Abstract
Inactivation of poly(ADP-ribose) polymerase 1 (PARP1) has been found to be protective in several disease models; however, the role of PARP1 in acute kidney injury-induced interstitial fibrosis has not been studied. Herein, we tested whether PARP1 inactivation by treatment with PJ34 (a PARP1 inactivator; 10 mg/kg body weight/day, intraperitoneal implantation of a miniosmotic pump at 2 days after the onset) contributed to the decrease in interstitial fibrosis induced by ischemia-reperfusion injury (IRI) in mouse kidneys. IRI increased PARP1 activation represented by poly(ADP-ribose) expression from 4 to 16 days postinjury, whereas treatment with PJ34 at 2 days after the onset efficaciously abolished the increase in PARP1 activation at 4, 8 and 16 days after IRI. Pharmacological inactivation of PARP1 significantly reduced interstitial fibrosis as represented by the collagen deposition and transforming growth factor-β1 level at 8 and 16 days after IRI. Consistent with collagen deposition, myofibroblast activation represented by α-smooth muscle actin expression was also reduced by PARP1 inactivation at 8 and 16 days after IRI. Furthermore, IRI enhanced macrophage influx, but PARP1 inactivaton remarkably reduced macrophage influx for 4 through 16 days after the injury. Among the chemoattractants for monocytes/macrophages and neutrophils, monocyte chemotactic protein-1 (MCP-1) production in IRI kidneys was significantly reduced by PARP1 inactivation from 4 to 16 days postinjury. These data demonstrate that PARP1 activation contributes to IRI-induced MCP-1 production and in turn to macrophage influx, resulting in the promotion of interstitial fibrosis.
Keywords: Poly(ADP-ribose) polymerase 1, Ischemia-reperfusion injury, Acute kidney injury, Interstitial fibrosis, Chronic kidney injury, Inflammation, Collagen, Macrophage, Monocyte chemotactic protein-1, PJ34
Introduction
Incomplete restoration of acute kidney injury (AKI) has a risk of progression to chronic kidney disease (CKD) and even end-stage renal disease [1]. However, the mechanisms of the relationship between AKI and CKD remain poorly defined. The presence of interstitial fibrosis is a key structural component of CKD and a major cause of CKD [2]. Several molecules induced by ischemia-reperfusion injury (IRI) are implicated in injury and inflammation as well as in repair. These molecules include various cytokines/chemokines, growth factors, angiogenic factors and the renin-angiotensin system [3–5]. The interaction between these molecules and their downstream signaling pathways in the injured or regenerating tubular epithelium, capillary and interstitial cells could evoke inflammation, myofibroblast activation, fibroblast proliferation and extracellular matrix deposition. However, pharmacological intervention based on the function of identified molecules is inadequate; hence, the development of effective drugs remains an elusive goal.
Poly(ADP-ribose) polymerase 1 (PARP1) is a nuclear protein that can regulate gene expression as a transcriptional coactivator and protein functions via poly(ADP-ribosyl)ation [6, 7]. Poly(ADP-ribosyl)ation can have different effects on various proteins including activation, downregulation, changes in protein conformation and promotion of protein-protein interactions [8]. Additionally, the role of PARP1 as a transcriptional regulator is confirmed by pharmacological inhibition studies, demonstrating its influence on the expression of inflammatory genes [9–11]. In previous studies, we and other investigators have demonstrated that pharmacological or genetic inhibition of PARP1 protects kidneys against IRI, cisplatin nephrotoxicity and ureteral obstruction [12–15]. Given the pronounced effect of PARP1, we sought to determine whether PARP1 activation induced by ischemic AKI contributed to interstitial fibrosis. Especially since respective PARP1 deficiency and preconditioning treatment of a PARP1 inhibitor protect against renal IRI [12, 13], their approaches are not adequate for an IRI-induced CKD model. Therefore, we investigated the effect of postconditioning treatment with a PARP1 inhibitor at the beginning of interstitial fibrosis after acute IRI.
Materials and methods
Mice and surgical preparation
Male C57BL/6 mice aged 8–10 weeks were purchased from Orient Bio (Seongnam, Republic of Korea). All mouse experiments were performed in accordance with the animal protocols approved by the Institutional Animal Care and Use Committee of Jeju National University. Mice were subjected to 30 min of left renal ischemia followed by 4, 8 or 16 days of reperfusion or sham operation under anesthesia through an intraperitoneal injection of a cocktail containing ketamine (200 mg/kg body weight) and xylazine (16 mg/kg body weight), as previously described [16]. Sham-operated mice underwent the same surgical procedure except for the induction of ischemia. Mice were given PJ34 (R&D Systems, 10 mg/kg body weight per day [14]) for inhibition of PARP1 or 0.9 % saline via intraperitoneal implantation of the miniosmotic pump (Alzet, Palo Alto, CA) at 2 days after IRI or sham operation.
Collagen deposition
Collagen deposition was assessed by both Sirius Red staining and hydroxyproline assay as previously described [15].
Histology
Immunohistochemical and immunofluorescent stains of the kidneys were performed on paraffin sections as previously described [17]. Briefly, 4 % paraformaldehyde-fixed kidney sections were rehydrated and labeled with antibodies against F4/80 (Abcam, Cambridge, MA) and α-smooth muscle actin (α-SMA; Sigma, St. Louis, MO). The sections were then incubated with peroxidase- or FITC-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA). The F4/80- or α-SMA-positive area was measured in ten randomly chosen high-power (×200 magnification) fields per kidney using NIH Image J software.
Western blot
We performed electrophoresis of protein extracts derived from whole kidneys using a Tris-glycine buffer system and subsequent blotting as previously described [18]. Membranes were incubated with antibodies against poly(ADP-ribose) (PAR; BD Pharmingen, San Jose, CA) and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Peroxidase-conjugated secondary antibodies (Vector Laboratories) were applied, and a chemiluminescence reagent (PerkinElmer, Boston, MA) was used to detect proteins. The bands were quantified using Lab Works analysis software (Ultra-Violet Products, Cambridge, UK).
Elisa
The levels of transforming growth factor-β1 (TGF-β1), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), interferon-γ-inducible protein-10 (IP-10), lipopolysaccharide-induced CXC chemokine (LIX) and keratinocyte chemoattractant (KC) in whole kidneys were measured using a multiplex immunoassay (Millipore, Bedford, MA).
Renal function
Renal function was assessed by plasma concentrations of creatinine and blood urea nitrogen (BUN) using QuantiChromTM Creatinine and Urea Assay kits (BioAssay Systems, Hayward, CA), respectively. Blood samples were taken from the retroocular vein plexus at 24 h after contralateral nephrectomy.
Statistical analyses
Analysis of variance was used to compare data among groups. Differences between the two groups were assessed by two-tailed unpaired Student’s t test. P values less than 0.05 were considered statistically significant.
Results
IRI induces PARP1 activation in mouse kidneys
To study the role of PARP1 in interstitial fibrosis induced by IRI, we first assessed PARP1 activation. Because catalytic activation of PARP1 adds PAR polymers to itself, the assessment of PARP1 activation was accomplished by Western blot analysis of the total poly(ADP-ribosyl)ated PARP1 level. PARP1 activation at approximately 116 kDa was increased at 4, 8 and 16 days after IRI compared to that in sham kidneys (Fig. 1). However, treatment with PJ34, a PARP1 inactivator, abolished the increase in PARP1 activation during reperfusion after ischemia (Fig. 1), indicating that the pharmacological inactivation of PARP1 is efficacious against IRI-induced PARP1 activation.
Fig. 1.
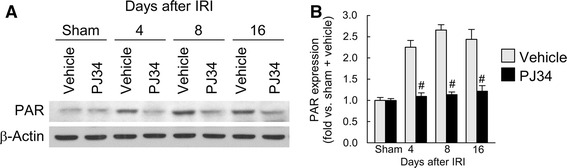
Treatment with PJ34 prevents the increase in PARP activation induced by IRI in mouse kidneys. a PAR expression examined by Western blot analysis using anti-PAR antibody. Anti-β-actin antibody was used as a loading control. b Protein bands were quantified using Lab Works analysis software. Error bars represent SD (n = 5). # P < 0.05 versus vehicle
PARP1 inactivation reduces interstitial fibrosis induced by IRI
To explore whether PARP1 is involved in the pathogenesis of interstitial fibrosis induced by IRI, we measured collagen deposition in mouse kidneys. IRI-subjected kidneys showed a time-dependent increase in the Sirius Red-positive area at 4, 8 and 16 days after onset compared to that in sham kidneys (Fig. 2a, b). However, treatment with PJ34 significantly attenuated the increase in the Sirius Red-positive area at 8 and 16 days after IRI (Fig. 2a, b). Consistent with the results of the Sirius Red stain, IRI augmented the hydroxyproline content in mouse kidneys at 4, 8 and 16 days after onset, but the augmentation of hydroxyproline content was diminished by treatment with PJ34 at 8 and 16 days after IRI (Fig. 2c). Furthermore, treatment with PJ34 significantly reduced TGF-β1 production at 8 and 16 days after IRI (Fig. 2d). In the sham kidneys, the Sirius Red-positive area, hydroxyproline content and TGF-β1 production were not significantly altered between vehicle- and PJ34-treated mice (Fig. 2b, c). These data suggest that PARP1 inactivation reduces interstitial fibrosis at a later stage after IRI.
Fig. 2.
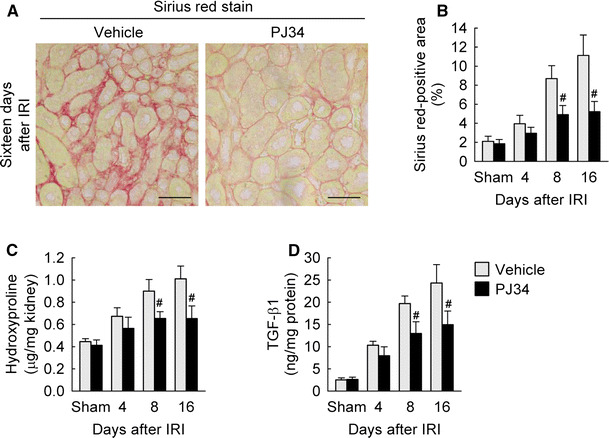
PARP1 inactivation decreases collagen deposition during the period of kidney interstitial fibrosis after IRI. a Collagen deposition using Sirius Red stain in kidney sections at 16 days after IRI. Scale bars indicate 50 μm. b Percentage of Sirius Red-positive area in the kidney sections. c Collagen content represented by hydroxyproline level in the kidneys. d Levels of TGF-β1 protein in the kidneys using a multiplex immunoassay kit. Error bars represent SD (n = 5). # P < 0.05 versus vehicle
PARP1 inactivation reduces myofibroblast activation induced by IRI
Since the α-SMA-positive myofibroblast is one of the principal cells responsible for the production of the extracellular matrix including collagen in renal fibrosis [19, 20], we investigated the magnitude of myofibroblast activation induced by IRI. The percentage of α-SMA-positive area was markedly increased in mouse kidneys at 4, 8 and 16 days after IRI compared to that in sham kidneys, whereas the increase in the α-SMA-positive area was significantly inhibited by treatment with PJ34 at 8 and 16 days after IRI (Fig. 3). In the sham kidneys, the percentage of α-SMA-positive area was not significantly altered between vehicle- and PJ34-treated mice (Fig. 3). These data indicate that PARP1 inactivation reduces α-SMA-positive myofibroblast activation at a later stage of interstitial fibrosis after IRI.
Fig. 3.
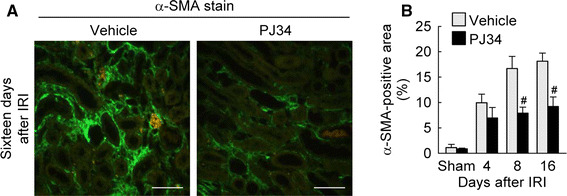
PARP1 inactivation reduces α-SMA expression during the period of kidney interstitial fibrosis after IRI. a Expression of α-SMA was detected by immunofluorescent stain on kidney sections at 16 days after IRI. Scale bars indicate 50 μm. b Percentage of α-SMA-positive area on the kidney sections. Error bars represent SD (n = 5). # P < 0.05 versus vehicle
PARP1 inactivation reduces inflammation induced by IRI
Macrophages play a key role in fibrosis by releasing fibrogenic factors, which stimulate myofibroblast activation and collagen deposition [21]. Here, we tested whether treatment with PJ34 inhibited macrophage influx in fibrotic kidneys after IRI using the immunohistochemical analysis of F4/80-positive macrophages. Four, 8 and 16 days after IRI, prominent infiltration of F4/80-positive macrophages occurred in vehicle-treated mouse kidneys, but PJ34-treated mice showed a significant reduction in F4/80-positive macrophages in IRI-subjected kidneys at 4, 8 and 16 days (Fig. 4). In the sham kidneys, the percentage of F4/80-positive area was not significantly altered between vehicle- and PJ34-treated mice (Fig. 4). These data suggest that PARP1 inactivation reduces inflammation by decreasing macrophage influx during the period of interstitial fibrosis after IRI.
Fig. 4.
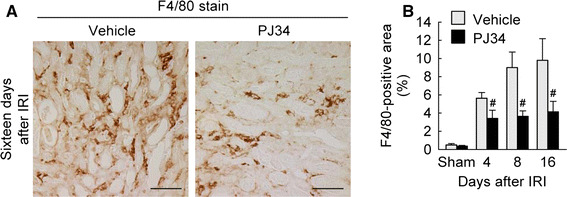
PARP1 inactivation reduces macrophage influx during the period of kidney interstitial fibrosis after IRI. a Macrophages were detected by immunohistochemistry using anti-F4/80 antibody in kidney sections at 16 days after IRI. Scale bars indicate 50 μm. b Percentage of F4/80-positive area in the kidney sections. Error bars represent SD (n = 5). # P < 0.05 versus vehicle
PARP1 inactivation modulates MCP-1 production induced by IRI
To determine whether PARP1 activation contributes to the production of chemokines implicated in macrophage influx, we measured the levels of chemotactic cytokines involved in macrophage influx in mouse kidneys. As demonstrated in Fig. 5a, IRI caused marked increases in levels of MCP-1, MIP-1β and IP-10 protein among the chemoattractants for monocytes/macrophages at 4, 8 and 16 days after onset. Treatment with PJ34 significantly diminished the IRI-induced level of MCP-1, but not that of MIP-1β and IP-10. Additionally, we measured the levels of chemoattractants for neutrophils in mouse kidneys. No significant difference was found in LIX and KC levels in IRI or sham kidneys in mice treated with vehicle or PJ34 (Fig. 5b). These data suggest that PARP1 inactivation modulates MCP-1 production during the period of interstitial fibrosis after IRI.
Fig. 5.
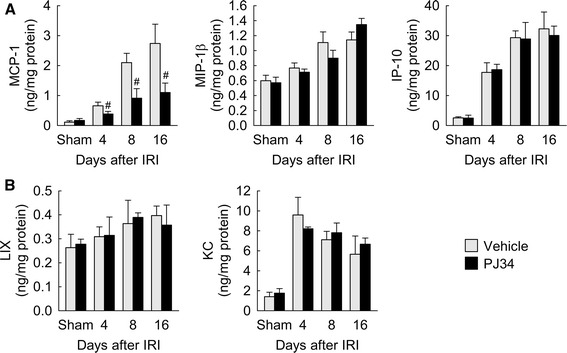
Levels of chemokines during the period of kidney interstitial fibrosis after IRI. a Levels of monocyte/macrophage chemoattractants including MCP-1, MIP-1β and IP-10 protein in the kidneys using a multiplex immunoassay kit. b Levels of neutrophil chemoattractants including LIX and KC protein in the kidneys using a multiplex immunoassay kit. Error bars represent SD (n = 5). # P < 0.05 versus intact
PARP1 inactivation ameliorates renal dysfunction induced by IRI
To assess the renal function, IRI- and sham-subjected mice were used to measure plasma concentrations of creatinine and BUN at 24 h after contralateral nephrectomy. Treatment with PJ34 significantly decreased the levels of plasma creatinine and BUN at 4, 8 and 16 days after IRI (Fig. 6), indicating that PARP1 inactivation ameliorates renal dysfunction during a period of interstitial fibrosis after IRI.
Fig. 6.
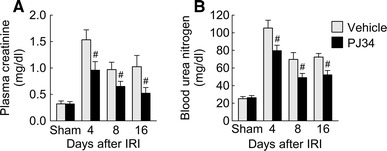
PARP1 inactivation restores renal function during the period of kidney interstitial fibrosis after IRI. Plasma concentrations of creatinine a and blood urea nitrogen b were measured using creatinine and urea assay kits, respectively. Error bars represent SD (n = 5). # P < 0.05 versus vehicle
Discussion
In this study, we have shown that PARP1 plays a role in the pathogenesis of interstitial fibrosis in the IRI model of renal injury, as indicated by the appearance of 2.5-fold increased PARP1 activation. Furthermore, PARP1 inactivation markedly attenuates renal inflammation, especially macrophage influx and MCP-1 production, after 4, 8 and 16 days of IRI, whereas PARP1 inactivation reduces renal fibrosis during the late stage after IRI. Taken together, these results indicate that the prevention of MCP-1-dependent renal inflammation by pharmacological inactivation of PARP1 is the primary pathway leading to attenuated interstitial fibrosis.
Inflammatory response results in overproduction and deposition of collagen, which causes tissue fibrosis. Recent evidence demonstrates upregulation of a variety of chemokines/cytokines including TGF-β1, connective tissue growth factor (CTGF), intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion protein-1 (VCAM-1) during renal fibrosis [22–24]. Interstitial fibrosis is particularly characterized by macrophage accumulation. The influx of macrophages and the macrophage-secreted cytokines including TGF-β promote renal fibrosis [25–27]. In studies from several laboratories including ours, PARP1 has been shown to be involved in the regulation of the inflammatory processes, being functionally associated with recruitment of macrophages and proinflammatory proteins: For example, genetic deletion or pharmacological inhibition of PARP1 suppresses macrophage influx and upregulation of ICAM-1, MCP-1 and inducible nitric oxide synthase (iNOS) in an exacerbated tissue or systemic inflammatory disorder [28–30]. Consistent with previous reports in other disease and tissue models, our present data have demonstrated that the pharmacological inactivation of PARP1 resulted in the inhibition of macrophage influx at 4, 8 and 16 days after IRI, suggesting that endogenous PARP1 actually accelerates renal inflammation from the early stage of renal fibrosis.
MCP-1 is a secreted protein that attracts circulating monocytes and tissue macrophages via interaction with CCR2 [31]. Kidney proximal tubular cells can produce MCP-1 in response to a variety of proinflammatory stimuli [32]. Elements of the diabetic milieu are known to induce MCP-1 mRNA synthesis and protein secretion by cultured renal parenchymal cells [33, 34], suggesting that injured kidney tubular cells can stimulate renal macrophage influx. Genetic or pharmacological inhibition of MCP-1 in type 1 diabetes and crescentic nephritis models also reduces extracellular matrix deposition and TGF-β1 expression, as well as a marked decline in the number of macrophages in mouse kidneys [35–37], suggesting that MCP-1-mediated macrophage influx may cause renal fibrosis. In inflammatory disease models, MCP-1 production is strongly reduced by PARP1 deletion or inactivation [38, 39]. The MCP-1 gene can be transactivated by NF-κB, activator protein-1 or p53 transcription factors [40–42]. Although it has been reported that PARP1 is involved in the regulation of NF-κB and activator protein-1 transcription [43, 44], the role of PARP1 and effect of ADP-ribosylation on functions of transcription factors are not completely understood. In our study, pharmacological inhibition of PARP1 markedly attenuated MCP-1 production from the early stage of interstitial fibrosis after IRI, but only MCP-1 among NF-κB target genes including MIP-1β, IP-10, LIX and KC was reduced by PARP1 inactivation, suggesting that PARP1-mediated MCP-1 transactivation is independent of the NF-κB transcriptional pathway. Taken together, MCP-1 production by PARP activation may cause macrophage influx during the period of interstitial fibrosis after IRI.
In conclusion, this study provides unswerving evidence that PARP1 activation contributes to fibrogenesis in the IRI kidney. Our data implicate PARP1 activation as a trigger of MCP-1 production, macrophage influx and interstitial fibrogenesis. Inhibiting the action of PARP1 might represent a major effective therapeutic strategy to prevent or limit progression of renal fibrogenesis at its onset in IRI-induced CKD.
Acknowledgments
This work was supported by a research grant from the Jeju National University Hospital Research Fund of Jeju National University in 2013 (to J.K.).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the transplant registry: the 2006 annual report of the North American pediatric renal trials and collaborative studies (NAPRTCS) Pediatr Transplant. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol . 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 6.Krishnakumar R, Gamble MJ, Frizzell KM, Berrocal JG, Kininis M, Kraus WL. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–821. doi: 10.1126/science.1149250. [DOI] [PubMed] [Google Scholar]
- 7.Tulin A, Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–562. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- 8.Zaniolo K, Desnoyers S, Leclerc S, Guerin SL. Regulation of poly(ADP-ribose) polymerase-1 (PARP-1) gene expression through the post-translational modification of Sp1: a nuclear target protein of PARP-1. BMC Mol Biol. 2007;8:96. doi: 10.1186/1471-2199-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp C, Warren A, Oshima T, Williams L, Li JH, Alexander JS. Poly ADP ribose-polymerase inhibitors prevent the upregulation of ICAM-1 and E-selectin in response to Th1 cytokine stimulation. Inflammation. 2001;25:157–163. doi: 10.1023/A:1011032313445. [DOI] [PubMed] [Google Scholar]
- 10.Zingarelli B, Cuzzocrea S, Zsengeller Z, Salzman AL, Szabo C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc Res. 1997;36:205–215. doi: 10.1016/S0008-6363(97)00137-5. [DOI] [PubMed] [Google Scholar]
- 11.Zingarelli B, O’Connor M, Hake PW. Inhibitors of poly (ADP-ribose) polymerase modulate signal transduction pathways in colitis. Eur J Pharmacol. 2003;469:183–194. doi: 10.1016/S0014-2999(03)01726-6. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J, Devalaraja-Narashimha K, Singaravelu K, Padanilam BJ. Poly(ADP-ribose) polymerase-1 gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2005;288:F387–F398. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 13.Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1834–R1840. doi: 10.1152/ajpregu.2000.279.5.R1834. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012;82:193–203. doi: 10.1038/ki.2012.64. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011;301:F450–F459. doi: 10.1152/ajprenal.00059.2011. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Padanilam BJ (2014) Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 17.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24:229–242. doi: 10.1681/ASN.2012070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Lim JY, Kim J (2014) Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. Vitro Cell Dev Biol Anim [Epub ahead of print] [DOI] [PubMed]
- 19.Ina K, Kitamura H, Tatsukawa S, Fujikura Y. Significance of alpha-SMA in myofibroblasts emerging in renal tubulointerstitial fibrosis. Histol Histopathol. 2011;26:855–866. doi: 10.14670/HH-26.855. [DOI] [PubMed] [Google Scholar]
- 20.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 22.El Chaar M, Chen J, Seshan SV, Jha S, Richardson I, Ledbetter SR, Vaughan ED, Jr, Poppas DP, Felsen D. Effect of combination therapy with enalapril and the TGF-beta antagonist 1D11 in unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2007;292:F1291–F1301. doi: 10.1152/ajprenal.00327.2005. [DOI] [PubMed] [Google Scholar]
- 23.Shappell SB, Mendoza LH, Gurpinar T, Smith CW, Suki WN, Truong LD. Expression of adhesion molecules in kidney with experimental chronic obstructive uropathy: the pathogenic role of ICAM-1 and VCAM-1. Nephron. 2000;85:156–166. doi: 10.1159/000045649. [DOI] [PubMed] [Google Scholar]
- 24.Yokoi H, Mukoyama M, Sugawara A, Mori K, Nagae T, Makino H, Suganami T, Yahata K, Fujinaga Y, Tanaka I, Nakao K. Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2002;282:F933–F942. doi: 10.1152/ajprenal.00122.2001. [DOI] [PubMed] [Google Scholar]
- 25.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 26.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2008;23:842–852. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 27.Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- 28.Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C. Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165:372–377. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- 29.Mabley JG, Jagtap P, Perretti M, Getting SJ, Salzman AL, Virag L, Szabo E, Soriano FG, Liaudet L, Abdelkarim GE, Hasko G, Marton A, Southan GJ, Szabo C. Anti-inflammatory effects of a novel, potent inhibitor of poly (ADP-ribose) polymerase. Inflamm Res. 2001;50:561–569. doi: 10.1007/PL00000234. [DOI] [PubMed] [Google Scholar]
- 30.Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. Embo J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C–C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992;148:2148–2153. [PubMed] [Google Scholar]
- 33.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 34.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 35.Giunti S, Tesch GH, Pinach S, Burt DJ, Cooper ME, Cavallo-Perin P, Camussi G, Gruden G. Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia. 2008;51:198–207. doi: 10.1007/s00125-007-0837-3. [DOI] [PubMed] [Google Scholar]
- 36.Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, Takahashi T, Abe H, Iehara N, Fukatsu A, Okamoto H, Kita T, Doi T, Arai H. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360:772–777. doi: 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia S, Bodano A, Gonzalez A, Forteza J, Gomez-Reino JJ, Conde C. Partial protection against collagen antibody-induced arthritis in PARP-1 deficient mice. Arthritis Res Ther. 2006;8:R14. doi: 10.1186/ar1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oumouna-Benachour K, Hans CP, Suzuki Y, Naura A, Datta R, Belmadani S, Fallon K, Woods C, Boulares AH. Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: effects on macrophage recruitment, nuclear factor-kappaB nuclear translocation, and foam cell death. Circulation. 2007;115:2442–2450. doi: 10.1161/CIRCULATIONAHA.106.668756. [DOI] [PubMed] [Google Scholar]
- 40.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 41.Finzer P, Soto U, Delius H, Patzelt A, Coy JF, Poustka A, zur Hausen H, Rosl F. Differential transcriptional regulation of the monocyte-chemoattractant protein-1 (MCP-1) gene in tumorigenic and non-tumorigenic HPV 18 positive cells: the role of the chromatin structure and AP-1 composition. Oncogene. 2000;19:3235–3244. doi: 10.1038/sj.onc.1203643. [DOI] [PubMed] [Google Scholar]
- 42.Hacke K, Rincon-Orozco B, Buchwalter G, Siehler SY, Wasylyk B, Wiesmuller L, Rosl F. Regulation of MCP-1 chemokine transcription by p53. Mol Cancer. 2010;9:82. doi: 10.1186/1476-4598-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreone TL, O’Connor M, Denenberg A, Hake PW, Zingarelli B. Poly(ADP-ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J Immunol. 2003;170:2113–2120. doi: 10.4049/jimmunol.170.4.2113. [DOI] [PubMed] [Google Scholar]


