Abstract
The effects of deconditioning on exercise-induced bone gains in rats were investigated in 12-week-old female WKY rats performing a standard jumping exercise regimen for either 8, 12 or 24 weeks, followed by sedentary periods of either 24, 12 or 0 weeks, respectively. Age-matched controls received no exercise over the same period. At the end of the training/sedentary period, the tibiae were harvested for analyses of bone parameters. Gains in tibial fat-free dry weight decayed within 12 weeks of deconditioning, but gains in tibial ultimate bending force (strength), maximum diameter and cortical area were still present at 12 weeks of deconditioning. With the exception of cortical area, all other exercise-induced bone gains decayed by the 24th week of deconditioning. It appears that the decay in exercise-induced bone gains in strength, physical and morphological properties is not uniform, and that gains in fat-free dry weight seem to decay earlier.
Keywords: Cortical area, Deconditioning, Fat-free dry weight, Rats, Tibia, Ultimate bending force
Introduction
It is now well established that bone gains, often defined as increases in bone mineral density, bone morphology and mechanical properties secondary to regular physical activities, decay upon cessation of physical activity. However, the reported duration required for the complete decay of these bone gains seems to vary. In humans, for example, durations of cessation of exercise of between 3 and 13 months have been reported for complete decay of exercise-induced bone gains. In postmenopausal women with or without osteoporosis, for example, increases in lumber bone mineral content induced by 9–12 months of jogging, brisk walking, gymnastic training, and stair climbing exercises were all lost over a period of 13 months following cessation of these activities [1, 2]. Other studies, however, report earlier loss of exercise-induced bone gains. Gains in bone mineral density and content in trained lower limbs, induced by unilateral strength training in women aged between 19 and 27 years, were found to decline towards baseline values within 3 months of cessation of training [3]. Another study found that bone gains, resulting from a 12-month combined exercise programme that consisted of jumping and lower body resistance training in women between 30 and 45 years of age, were lost within 6 months of cessation of exercise [4].
Similar variations in the duration of decay in exercise-induced bone gains have also been reported in animal studies. Gains in femoral wet weight following 8 weeks of treadmill running in female rats were lost within 4 weeks of deconditioning [5]. Increases in bone mineral content [6] and bone mass [7] of the femur induced by treadmill running in female rats were also lost after 4 weeks of deconditioning. Similar losses in bone gains following treadmill running were also reported in male mice [8]. In contrast, exercise-induced gains in tibial fat-free dry mass, cortical area, periosteal perimeter and ultimate bending force accrued during 4 weeks of jumping exercise were still maintained 4 weeks after the cessation of exercise in rats [9]. Significant retention of treadmill running exercise-induced bone fat-free dry weight even after 10 weeks of deconditioning in rats has also been reported [10]. A number of other animal studies also report deconditioning periods ranging from 14 to 28 weeks before any significant decay in femoral gains became evident [11–13]. Collectively, while all these studies confirm that physical exercise induces bone changes, and that these gains decay following cessation of exercise, there is, however, little consensus on the exact time frame for the complete decay of these bone gains following cessation of jumping exercise. Moreover, the reason for the variation in the rate of decay of bone gains is unclear. It might be related to the duration of exercise, type of exercise or even the age of the animal. It is also possible that some of the gains in bone, particularly related to morphology, might not be easily reversible, and this might therefore influence the rate of decay of some of the bone properties. The present study, therefore, attempts to examine the effects of 12 and 24 weeks of cessation of exercise on the pattern of decay of bone gains accrued during 8 weeks of jumping exercise in rats.
Methods
Animal grouping and exercise regimen
Ninety, 12-week-old, Wistar–Kyoto female rats with a mean initial body weight of 181.0 ± 14.6 g, were randomly divided into nine equal groups that consisted of: baseline control (C); 8 weeks sedentary control (8S) (sedentary, free cage activity); 8 weeks of jumping exercise (8Ex); 20 weeks sedentary control (20S) (S, free cage activity); 8 weeks of jumping exercise followed by 12 weeks of sedentary activity (8Ex12S); 20 weeks of continuous jumping exercise (20Ex); 32 weeks sedentary control (32S); 8 weeks of jumping exercise followed by 24 weeks of sedentary activity (8Ex24S); 32 weeks of continuous jumping exercise (32Ex) (Fig. 1). The total length of exercise period for 8Ex, 20Ex and 32Ex was 8, 20 and 32 weeks, respectively. The cessation period for 8Ex12S and 8Ex24S was 12 weeks and 24 weeks, respectively. Jumping exercise (Ex) consisted of 40 jumps day−1 (40 J/d) for 5 days week−1 (5 d/w), with a jumping height of 40 cm. All animals were housed in cages (55 cm × 33 cm × 19 cm) with five animals per cage. They were exposed to a constant 12:12 light:dark cycle and had ad libitum access to water and chow (Gold Coin, Port Klang, Malaysia) throughout the study. Rats in the baseline control (C) group were sacrificed on day 1 of the study. Rats in groups 8S and 8Ex were euthanised at the end of the eighth week. Rats in groups 24S, 8Ex12S and 20Ex were sacrificed at the end of the 20th week, and rats in groups 32S, 8Ex24S and 32Ex were sacrificed at the end of the 32nd week. On the day of sacrifice, the rats were weighed (Ohaus Navigator™ Balance, USA) to obtain the final body weight before being lightly anaesthetised with diethyl ether for decapitation using an animal guillotine (Scientific Research Instruments, UK). Bone harvesting was then carried out for subsequent analysis. The Animal Research Ethics Committee of Universiti Sains Malaysia approved the study and experimental protocol.
Fig. 1.
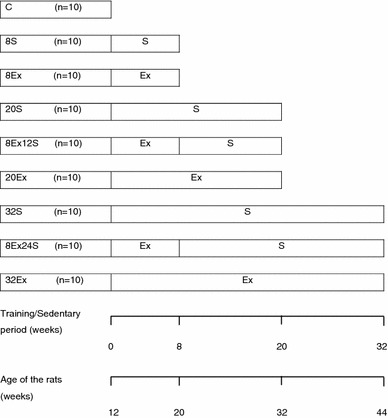
The experimental design of the study, including the training and sedentary periods, and age of the rats. The abbreviations denote the following groups: baseline control (C), 8 weeks sedentary control (8S), 8 weeks of jumping exercise (8Ex) for 5 d/w (d/w = number of days per week) at 40 J/d (J/d = number of jumps per day), 20 weeks sedentary control (20S), 8 weeks of jumping exercise followed by 12 weeks sedentary (8Ex12S), 20 weeks of jumping exercise at 5 d/w and 40 J/d (20Ex), 32 weeks sedentary control (32S), 8 weeks of jumping exercise followed by 24 weeks sedentary (8Ex24S), 32 weeks of jumping exercise at 5 d/w and 40 J/d (32Ex). S sedentary, Ex exercise, number before S or Ex number of weeks of sedentary or exercise. These abbreviations will be used in all the other figures and tables in this study
Jump training
The intensity and frequency of the exercise consisted of 40 jumps per day and for 5 days per week (40 J/d, 5 d/w) for either 8, 20 or 32 weeks, using a previously described protocol [14, 15]. Briefly, each rat in the jumping exercise groups was placed at the bottom of a specially designed wooden box, measuring 30.5 cm × 30.5 cm and 40 cm in length, width and height, respectively. The jumping exercise was initiated by applying an electrical current to the wired floor (electrical grid) of the box through a stimulator. When stimulated, each rat jumped from the floor of the box to catch the top edge of the box with its forepaws. The rat was then immediately returned to the floor of the box to repeat the procedure. The time required per jump was about 4 s. After a few days of training, the rats jumped without electrical stimulation. Rats in the sedentary groups were not given any electrical stimulus but to mimic the stress induced by handling, before and after jumping exercise, the sedentary rats were also just handled for the duration of the study.
Measurements of bone length and maximum diameter
Immediately after the rats were euthanised, the tibias and fibulas were dissected from the right hind limbs. After careful removal of the soft tissue, the fibula was separated from the tibia. The length and maximum diameter of the tibia were measured with a digital sliding caliper (ASM, Series 600; Germany). Tibial length, to the nearest 0.01 mm, was measured from the top of the tibial head (medial condyle) to the distal point of the tibia (medial malleolus) using a digital sliding caliper (ASM). At the mid-point of the tibial length, tibial maximum diameter to the nearest 0.01 mm was then measured. Tibial maximum diameter was measured from anterior to posterior regions of the bone. The bones (tibias and fibulas) were then wrapped in saline-soaked gauze pads, to prevent dehydration, and put into labeled plastic bags and stored at −80°C (Heto Ultra Freezer 3410; Denmark) for the measurement of mechanical property and morphological characteristics at a later date. This method of storing has been shown not to affect biomechanical properties of bone [13].
Measurement of bone mechanical property
On the day of mechanical testing, the stored tibias were thawed for approximately 1 h at room temperature, and then soaked in 0.9% saline as described earlier [16]. They were then wiped dry and each tibia was loaded onto an electromechanical testing system (Bone Strength Tester; TK-252C; Muromachi Kikai, Japan), and subjected to a three-point bending until fracture for the determination of bone ultimate bending force as previously described [14, 15]. The distance between the two bottom supports of the tester was set 16 mm apart, and the cross-head speed was set at 10 mm min−1. After positioning each bone on the support, a force was applied to the tibial mid-shaft from lateral to the medial surface. The ultimate bending force was then obtained from the load-deformation curves that were continuously recorded by a computer linked to the load tester.
Bone mass and morphometric properties measurements
After estimation of the mechanical properties, the broken tibias together with the fibulas were then immersed in chloroform: methanol solvent (2:1 by volume, respectively) for 1 week to remove the fat from the bones [14, 15]. Following which, the bones were oven-dried at 80°C for 24 h (Isuzu Model 2-2020; Isuzu Seisakusho, Japan). After drying, fat-free dry weight to the nearest 0.01 mg was determined on an electronic balance (ER-180A; A&D, Japan). For mid-shaft cross-sectional morphometry measurements, broken tibias were bonded at the site of breakage with bonding glue, and then embedded in synthetic resins (Rigolac 2004, Promoter E and Permeck N hardeners; Okenshoji, Tokyo, Japan) by submersion at room temperature for 1 week. The mid-shaft of each tibia in the resin-filled tube was then cut into 1-mm-thick cross sections, about 1 mm distal to the site of the fracture, with an electrical rotary saw (Maruto, Japan). The bone segments were then magnified 50 times on a microscope projector (Nikon Profile Projector, V12, Japan) and the bone images were traced. A digitising pad (MyPad-A3 Logitec Digitiser, Model K-510mk2; Japan) connected to a computer, installed with the appropriate software [NECN-88 Basic (86) Version 6.0], was used to measure the tibial mid-shaft cross-sectional periosteal and endosteal perimeters, and cortical and medullary areas. These were made by tracking a digitising pen over the magnified bone tracings of the outer and inner bone perimeters.
Statistical analysis
Statistical tests contained in the Statistical Package for Social Sciences (SPSS) v.10.0 were used for statistical analysis. All data are reported as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to determine the significance of the differences between groups. When the one-way ANOVA revealed a significant difference, post hoc (least significant difference test) was used to determine the differences between specific means. Pearson correlation coefficient was performed to explore the relationship between the various measured parameters. A significant level of p < 0.05 was used for all the comparisons.
Results
In the present study, a particular bone gain was considered maintained if the percentage difference between 8Ex12S or 8Ex24S group and their age-matched sedentary controls was at least equal to or more than the percentage difference observed between 8Ex and 8S.
Body weight, tibial length and mid shaft maximum diameter
Analysis of the data revealed that there were no significant differences in final body weight between the exercised rats and their age-matched sedentary controls (Table 1).
Table 1.
Final body weight and bone physical dimensions: means final body weight, tibial length and maximum diameter at the mid shaft of the right tibias of the rats
| Groups | No. of rats (n) | Age of rats at sacrifice (weeks) | Initial body weight (g) | Final body weight (g) | Tibial length (mm) | Tibial maximum diameter (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | |||||
| C | 10 | 12 | 184.9 ± 17.3 | 184.9 ± 17.3 | 35.92 ± 1.17 | 2.52 ± 0.14 | ||||
| 8S | 10 | 20 | 179.9 ± 12.0 | 225.2 ± 21.3 | 37.94 ± 1.08 | +5.62 | 2.62 ± 0.15 | +3.97 | ||
| 8Ex | 10 | 20 | 181.4 ± 18.6 | 227.3 ± 16.8 | 38.25 ± 0.57 | +0.8 | +6.49 | 2.80 ± 0.10a | +6.9 | +11.11 |
| 20S | 10 | 32 | 185.2 ± 22.2 | 244.0 ± 26.0 | 38.32 ± 1.19 | +6.68 | 2.61 ± 0.26 | +3.57 | ||
| 8Ex12S | 10 | 32 | 180.9 ± 13.0 | 249.0 ± 26.0 | 38.43 ± 1.28 | +0.3 | +6.99 | 2.81 ± 0.21a | +7.7 | +11.51 |
| 20Ex | 10 | 32 | 180.6 ± 17.3 | 258.1 ± 23.6 | 39.11 ± 0.82 | +2.1 | +8.88 | 2.92 ± 0.13a | +11.9 | +15.87 |
| 32S | 10 | 44 | 178.4 ± 8.6 | 257.8 ± 15.1 | 38.84 ± 1.41 | +8.13 | 2.66 ± 0.17 | +5.56 | ||
| 8Ex24S | 10 | 44 | 179.0 ± 10.5 | 270.8 ± 13.9 | 39.12 ± 0.72 | +0.7 | +8.91 | 2.80 ± 0.10 | +5.3 | +11.11 |
| 32Ex | 10 | 44 | 179.4 ± 10.8 | 264.4 ± 18.5 | 38.92 ± 0.84 | +0.2 | +8.35 | 2.99 ± 0.31a,b | +12.4 | +18.85 |
Values are means ± SD
a p < 0.05 compared to their age-matched sedentary controls
b p < 0.05 compared to 8Ex24S
Tibial length was greater with each increasing age category in all the groups. There was, however, no significant difference in mean tibial length between the exercised rats and age-matched sedentary controls (Table 1). Tibial maximum diameter was also larger with each increasing age category of rats in all the groups. Mean tibial maximum diameters in the exercised groups, however, were significantly greater than those in their respective sedentary controls (p < 0.05) (Table 1). In rats that were deconditioned for 12 weeks (8Ex12S), tibial maximum diameter was still significantly larger than that in the 20S group (+7.7%; p < 0.01). No significant difference in mean maximum tibial diameter was evident between rats that were deconditioned for 24 weeks (8Ex24S) and their age-matched sedentary controls (32S), although the mean was still slightly greater in the former. The difference was nevertheless lower than the difference between 8S and 8Ex (+5.3 vs. +6.9%; Table 1). Body weight increased significantly with age in all rats and the increases in body weight were, however, not significantly different between the exercised rats and their age-matched sedentary controls (Table 1). Similarly, age-related increase in tibial length was not different between the three groups (Table 1).
Tibial fat-free dry weight and mechanical property
Tibial fat-free dry weight was greater with each increasing age category in both the groups (Table 2). In addition, tibial fat-free dry weight was significantly greater in groups that had continued to exercise (20Ex and 32Ex) when compared to their age-matched sedentary controls (20S and 32S, respectively). Tibial fat-free dry weight was significantly lower in rats that were deconditioned for 12 (8Ex12S) and 24 (8Ex24S) weeks when compared to the groups that continued to exercise (20Ex and 32Ex, respectively), but it was not significantly different from their respective sedentary controls (20S and 32S, respectively).
Table 2.
Bone mass and mechanical properties: means fat-free dry weight and ultimate bending force of the right tibias of the rats
| Groups | Tibial fat-free dry weight (mg) | Tibial ultimate bending force (N) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | |
| C | 315.91 ± 22.68 | 72.10 ± 7.08 | ||||
| 8S | 393.25 ± 42.49 | +24.48 | 91.60 ± 14.08 | +27.05 | ||
| 8Ex | 424.47 ± 27.57a | +7.9 | +34.36 | 103.20 ± 9.31a | +12.7 | +43.13 |
| 20S | 417.86 ± 48.51 | +32.27 | 90.70 ± 13.87 | +25.80 | ||
| 8Ex12S | 424.54 ± 55.14 | +1.6 | +34.39 | 103.70 ± 14.28a | +14.3 | +43.83 |
| 20Ex | 480.97 ± 35.93a,b | +15.1 | +52.25 | 122.90 ± 13.25a,b | +35.5 | +70.46 |
| 32S | 430.04 ± 30.00 | +36.13 | 104.60 ± 12.39 | +45.08 | ||
| 8Ex24S | 452.67 ± 24.75 | +5.3 | +43.29 | 110.70 ± 14.17 | +5.8 | +53.54 |
| 32Ex | 497.51 ± 50.09a,c | +15.7 | +57.48 | 126.10 ± 16.56a,c | +20.6 | +74.90 |
Values are means ± SD
a p < 0.05 compared to their age-matched sedentary controls
b p < 0.05 compared to 8Ex12S
c p < 0.05 compared to 8Ex24S
Mean tibial ultimate bending force, an indicator of bone strength, was greater with each increasing age category of rats in both the groups (Table 2). It was also significantly greater in all exercised rats when compared to their sedentary controls. Mean tibial ultimate bending force was significantly greater in 20Ex and 32Ex groups when compared to that in the deconditioned groups (8Ex12S and 8Ex24S groups, respectively). Ultimate bending force was also significantly greater in rats kept sedentary for 12 weeks after 8 weeks of exercise (8Ex12S) when compared to that in their sedentary controls (20S). The fractional difference was slightly greater than that between 8S and 8Ex. Mean ultimate bending load in rats kept sedentary for 24 weeks after 8 weeks of exercise (8Ex24S) was not significantly different from their age-matched sedentary controls (32S). Moreover, the fractional difference was also smaller than that observed between 8S and 8Ex (Table 2). Although fat-free dry weight and ultimate bending force increased in all rats with age, the increases were, however, greater in exercised and deconditioned rats than those in the sedentary control rats (Table 2).
Tibial mid-shaft cross-sectional morphometric properties
Schematic illustration of bone morphometry of all the experimental groups is presented in Fig. 2. Mean cross-sectional perimeters, and cortical and medullary areas of the mid-shaft of the tibias of the various groups are presented in Table 3. Mean tibial periosteal perimeter was greater with each increasing age category of rats. It was also significantly greater in the exercised rats, being highest in the 32Ex group, when compared to that in age-matched sedentary controls. Mean tibial periosteal perimeter was significantly greater in the 8Ex12S and 8Ex24S groups when compared to their 20S and 32S sedentary controls, respectively. The fractional differences in mean tibial periosteal perimeter between the deconditioned rats and their age-matched sedentary controls was, however, lower than the mean difference between 8S and 8Ex groups.
Fig. 2.
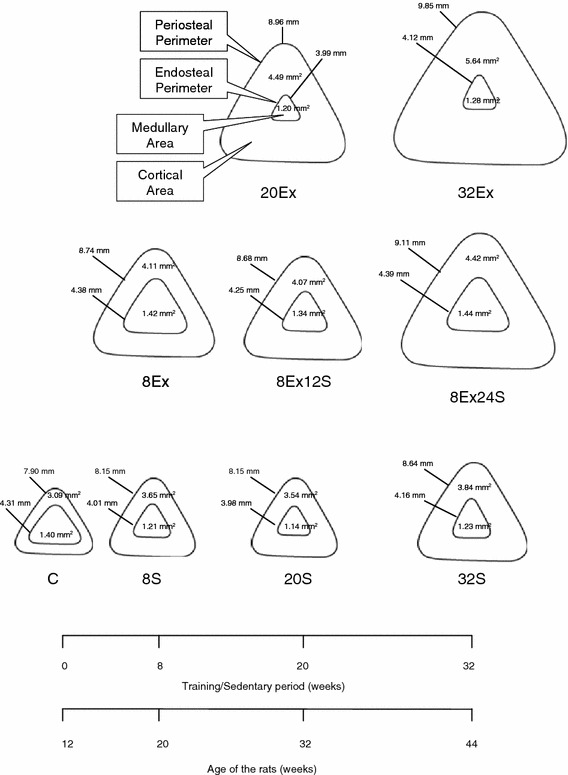
Schematic illustration of bone morphometry of the experimental groups (values were adapted from Table 3)
Table 3.
Bone morphometry: means mid shaft cross-sectional perimeters and areas of the right tibias of the rats
| Groups | Tibial periosteal perimeter (mm) | Tibial endosteal perimeter (mm) | Tibial cortical area (mm2) | Tibial medullary area (mm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | Mean ± SD | Percent difference compared to age-matched sedentary control (%) | Percent difference compared to baseline control (%) | |
| C | 7.90 ± 0.33 | 4.31 ± 0.52 | 3.09 ± 0.21 | 1.40 ± 0.33 | ||||||||
| 8S | 8.15 ± 0.58 | +3.16 | 4.01 ± 0.60 | −6.96 | 3.65 ± 0.37 | +18.12 | 1.21 ± 0.34 | −13.57 | ||||
| 8Ex | 8.74 ± 0.32a | +7.2 | +10.63 | 4.38 ± 0.25a | +9.2 | +1.62 | 4.11 ± 0.21a | +12.6 | +33.01 | 1.42 ± 0.14 | +17.4 | +1.43 |
| 20S | 8.15 ± 0.39 | +3.16 | 3.98 ± 0.27 | −7.66 | 3.54 ± 0.36 | +14.56 | 1.14 ± 0.14 | −18.57 | ||||
| 8Ex12S | 8.68 ± 0.59a | +6.5 | +9.87 | 4.25 ± 0.36 | +6.8 | −1.39 | 4.07 ± 0.50a | +15.0 | +31.72 | 1.34 ± 0.22 | +17.5 | −4.29 |
| 20Ex | 8.96 ± 0.41a | +9.9 | +13.42 | 3.99 ± 0.49 | +0.3 | −7.42 | 4.49 ± 0.59a | +26.8 | +45.31 | 1.20 ± 0.27 | +5.3 | −14.29 |
| 32S | 8.64 ± 0.31 | +9.37 | 4.16 ± 0.36 | −3.84 | 3.84 ± 0.35 | +24.27 | 1.23 ± 0.23 | −12.14 | ||||
| 8Ex24S | 9.11 ± 0.34a | +5.4 | +15.32 | 4.39 ± 0.34 | +5.5 | +1.86 | 4.42 ± 0.30a | +15.1 | +43.04 | 1.44 ± 0.22 | +17.1 | +2.86 |
| 32Ex | 9.85 ± 0.70a,b | +14.0 | +24.68 | 4.12 ± 0.47 | −1.0 | −4.41 | 5.64 ± 0.75a,b | +46.9 | +85.52 | 1.28 ± 0.33 | +4.1 | −8.57 |
Values are means ± SD
a p < 0.05 compared to their age-matched sedentary controls
b p < 0.05 compared to 8Ex24S
Tibial cortical area was greater with each increasing age category of rats and was significantly greater in the exercised groups when compared to their age-matched sedentary control groups. Although mean tibial cortical areas in the 8Ex12S and 8Ex24S groups were lower than those in the 20Ex and 32Ex groups, respectively, they were nevertheless still significantly greater than those in the 20S and 32S groups, respectively. The difference between the deconditioned rats and their respective sedentary controls was also slightly greater than that between 8S and 8Ex (Table 3). Age-related increases in tibial periosteal perimeter and cortical area were evident in all groups, but the increases in these were greater in exercised and deconditioning rats (Table 3).
Relationship between the measured parameters
Pearson correlation coefficient analysis showed that bone ultimate bending force correlated significantly with fat-free dry weight (r = 0.808, p < 0.01), tibial maximum diameter (r = 0.820, p < 0.01), and bone cortical area (r = 0.690, p < 0.05) in rats following 8 weeks of jumping exercise (8Ex). Similarly, 20 weeks of continuous exercise (20Ex) also elicited significant correlations between bone ultimate bending force and fat-free dry weight (r = 0.888, p < 0.01), and tibial maximum diameter (r = 0.759, p < 0.05), respectively. Significant correlations between ultimate bending load and fat-free dry weight (r = 0.929, p < 0.001), maximum tibial diameter (r = 0.741, p < 0.05), and periosteal perimeter (r = 0.720, p < 0.05) were also evident in rats given 32 weeks of continuous exercise (32Ex). In addition, highly significant correlation (r = 0.874, p < 0.01) was also evident between bone ultimate bending load and tibial maximum diameter following 12 weeks of cessation of exercise (8Ex12S) but not after 24 weeks of cessation of exercise (8Ex24S).
In the deconditioned groups, the correlation between bone cortical area and ultimate bending load was reduced from r = 0.672 (p < 0.05) in the rats following 12 weeks of deconditioning (8Ex12S) to r = 0.310 (p = 0.383) in the rats following 24 weeks of deconditioning (8Ex24S).
Discussion
Based on the criterion mentioned earlier, two major observations of note emerged from this study. The first was that, with the exception of bone cortical area, all other measured bone gains achieved during 8-week jumping exercise regimen decayed during 12 and 24 weeks of cessation of exercise. The second notes that the decay in these gains was not uniform, e.g. gain in fat-free dry weight decayed by the 12th week of cessation of exercise, whereas gains in ultimate bending load (bone strength), maximum diameter decayed sometime between the 12th and 24th week of cessation of exercise. Rats that continued to exercise for either 12 or 24 weeks continued to accrue the bone gains.
Decay of exercise-induced bone gains following treadmill exercise in rats has been well documented [5–8, 11–13]. The reason for the decay of bone gains upon cessation of exercise is not clearly understood. It might stem from some evolutionary reasons, including (1) to minimize bone mass, primarily to reduce metabolic costs, as bone tissue is twice as dense when compared to soft tissue and metabolically expensive, and/or (2) to minimize bone flaws as per Weibull theory, which predicts that brittle fracture strength is a function of size, stress distribution, and stress state. Regardless of the reasons, the amount of bone at any one time is usually adjusted through remodelling to a minimum level required to maintain strains at a “set point” value [17, 18]. Although the precise mechanism for this is still unclear, it has nevertheless been hypothesised that the strain applied to the bone is compared to a “set point”, and if the two do not agree, bone remodelling, through adjustment of osteoclast and osteoblast activities, is initiated to correct the strain. This also forms the basis of Frost’s mechanostat theory [19, 20], which describes the relationship between bone strain and bone response in four windows or phases i.e., the ‘disuse window’, where the strain below the set point leads to bone dissolution; the ‘physiological window’ where the strain is sufficient to effectively maintain a balance between bone formation and resorption; the ‘overuse window’ where the strain is sufficiently high to induce net bone formation; and the ‘pathological window’ where there is formation of woven bone. In this study, it seems that during the deconditioning period the strains elicited by daily free cage activity on bone were below the required load or strain (physiological window) for the conservation mode, and the rats were therefore in the relative ‘disuse window’ phase, consequently resulting in poor maintenance of the bone gains acquired during training.
While poor maintenance or retention of exercise-induced bone gains is usually expected during deconditioning, it, however, remains unclear if all the gains in bone properties decay at the same rate. Of the measured parameters, gain in fat-free dry weight was the first to decay completely within 12 weeks of cessation of exercise compared to the other morphological and mechanical adaptations. The mode of decay was not analysed in this study. In the present study, despite the decay in fat-free dry weight, the mechanical and morphological gains, including bone strength, were still maintained after 12 weeks of deconditioning, and only completely decaying sometime between 12 and 24 weeks of deconditioning, with the exception of gains in tibial cortical and medullary areas. Similar decay in exercise-induced gain in bone mass after a period of deconditioning has been reported previously in a number of other studies in the rat, albeit in the femoral neck and shaft [11, 13]. While the cause for the overall decay in bone gains can be attributed to the cessation of exercise, the reason for the variable pattern of decay in the bone gains is unclear. Although the major determinant of bone strength is bone mineral mass or density, there are a number of other factors that probably also contribute independently to bone strength, including organic matrix, structural properties, and bone architecture such as area, diameter and perimeter of structures [21–24]. In this regard, maximum tibial diameter was significantly greater in the 8Ex12S group when compared to the age-matched 20S group, and the percentage difference was also slightly greater than the difference between 8S and 8Ex, suggesting that bone physical characteristics might also contribute to bone strength, as significant high correlations between bone strength, mass and physical dimensions were observed in this study. Collectively, these findings seem to confirm the close relationship between bone strength, mass and morphology, and indicate that increases in bone mass and morphological properties contribute to the increase in bone strength following exercise, and the reason for the delayed decay in bone strength despite a decay in fat-free dry weight is perhaps related to the slower rate of decay in the other bone physical dimensions. The reason for the slower decay in bone morphological gains when compared to the decay in gains in bone mass is unclear. It is possible that some of the morphological gains might not be easily reversible or require a longer time to decay. Clearly, this needs to be further investigated. Two recent jumping studies [25, 26] found that the beneficial effects of training on bone properties were still preserved after 24 weeks of detraining. The reason for the difference between the findings of the present study and these two studies is, however, unclear. The exercise regimen was somewhat similar but it is unclear if the composition of the diet consumed was the same in both the studies. These were, however, not reported in any of the studies.
Comparing the findings of the present animal study with those in some previous human studies [1–4], whilst further confirming that decay in bone gains follows the cessation of exercise, they, however, also suggest that this decay occurs even if growth of bone is still present. The reported duration of between 3 and 13 months of cessation of exercise required for the complete decay in exercise-induced gains in bone mineral content in humans, however, appears a little different. There appears to be a longer duration before the gains completely decay in humans. The reason for this is not immediately evident, but it might be related to the size of the skeleton, subsequent level of weight-bearing activities, age, life-span and perhaps the differences in skeletal growth pattern between humans and rats.
In the present study, it was found that both bone tibial periosteal perimeter and cortical area increased with age in the rats (Fig. 2), and tibial periosteal perimeter was also significantly greater in exercised rats than that in age-matched sedentary and control rats. Mean tibial endosteal perimeter, on the other hand, did not show a significant age-related increase, and while it was significantly higher in 8Ex, when compared to 8S, it was, however, lower in 32Ex when compared to the 32S group, although the difference was not statistically significant. A similar trend was also evident in the tibial medullary area. This is expected as the tibial medullary and cortical areas were calculated from the periosteal and endosteal perimeter measurements. Increases in endosteal perimeter would therefore increase the medullary area, whereas decreases would decrease the medullary area. With regard to tibial endosteal perimeter and the medullary area, it seems that a short duration of exercise in young rats initially causes an increase in these two parameters, but with more prolonged exercise, and as the rat gets older, increases in endosteal perimeter and medullary area become somewhat less. The reason for this is unclear but may reflect the maximum possible changes that could occur in bone following exercise.
The greater mean tibial cortical area in exercised rats was probably due to the expanding periosteal perimeter and slightly decreasing endosteal perimeter and medullary area. Overall, 12 or 24 weeks of deconditioning does not seem to have affected tibial medullary or cortical areas, as the gain, particularly in the latter, was still present despite 24 weeks of deconditioning. This could be because the jumping exercise-induced change in periosteal perimeter is either not reversible or requires a lot longer duration for it to decay. Whether the retained cortical area contributes to the delayed decay in bone strength in the present study is unclear, but, interestingly, the relationship between cortical area and ultimate bending load was found to be reducing when deconditioning period was extended from 12 to 24 weeks in the present study. It seems that bone ultimate bending load (strength), particularly when measured with force applied to the mid-shaft, might have become less related to bone cortical area after a longer period of deconditioning. Whether a similar finding between bone strength and cortical area will be evident if bone strength was measured using the longitudinal press method is unclear.
Conclusion
In conclusion, it appears that 8-week jumping exercise regimen (8Ex), at 40 jumps day−1 for 5 days week−1, results in significant gains in a number of parameters in the tibias of rats. However, most of these bone gains decay with cessation of exercise but the decay is not uniform or equal for all parameters. Gains in ultimate bending load, maximum diameter and cortical area are maintained for a longer period than gains in fat-free dry weight. The precise reason for this non-uniform decay of these parameters is unclear. While bone density measurements, and perhaps more measurements of bone strength, are required to confirm our observations, our data nevertheless seem to suggest the need for measurement of a number of bone parameters when assessing for benefits of exercise or consequences of decreased exercise on bone.
Acknowledgments
This work was supported by a grant from the Ministry of Science, Technology and the Environment, Malaysia (Project number: 06-02-05-2159 EA 009) and Universiti Sains Malaysia, Malaysia (Grant number: 304/PPSP/6131355).
Conflict of interest
None.
References
- 1.Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ., Jr Weight bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med. 1988;108:824–828. doi: 10.7326/0003-4819-108-6-824. [DOI] [PubMed] [Google Scholar]
- 2.Iwamoto J, Takeda T, Ichimura S. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J Orthop Sci. 2001;6:128–132. doi: 10.1007/s007760100059. [DOI] [PubMed] [Google Scholar]
- 3.Vuori I, Heinonen A, Sievänen H, Kannus P, Pasanen M, Oja P. Effects of unilateral strength training and detraining on bone mineral density and content in young women: a study of mechanical loading and deloading on human bones. Calcif Tissue Int. 1994;55:59–67. doi: 10.1007/BF00310170. [DOI] [PubMed] [Google Scholar]
- 4.Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000;15:2495–2503. doi: 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto J, Yeh JK, Aloia JF. Effect of deconditioning on cortical and cancellous bone growth in the exercise trained young rats. J Bone Miner Res. 2000;15:1842–1849. doi: 10.1359/jbmr.2000.15.9.1842. [DOI] [PubMed] [Google Scholar]
- 6.Shimamura C, Iwamoto J, Takeda T, Ichimura S, Abe H, Toyama Y. Effect of decreased physical activity on bone mass in exercise-trained young rats. J Orthop Sci. 2000;7:358–363. doi: 10.1007/s007760200060. [DOI] [PubMed] [Google Scholar]
- 7.Yeh JK, Aloia JF. Deconditioning increases bone resorption and decreases bone formation in the rat. Metabolism. 1990;39:659–663. doi: 10.1016/0026-0495(90)90036-C. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Wang XX, Higuchi M, Yamada K, Ishimi Y. High bone mass gained by exercise in growing male mice is increased by subsequent reduced exercise. J Appl Physiol. 2004;97:806–810. doi: 10.1152/japplphysiol.01169.2003. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Umemura Y, Honda A, Nagasawa S. Maintenance of bone mass and mechanical properties after short-term cessation of high impact exercise in rats. Int J Sports Med. 2002;23:1–5. doi: 10.1055/s-2002-20128. [DOI] [PubMed] [Google Scholar]
- 10.Kiuchi A, Arai Y, Katsuta S. Detraining effects on bone mass in young male rats. Int J Sports Med. 1998;19:245–249. doi: 10.1055/s-2007-971912. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen TL, Pajamäki I, Sievänen H, Vuohelainen T, Tuukkanen J, Järvinen M, Kannus P. Femoral neck response to exercise and subsequent deconditioning in young and adult rats. J Bone Miner Res. 2003;18:1292–1299. doi: 10.1359/jbmr.2003.18.7.1292. [DOI] [PubMed] [Google Scholar]
- 12.Kannus P, Järvinen TLN, Sievänen H, Kvist M, Rauhaniemi J, Maunu V, Hurme T, Jozsa L, Järvinen M. Effects of immobilization, three forms of remobilization, and subsequent deconditioning on bone mineral content and density in rat femora. J Bone Miner Res. 1996;11:1339–1346. doi: 10.1002/jbmr.5650110919. [DOI] [PubMed] [Google Scholar]
- 13.Pajamäki I, Kannus K, Vuohelainen T, Sievänen H, Tuukkanen J, Järvinen M, Järvinen TL. The bone gain induced by exercise in puberty is not preserved through a virtually life-long deconditioning: a randomized controlled experimental study in male rats. J Bone Miner Res. 2003;18:544–552. doi: 10.1359/jbmr.2003.18.3.544. [DOI] [PubMed] [Google Scholar]
- 14.Umemura Y, Ishiko T, Tsujimoto H, Miura H, Mokushi N, Suzuki H. Effect of jump training on bone hypertrophy in young and old rats. Int J Sports Med. 1995;16:364–367. doi: 10.1055/s-2007-973021. [DOI] [PubMed] [Google Scholar]
- 15.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12:1480–1485. doi: 10.1359/jbmr.1997.12.9.1480. [DOI] [PubMed] [Google Scholar]
- 16.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 17.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 18.Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci. 1998;3:346–355. doi: 10.1007/s007760050064. [DOI] [PubMed] [Google Scholar]
- 19.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 20.Frost HM. Perspectives: bone’s mechanical usage windows. Bone Miner. 1992;19:257–271. doi: 10.1016/0169-6009(92)90875-E. [DOI] [PubMed] [Google Scholar]
- 21.Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;12:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- 22.Frost HM. Strain and other mechanical influences on bone strength and maintenance. Curr Opin Orthop. 1997;8:60–70. doi: 10.1097/00001433-199710000-00010. [DOI] [Google Scholar]
- 23.Kimmel DB. A paradigm for skeletal strength homeostasis. J Bone Miner Res. 1993;8:S515–S522. doi: 10.1002/jbmr.5650081317. [DOI] [PubMed] [Google Scholar]
- 24.Orwoll ES. Towards an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18:949–954. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- 25.Honda A, Sogo N, Nagasawa S, Kato T, Umemura Y. Bone benefits gained by jump training are preserved after detraining in young and adult rats. J Appl Physiol. 2008;105:849–853. doi: 10.1152/japplphysiol.00902.2007. [DOI] [PubMed] [Google Scholar]
- 26.Umemura Y, Nagasawa S, Sogo N, Honda A. Effects of jump training on bone are preserved after training, regardless of estrogen secretion state in rats. J Appl Physiol. 2008;104:1116–1120. doi: 10.1152/japplphysiol.00937.2007. [DOI] [PubMed] [Google Scholar]


