any or all of these mechanisms could be relevant in explaining the beneficial effects found in the current study.
Conclusions
We conclude that oleuropein as an antioxidant agent could decrease oxidative stress and cognitive dysfunction induced by xylazine and ketamine in rats. However, further studies in humans will be needed to confirm the ability of oleuropein to protect brain tissue from oxidative stress.
Acknowledgments
This study was financially supported by research project No. 91/17 of Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran. We are most grateful to Dr. Nikahval for his kindly cooperation in providing of propofol. Dedicate to Arshida and Aderian, flavor of my life.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Animal care
All rats were treated humanely and in compliance with the recommendations of Animal Care Committee for Lorestan University of Medical Sciences (Khorramabad, Iran) with Approval Number: SM 91/17.
References
- 1.Hajighahramani S, Vesal N. Evaluation of several drug combinations for intraperitoneal anaesthesia in adult male rats. Iran J Vet Res. 2007;8:106–115. [Google Scholar]
- 2.Gelb AW, Bayona NA, Wilson JX, Cechetto DF. Propofol anesthesia compared to awake reduces infarct size in rats. Anesthesiology. 2002;96:1183–1190. doi: 10.1097/00000542-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Gelb AW, Wilson JX, Cechetto DF. Anesthetics and cerebral ischemia: should we continue to dream the impossible dream? Can J Anesth. 2001;48:727–731. doi: 10.1007/BF03016685. [DOI] [PubMed] [Google Scholar]
- 4.De La Cruz JP, Villalobos MA, Sedeno G, De La Cuesta FS. Effect of propofol on oxidative stress in an in vitro model of anoxia-reoxygenation in the rat brain. Brain Res. 1998;800:136–144. doi: 10.1016/S0006-8993(98)00516-2. [DOI] [PubMed] [Google Scholar]
- 5.Wixson SK, White WJ, Hughes HC, Jr, Lang CM, Marshall WK. A comparison of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam anesthesia in adult male rats. Lab Anim Sci. 1987;37:726–730. [PubMed] [Google Scholar]
- 6.Green TR, Bennett SR, Nelson VM. Specificity and properties of propofol as an antioxidant free radical scavenger. Toxicol App Pharmacol. 1994;129:163–169. doi: 10.1006/taap.1994.1240. [DOI] [PubMed] [Google Scholar]
- 7.Alirezaei M, Dezfoulian O, Kheradmand A, Neamati SH, Khonsari A, Pirzadeh A. Hepatoprotective effects of purified oleuropein from olive leaf extract against ethanol-induced damages in the rat. Iran J Vet Res. 2011;13:218–226. [Google Scholar]
- 8.Alirezaei M, Dezfoulian O, Neamati S, Rashidipour M, Tanideh N, Kheradmand A. Oleuropein prevents ethanol-induced gastric ulcers via elevation of antioxidant enzyme activities in rats. J Physiol Biochem. 2012;68:583–592. doi: 10.1007/s13105-012-0177-8. [DOI] [PubMed] [Google Scholar]
- 9.Alirezaei M, Dezfoulian O, Sookhtehzari A, Asadian P, Khoshdel Z. Antioxidant effects of oleuropein versus oxidative stress induced by ethanol in the rat intestine. Comp Clinl Pathol. 2014;23:1359–1365. doi: 10.1007/s00580-013-1791-8. [DOI] [Google Scholar]
- 10.Alirezaei M, Kheradmand A, Heydari R, Tanideh N, Neamati S, Rashidipour M. Oleuropein protects against ethanol-induced oxidative stress and modulates sperm quality in the rat testis. Mediterr J Nutr Metab. 2012;5:205–211. doi: 10.1007/s12349-011-0079-2. [DOI] [Google Scholar]
- 11.Morris R, Garrud P, Rawlins J, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 12.Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+ -activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezaei M, Alirezaei M. Protective effects of Althaea officinalis L. extract in 6-hydroxydopamine-induced hemi-Parkinsonism model: behavioral, biochemical and histochemical evidence. J Physiol Sci. 2014;64:171–176. doi: 10.1007/s12576-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alirezaei M, Khoshdel Z, Dezfoulian O, Rashidipour M, Taghadosi V. Beneficial antioxidant properties of betaine against oxidative stress mediated by levodopa/benserazide in the brain of rats. J Physiol Sci. 2015;65:243–252. doi: 10.1007/s12576-015-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem. 1990;55:342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 17.Claiborne A, Greenwald RA. CRC handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1986. pp. 283–284. [Google Scholar]
- 18.Al-Azzawie HF, Alhamdani M-SS. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006;78:1371–1377. doi: 10.1016/j.lfs.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Manna C, D’Angelo S, Migliardi V, Loffredi E, Mazzoni O, Morrica P, Galletti P, Zappia V. Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J Agric Food Chem. 2002;50:6521–6526. doi: 10.1021/jf020565+. [DOI] [PubMed] [Google Scholar]
- 20.Manna C, Migliardi V, Golino P, Scognamiglio A, Galletti P, Chiariello M, Zappia V. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J Nutr Biochem. 2004;15:461–466. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Visioli F, Bellomo G, Galli C. Free radical-scavenging properties of olive oil polyphenols. Biochem Biophys Res Commun. 1998;247:60–64. doi: 10.1006/bbrc.1998.8735. [DOI] [PubMed] [Google Scholar]
- 22.Visioli F, Caruso D, Galli C, Viappiani S, Galli G, Sala A. Olive oils rich in natural catecholic phenols decrease isoprostane excretion in humans. Biochem Biophys Res Commun. 2000;278:797–799. doi: 10.1006/bbrc.2000.3879. [DOI] [PubMed] [Google Scholar]
- 23.Sarbishegi M, Mehraein F, Soleimani M. Antioxidant role of oleuropein on midbrain and dopaminergic neurons of substantia nigra in aged rats. Irann Biomed J. 2014;18:16. doi: 10.6091/ibj.1274.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Çoban J, Öztezcan S, Doğru‐Abbasoğlu S, Bingül I, Yeşil‐Mizrak K, Uysal M (2014) Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriat Gerontol Inter 996–1002 [DOI] [PubMed]
- 25.Khalatbary AR, Ahmadvand H. Neuroprotective effect of oleuropein following spinal cord injury in rats. Neurol Res. 2012;34:44–51. doi: 10.1179/1743132811Y.0000000058. [DOI] [PubMed] [Google Scholar]
- 26.Michowiz SD, Melamed E, Pikarsky E, Rappaport ZH. Effect of ischemia induced by middle cerebral artery occlusion on superoxide dismutase activity in rat brain. Stroke. 1990;21:1613–1617. doi: 10.1161/01.STR.21.11.1613. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland G, Bose R, Louw D, Pinsky C (1991) Global elevation of brain superoxide dismutase activity following forebrain ischemia in rat. Neurosci lett128:169–172 [DOI] [PubMed]
- 28.Spranger M, Krempien S, Schwab S, Donneberg S, Hacke W. Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury correlation with clinical course and infarct size. Stroke. 1997;28:2425–2428. doi: 10.1161/01.STR.28.12.2425. [DOI] [PubMed] [Google Scholar]
- 29.De La Cruz JP, Carmona JA, Paez MV, Blanco E, De La Cuesta FS. Propofol inhibits in vitro platelet aggregation in human whole blood. Anesth Analg. 1997;84:919–921. doi: 10.1213/00000539-199704000-00040. [DOI] [PubMed] [Google Scholar]
- 30.De la Cruz JP, Carrasco T, Ortega G, De la Cuesta FS. Inhibition of ferrous-induced lipid peroxidation by pyrimido-pyrimidine derivatives in human liver membranes. Lipids. 1992;27:192–194. doi: 10.1007/BF02536177. [DOI] [PubMed] [Google Scholar]
- 31.Fo Sztark, Fo Ichas, Ouhabi R, Dabadie P, Mazat J-P. Effects of the anaesthetic propofol on the calcium-induced permeability transition of rat heart mitochondria: direct pore inhibition and shift of the gating potential. FEBS Lett. 1995;368:101–104. doi: 10.1016/0014-5793(95)00610-L. [DOI] [PubMed] [Google Scholar]
- 32.Frenkel C, Urban BW. () Interactions of intravenous anesthetics with human CNS ion channels. Electrophysiologic studies with a new type of voltage clamp technique. Der Anaesthesist. 1994;43:229–234. doi: 10.1007/s001010050052. [DOI] [PubMed] [Google Scholar]
- 33.Minami K, Yanagihara N, Segawa K, Tsutsui M, Shigematsu A, Izumi F. Inhibitory effects of propofol on catecholamine secretion and uptake in cultured bovine adrenal medullary cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 1996;353:572–578. doi: 10.1007/BF00169178. [DOI] [PubMed] [Google Scholar]
- 34.Ratnakumari L, Hemmings HC., Jr Effects of propofol on sodium channel-dependent sodium influx and glutamate release in rat cerebrocortical synaptosomes. Anesthesiology. 1997;86:428–439. doi: 10.1097/00000542-199702000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Rehberg B, Duch DS. Suppression of central nervous system sodium channels by propofol. Anesthesiology. 1999;91:512–520. doi: 10.1097/00000542-199908000-00026. [DOI] [PubMed] [Google Scholar]


