Abstract
Although an acute arterial pressure (AP) elevation induced by intravenous angiotensin II (ANG II) does not inhibit sympathetic nerve activity (SNA) compared to an equivalent AP elevation induced by phenylephrine, there are conflicting reports as to how circulating ANG II affects the baroreflex control of SNA. Because most studies have estimated the baroreflex function under closed-loop conditions, differences in the rate of input pressure change and the magnitude of pulsatility may have biased the estimation results. We examined the effects of intravenous ANG II (10 μg kg−1 h−1) on the open-loop system characteristics of the carotid sinus baroreflex in anesthetized and vagotomized rats. Carotid sinus pressure (CSP) was raised from 60 to 180 mmHg in increments of 20 mmHg every minute, and steady-state responses in systemic AP, splanchnic SNA and heart rate (HR) were analyzed using a four-parameter logistic function. ANG II significantly increased the minimum values of AP (67.6 ± 4.6 vs. 101.4 ± 10.9 mmHg, P < 0.01), SNA (33.3 ± 5.4 vs. 56.5 ± 11.5%, P < 0.05) and HR (391.1 ± 13.7 vs. 417.4 ± 11.5 beats/min, P < 0.01). ANG II, however, did not attenuate the response range for AP (56.2 ± 7.2 vs. 49.7 ± 6.2 mmHg), SNA (69.6 ± 5.7 vs. 78.9 ± 9.1%) or HR (41.7 ± 5.1 vs. 51.2 ± 3.8 beats/min). The maximum gain was not affected for AP (1.57 ± 0.28 vs. 1.20 ± 0.25), SNA (1.94 ± 0.34 vs. 2.04 ± 0.42%/mmHg) or HR (1.11 ± 0.12 vs. 1.28 ± 0.19 beats min−1 mmHg−1). It is concluded that high levels of circulating ANG II did not attenuate the response range of open-loop carotid sinus baroreflex control for AP, SNA or HR in anesthetized and vagotomized rats.
Keywords: Systems analysis, Open-loop gain, Equilibrium diagram, Carotid sinus baroreflex, Rats
Introduction
The arterial baroreflex is an important negative feedback system that stabilizes systemic arterial pressure (AP) during daily activities. The sympathetic arterial baroreflex can be divided into the neural and peripheral arc subsystems [1]. The neural arc characterizes the input–output relation between the baroreceptor pressure input and efferent sympathetic nerve activity (SNA), whereas the peripheral arc defines the input–output relation between SNA and AP. These subsystems operate as a controller and a plant, respectively, in the negative feedback loop. Although the input signal to the neural arc is primarily the absolute input pressure level, the rate of input pressure change [1–3] and the magnitude of pulsatility [4–7] are also important input signals that critically affect the baroreflex function. Many investigators employ pharmacologic interventions, such as intravenous phenylephrine and nitroprusside administration, to estimate baroreflex function under closed-loop conditions. The rate of input pressure change and the magnitude of pulsatility, however, may vary within and between studies, which could bias the estimation results. In addition, experiments performed under baroreflex closed-loop conditions do not usually permit an evaluation of the baroreflex control of AP, because measured AP cannot be separated into signals for the input pressure and output pressure. An open-loop experiment with isolated baroreceptor regions is therefore required to evaluate the baroreflex function precisely.
Angiotensin II (ANG II) can affect the arterial baroreflex by centrally increasing sympathetic outflow, stimulating sympathetic ganglia and the adrenal medulla, and facilitating neurotransmission at sympathetic nerve endings [8]. Although an acute AP elevation induced by intravenous ANG II does not inhibit SNA compared to an equivalent AP elevation induced by phenylephrine, how circulating ANG II affects the baroreflex control of SNA varies among reports, i.e., intravenous ANG has been shown to attenuate [9, 10] or not attenuate [11, 12] the baroreflex control of SNA. Because it is related to the pathologic sympathoexcitation observed in such cardiovascular diseases as chronic heart failure [13], analyzing the effects of circulating ANG II on the baroreflex open-loop system characteristics will deepen our understanding of the pathologic roles of ANG II. In the present study, we examined the effects of intravenous ANG II (10 μg kg−1 h−1 or 167 ng kg−1 min−1) on the open-loop system characteristics of the baroreflex neural and peripheral arcs in anesthetized rats. We hypothesized that ANG II would increase the minimum SNA and attenuate the range of SNA response because the maximum SNA may be saturated. Contrary to our hypothesis, ANG II increased both the minimum and maximum SNA, preserving the range of SNA response controlled by the arterial baroreflex.
Materials and methods
Animals were cared for in strict accordance with the guiding principles for the care and use of animals in the field of physiological sciences, which has been approved by the Physiological Society of Japan. All experimental protocols were reviewed and approved by the Animal Subjects Committee at the National Cardiovascular Center.
Baroreflex open-loop experiment
Male Sprague–Dawley rats (n = 8, 482 ± 14 g body weight, mean ± SE) were anesthetized with an intraperitoneal injection (2 ml/kg) of a mixture of urethane (250 mg/ml) and α-chloralose (40 mg/ml), and mechanically ventilated with oxygen-enriched room air. A venous catheter was inserted into the right femoral vein, and a tenfold dilution of the anesthetic mixture was administered (2 ml kg−1 h−1) to maintain an appropriate level of anesthesia. An arterial catheter was inserted into the right femoral artery to measure AP. A cardiotachometer was used to measure heart rate (HR). Another venous catheter was inserted into the left femoral vein to administer Ringer's solution with or without ANG II.
We exposed a postganglionic branch of the splanchnic nerve through a left flank incision and attached a pair of stainless steel wire electrodes (Bioflex wire AS633, Cooner Wire, CA) to record SNA. The nerve and electrodes were covered with silicone glue (Kwik-Sil, World Precision Instruments, Sarasota, FL) for insulation and fixation. To quantify the nerve activity, the preamplified nerve signal was band-pass filtered at 150–1,000 Hz, and then full-wave rectified and low-pass filtered with a cutoff frequency of 30 Hz. Pancuronium bromide (0.4 mg kg−1 h−1) was administered to prevent muscular activity from contaminating the SNA recording. At the end of the experiment, we confirmed the disappearance of SNA after an intravenous bolus injection of hexamethonium bromide (60 mg/kg) and recorded the noise level.
The vagal and aortic depressor nerves were sectioned at the neck to avoid reflexes from the cardiopulmonary region and aortic arch. The bilateral carotid sinuses were isolated from the systemic circulation according to previously reported procedures [14, 15]. Briefly, a fine needle with a 7-0 polypropylene suture (PROLENE, Ethicon, GA, USA) was passed through the tissue between the external and internal carotid arteries, and the external carotid artery was ligated close to the carotid bifurcation. The internal carotid artery was embolized using two or three bearing balls (0.8 mm in diameter, Tsubaki Nakashima, Nara, Japan), which were injected from the common carotid artery. The isolated carotid sinuses were filled with warmed Ringer's solution through catheters inserted via the common carotid arteries. Carotid sinus pressure (CSP) was controlled using a servo-controlled piston pump. Heparin sodium (100 U/kg) was given intravenously to prevent blood coagulation. Body temperature was maintained at approximately 38°C with a heating pad.
Protocols
Sympathetic nerve activity and AP responses to CSP perturbations were monitored for at least 30 min after the surgical preparation was completed. If these responses became smaller within this period, the animal was discarded from the study. Possible causes for deteriorations in the responses include surgical damage to the carotid sinus nerves and brain ischemia due to bilateral carotid occlusion.
The CSP was decreased to 60 mmHg for 4–6 min, and then increased every minute from 60 to 180 mmHg using 20-mmHg increments. At least four step cycles were performed under control conditions while Ringer’s solution was continuously administered (6 ml kg−1 h−1). After recording the control data, the intravenous Ringer’s solution was replaced with that containing ANG II (167 ng kg−1 min−1). The dose of ANG II was chosen to induce a significant pressor effect based on previous studies [16, 17]. At least three step cycles were performed during ANG II administration.
Data analysis
Data were sampled at 200 Hz using a 16-bit analog-to-digital converter and stored on the hard disk of a dedicated laboratory computer system. To quantify the open-loop static characteristics of the carotid sinus baroreflex, mean values of SNA, AP and HR were calculated during the last 10 s at each CSP level. The effects of ANG II were assessed during the third step cycle after ANG II administration began, at which point the hemodynamic responses to ANG II appeared to reach steady state. Comparisons were made against two control step cycles (control 1 and control 2, see Fig. 1). In each animal, the SNA noise level recorded after the administration of hexamethonium bromide was set to zero. The SNA values obtained at a CSP level of 60 mmHg during control 1 and control 2 were averaged and defined as 100%.
Fig. 1.
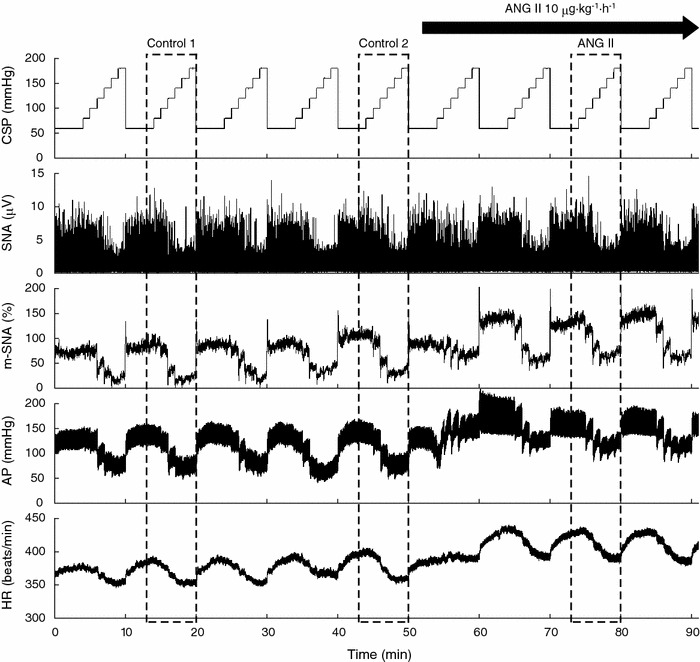
Typical recordings of carotid sinus pressure (CSP), splanchnic sympathetic nerve activity (SNA), the 5-s moving-average signal of the percentage of SNA (m-SNA), systemic arterial pressure (AP) and heart rate (HR). CSP was changed stepwise from 60 to 180 mmHg in 20-mmHg increments every minute. Angiotensin II (ANG II) was administered intravenously while the CSP perturbation was continued. ANG II significantly increased SNA, AP and HR. Reflex responses in SNA, AP and HR were not attenuated in the presence of ANG II. Dashed boxes indicate the step cycles used for the statistical analysis
The open-loop characteristics of the AP, SNA and HR responses as functions of CSP were quantified by fitting a four-parameter logistic function to the obtained data as follows [18]:
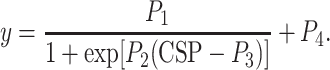 |
where y represents AP, SNA or HR; P 1 is the response range (the difference between the maximum and minimum values of y); P 2 is a slope coefficient; P 3 is the midpoint in CSP; P 4 is the minimum value of y. The maximum gain or maximum slope of the sigmoidal curve was obtained from P 1 P 2/4.
The open-loop characteristics of the baroreflex peripheral arc (i.e., SNA–AP relation) were quantified using linear regression analysis as follows:
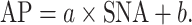 |
where a and b represent the slope and intercept of the regression line, respectively.
Statistical analysis
All parameters were compared among control 1, control 2 and ANG II conditions using repeated-measures analysis of variance [19]. When there was a significant difference among the three conditions, all pairwise comparisons were performed using the Student-Neuman-Keuls test. Differences were considered significant at P < 0.05. All data are expressed as mean and SE values.
Results
Typical experimental recordings are shown in Fig. 1. The stepwise input from 60 to 180 mmHg was imposed repeatedly on CSP. An increase in CSP decreased SNA. m-SNA represents the 5-s moving-average signal of the percentage of SNA. AP and HR were also decreased in response to increases in CSP. After ANG II administration was initiated, the levels of SNA, AP and HR all increased compared to the levels before ANG II administration. The responses in SNA, AP and HR to the CSP input appeared to be preserved. Data obtained from the three boxes with dashed lines (control 1, control 2 and ANG II) were used for the statistical analysis.
The open-loop characteristics of the total baroreflex revealed sigmoidal nonlinearity (Fig. 2a). No significant differences were observed between the two control conditions. ANG II significantly increased the minimum AP without affecting the response range, slope coefficient or midpoint in CSP (Table 1). The maximum gain of the total baroreflex was unchanged. The open-loop characteristics of the baroreflex control of HR also approximated sigmoidal nonlinearity (Fig. 2b), and no significant differences were observed between the two control conditions. ANG II significantly increased the minimum HR without affecting the response range, slope coefficient or midpoint in CSP (Table 1). The maximum slope of the baroreflex control of HR was unchanged.
Fig. 2.
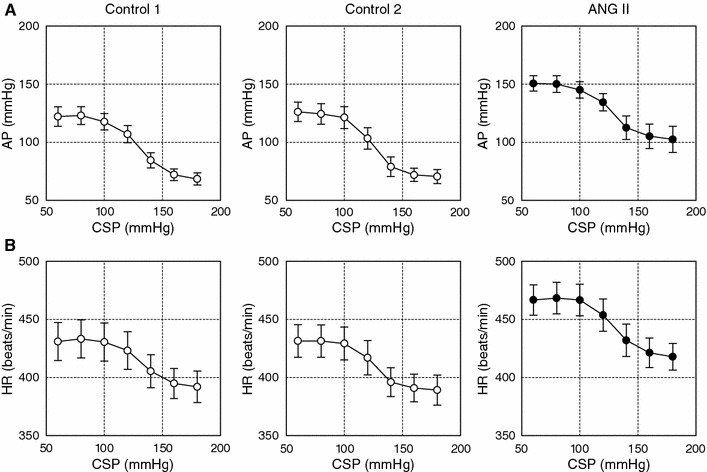
a Averaged input–output relation of the total baroreflex. AP decreased in response to an increase in the CSP. ANG II increased AP, while the range of the AP response was preserved. b Averaged input–output relation of the arterial baroreflex control of HR. HR decreased in response to an increase in the CSP. ANG II increased the HR, while the range of the HR response was preserved
Table 1.
Effects of intravenous angiotensin II (ANG II) on the parameters of logistic functions and regression lines of the open-loop baroreflex characteristics
| Control 1 | Control 2 | ANG II | |
|---|---|---|---|
| Total baroreflex, CSP–AP relation | |||
| P 1 (mmHg) | 56.2 ± 7.2 | 56.3 ± 6.4 | 49.7 ± 6.2 |
| P 2 (mmHg−1) | 0.116 ± 0.019 | 0.118 ± 0.015 | 0.094 ± 0.013 |
| P 3 (mmHg) | 129.2 ± 3.5 | 124.5 ± 2.8 | 125.7 ± 3.2 |
| P 4 (mmHg) | 67.6 ± 4.6 | 69.7 ± 5.8 | 101.4 ± 10.9**,†† |
| Maximum gain | 1.57 ± 0.28 | 1.58 ± 0.22 | 1.20 ± 0.25 |
| Baroreflex control of HR, CSP–HR relation | |||
| P 1(beats/min) | 41.7 ± 5.1 | 43.9 ± 6.2 | 51.2 ± 3.8 |
| P 2 (mmHg−1) | 0.123 ± 0.027 | 0.133 ± 0.018 | 0.099 ± 0.013 |
| P 3 (mmHg) | 131.8 ± 3.8 | 125.8 ± 3.6 | 129.1 ± 2.6 |
| P 4 (beats/min) | 391.1 ± 13.7 | 388.0 ± 12.6 | 417.4 ± 11.5**,†† |
| Maximum slope (beats min−1 mmHg−1) | 1.11 ± 0.12 | 1.39 ± 0.23 | 1.28 ± 0.19 |
| Neural arc, CSP–SNA relation | |||
| P 1 (%) | 69.6 ± 5.7 | 66.5 ± 7.4 | 78.9 ± 9.1 |
| P 2 (mmHg−1) | 0.110 ± 0.016 | 0.124 ± 0.015 | 0.098 ± 0.011 |
| P 3 (mmHg) | 133.2 ± 3.8 | 127.3 ± 3.1 | 126.0 ± 3.4* |
| P 4 (%) | 33.3 ± 5.4 | 35.0 ± 6.4 | 56.5 ± 11.5*,† |
| Maximum slope (%/mmHg) | 1.94 ± 0.34 | 2.02 ± 0.33 | 2.04 ± 0.42 |
| Peripheral arc, SNA–AP relation | |||
| Slope, a (mmHg/%) | 0.85 ± 0.09 | 0.86 ± 0.06 | 0.66 ± 0.10 |
| Intercept, b (mmHg) | 37.8 ± 5.2 | 36.9 ± 5.5 | 68.0 ± 10.6**,†† |
| AP at 100% SNA (mmHg) | 122.7 ± 9.9 | 122.7 ± 7.0 | 134.4 ± 4.9 |
| Operating point | |||
| AP (mmHg) | 111.4 ± 5.0 | 110.3 ± 5.1 | 128.1 ± 4.4**,†† |
| SNA (%) | 90.6 ± 7.4 | 85.8 ± 2.1 | 94.3 ± 5.9 |
Data are mean and SE values
CSP Carotid sinus pressure, AP arterial pressure, HR heart rate, SNA sympathetic nerve activity
* P < 0.05 and **P < 0.01 from control 1, † P < 0.05 and †† P < 0.01 from control 2
The total baroreflex was decomposed into the neural and peripheral arc subsystems. The open-loop characteristics of the baroreflex neural arc revealed sigmoidal nonlinearity (Fig. 3a). There were no significant differences between the two control conditions. ANG II significantly increased the minimum SNA (Table 1). Although the midpoint in CSP was lower in ANG II than in control 1, the difference was not significant when compared with control 2. ANG II did not affect the response range, slope coefficient or the maximum slope of the baroreflex control of SNA. The open-loop characteristics of the baroreflex peripheral arc approximated a straight line (Fig. 3b). There were no significant differences between the two control conditions. ANG II significantly increased the intercept of the regression line (Table 1). AP at 100% SNA did not change significantly, suggesting that the slope of the regression line could be shallower under the ANG II condition. The slope of the regression line, however, was not statistically different among the three conditions.
Fig. 3.
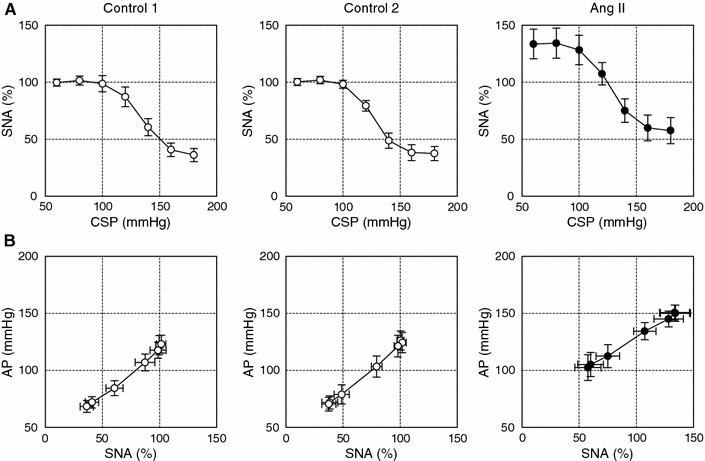
a Averaged input–output relation of the baroreflex neural arc or the arterial baroreflex control of SNA. SNA decreased in response to an increase in the CSP. ANG II increased SNA, while the range of the SNA response was preserved. b Averaged input–output relation of the baroreflex peripheral arc. AP increased in response to an increase in SNA. ANG II increased the AP, an effect that was greater for lower SNA
An equilibrium diagram or a balance diagram was obtained by drawing the neural and peripheral arcs using SNA as the common abscissa and CSP or AP as an ordinate [20–22]. Figure 4 illustrates the equilibrium diagrams under the control 2 (dashed line) and ANG II (solid line) conditions, which were drawn based on the mean parameter values from the logistic function and regression line. Open and filled circles represent the closed-loop operating points under the control 2 and ANG II conditions, respectively. Although AP at the closed-loop operating point was significantly increased by the intravenous ANG II, SNA at the closed-loop operating point was unchanged (Table 1). If ANG II affected the peripheral arc alone, the closed-operating point may have been located at the point depicted by the open triangle. If ANG II affected the neural arc alone, the closed-loop operating point may have been located at the point depicted by the filled triangle.
Fig. 4.
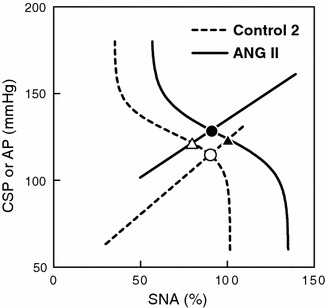
Equilibrium diagrams between the arterial baroreflex neural and peripheral arcs. The dashed and solid curves represent the open-loop characteristics of the baroreflex neural arc under the control and ANG II-treated conditions, respectively. The dashed and solid lines represent the open-loop characteristics of the baroreflex peripheral arc under the control and ANG II-treated conditions, respectively. The open circle indicates the closed-loop operating point under the control condition. ANG II causes an upward shift in the peripheral arc. If ANG II does not affect the neural arc, the closed-loop operating point would be at the point depicted by the open triangle. In this case, the estimation of baroreflex control of SNA based on the closed-loop operating points (the open circle and open triangle) approximates the slope of the baroreflex neural arc (dashed curve). ANG II, however, causes a rightward shift in the neural arc. Thus, the estimation of the baroreflex control of SNA based on closed-loop operating points (the open and filled circles) does not match the slope of the neural arc under either the control (dashed curve) or ANG II-treated condition (solid curve)
Discussion
Effects of ANG II on open-loop baroreflex control of SNA
Intravenous ANG II at 167 ng kg−1 min−1 shifted the open-loop baroreflex control of splanchnic SNA toward higher SNA values without attenuating the size of the response range (Fig. 3a; Table 1). The maximum slope was unaltered, which agreed with a previous study from our laboratory in which intravenous ANG II at 100 ng kg−1 min−1 did not change the dynamic gain of the neural arc in anesthetized rabbits [23]. In contrast, Sanderford and Bishop demonstrated that ANG II at 10 or 20 ng kg−1 min−1 significantly reduced the maximum renal SNA and attenuated the range of baroreflex control of renal SNA in conscious rabbits [9, 24]. On the other hand, Tan et al. [12] demonstrated that intravenous ANG II at 400 ng kg−1 min−1 did not increase the levels of renal SNA in anesthetized rats. The regional differences in SNA may partly explain the conflicting results, because Fukiyama [25] noted that ANG II infusion (3.5–9.5 ng kg−1 min−1) through the vertebral artery resulted in an increase in splanchnic SNA, a transient increase followed by a decrease in renal SNA, and no change in cardiac SNA in anesthetized dogs.
Activation of the renin–angiotensin system contributes to the pathologic sympathoexcitation observed in such cardiovascular diseases as chronic heart failure. In addition to the augmented cardiac sympathetic reflex, impairment of the arterial baroreflex is thought to contribute to sympathoexcitation [13]. The present results indicate that ANG II may increase SNA, but it does not attenuate baroreflex control of SNA such that the magnitude of the SNA response to the input pressure change is preserved (Fig. 3a). ANG II also did not attenuate the gain of the total baroreflex estimated by the magnitude of the AP response to the input pressure change (Fig. 2a). Therefore, the observed weakening of the baroreflex reported in patients with chronic heart failure may not be readily explainable by an acute effect of high circulating levels of ANG II.
Several studies have demonstrated that ANG II-induced hypertension does not decrease SNA via the arterial baroreflex compared to equivalent hypertension induced by phenylephrine [10, 12, 26]. Although those results seem to be consistent with the idea that ANG II blunts the arterial baroreflex, the experimental protocol is confusing, and the interpretation could be wrong as follows. The intersection between the neural and peripheral arcs in the baroreflex equilibrium diagram conforms to the closed-loop operating point [21, 27, 28]. In the present study, ANG II significantly increased AP without significant changes in SNA at the closed-loop operating point (Fig. 4, open vs. filled circles; Table 1). If we calculate the baroreflex control of SNA based on ANG II-induced hypertension, therefore, we would incorrectly conclude that the baroreflex does not control SNA. If we observe the SNA response to changes in CSP, however, the baroreflex should be able to control SNA in the presence of ANG II (Fig. 3a). Lumbers et al. [29] pointed out a problem regarding the use of ANG II-induced hypertension as an input perturbation to evaluate the baroreflex.
Effects of ANG II on the baroreflex peripheral arc
The open-loop system characteristics of the baroreflex peripheral arc, assessed using the AP response as a function of SNA, approximated a straight line under both control and ANG II-treated conditions (Fig. 3b), suggesting that the splanchnic SNA may represent changes in systemic SNA that controlled the AP. ANG II significantly increased the intercept of the regression line, reflecting its direct vasoconstrictive effect (Table 1). Because the AP at 100% SNA did not differ among the three conditions, the slope could be shallower in the presence of ANG II. In other words, ANG II appears to elevate the AP to a greater extent for the lower SNA range. Although both the modulation of sympathetic neurotransmission and direct vasoconstriction contribute to the elevation of AP, the fact that ANG II enhances the sympathetic neurotransmission more with a lower stimulation frequency [30, 31] may, in part, account for the greater ANG II-induced increase in AP for the lower SNA range.
Effects of ANG II on the open-loop sympathetic baroreflex control of HR
The baroreflex control of HR showed changes similar to those observed for SNA. Intravenous ANG II increased both the minimum and maximum HR while not significantly affecting the response range of HR or the maximum slope of the response (Fig. 2b; Table 1). The midpoint in CSP was not changed by ANG II. Therefore, the open-loop baroreflex control of HR shifted upward to higher HR values without a concomitant rightward shift to higher CSP values in the present study. In contrast, previous studies reported a rightward shift in the baroreflex control of HR toward higher input pressure values during acute [11, 32] and chronic [33] administration of ANG II in conscious rabbits. Reid and Chou [32] indicated that the inhibition of vagal tone to the heart played a significant role in resetting the baroreflex control of HR in conscious rabbits. It is likely that the rightward shift in the baroreflex control of HR by ANG II was not observed in the present study because the vagal nerves were sectioned.
Limitations
First, we performed the experiments in anesthetized animals, and comparisons with results obtained in conscious animals should be made carefully. Circulating levels of ANG II may vary under anesthesia, which could have affected the present results. For instance, reported plasma ANG II concentration in pithed rats is approximately 400 pg/ml [16], which exceeds the plasma ANG II concentration reported in rats with heart failure [34]. Second, although the dose of ANG II used in the present study was within or below those used in previous studies in rats [12, 16, 17], Brown et al. demonstrated that intravenous ANG II at 20 and 270 ng kg−1 min−1 increased the plasma ANG II concentration from approximately 80 pg/ml to 140 and 2,000 pg/ml, respectively [35]. Based on those data, the plasma ANG II concentration might have been increased beyond a physiologically relevant range to approximately 1,200 pg/ml in the present study. Therefore, the observed effect of ANG II on the arterial baroreflex should be interpreted as pharmacologic. Effects of circulating ANG II can be different when examined in different doses. Third, there was large variation in HR values among the animals (Fig. 2b). Increasing the number of animals would reduce this variation. Nevertheless, data from the eight rats was sufficient to perform statistical analyses and draw reasonable conclusions. Fourth, we occluded the common carotid arteries to isolate the carotid sinuses. Although the vertebral arteries were kept intact and the effects of ANG II were examined using the same preparation, the possibility cannot be ruled out that the carotid occlusion affected the present results. Finally, we cut the vagal nerves to obtain the open-loop condition for the carotid sinus baroreflex. Further studies are needed to clarify the effects of ANG II on the baroreflex control of the cardiovascular system through the vagal system.
Conclusion
The present study indicates that high circulating levels of ANG II significantly increased splanchnic SNA but did not acutely attenuate the range of arterial baroreflex control of SNA. The ranges of the total baroreflex response and the baroreflex control of HR were also preserved during ANG II administration. ANG II does modify the arterial baroreflex in that it increases SNA at a given baroreceptor pressure level but does not appear to attenuate the range of arterial baroreflex control of SNA, HR or AP.
Acknowledgments
This study was supported by Health and Labour Sciences Research Grants (H18-nano-Ippan-003, H19-nano-Ippan-009, H20-katsudo-Shitei-007, and H21-nano-Ippan-005) from the Ministry of Health, Labour and Welfare of Japan; by a Grant-in-Aid for Scientific Research (No. 20390462) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and by the Industrial Technology Research Grant Program from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
References
- 1.Ikeda Y, Kawada T, Sugimachi M, Kawaguchi O, Shishido T, Sato T, Miyano H, Matsuura W, Alexander J, Jr, Sunagawa K. Neural arc of baroreflex optimizes dynamic pressure regulation in achieving both stability and quickness. Am J Physiol. 1996;271:H882–H890. doi: 10.1152/ajpheart.1996.271.3.H882. [DOI] [PubMed] [Google Scholar]
- 2.Kawada T, Yamamoto K, Kamiya A, Ariumi H, Michikami D, Shishido T, Sunagawa K, Sugimachi M. Dynamic characteristics of carotid sinus pressure-nerve activity transduction in rabbits. Jpn J Physiol. 2005;55:157–163. doi: 10.2170/jjphysiol.R2122. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Kawada T, Shishido T, Miyano H, Inagaki M, Miyashita H, Sugimachi M, Kneupfer MM, Sunagawa K. Dynamic transduction properties of in situ baroreceptors of rabbit aortic depressor nerve. Am J Physiol Heart Circ Physiol. 1998;274:H358–H365. doi: 10.1152/ajpheart.1998.274.1.H358. [DOI] [PubMed] [Google Scholar]
- 4.Chapleau MW, Abboud FM. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs. Circ Res. 1987;61:648–658. doi: 10.1161/01.res.61.5.648. [DOI] [PubMed] [Google Scholar]
- 5.Kawada T, Fujiki N, Hosomi H. Systems analysis of the carotid sinus baroreflex system using a sum-of-sinusoidal input. Jpn J Physiol. 1992;42:15–34. doi: 10.2170/jjphysiol.42.15. [DOI] [PubMed] [Google Scholar]
- 6.Kawada T, Yanagiya Y, Uemura K, Miyamoto T, Zheng C, Li M, Sugimachi M, Sunagawa K. Input-size dependence of the baroreflex neural arc transfer characteristics. Am J Physiol Heart Circ Physiol. 2002;284:H404–H415. doi: 10.1152/ajpheart.00319.2002. [DOI] [PubMed] [Google Scholar]
- 7.Kawada T, Zheng C, Yanagiya Y, Uemura K, Miyamoto T, Inagaki M, Shishido T, Sugimachi M, Sunagawa K. High-cut characteristics of the baroreflex neural arc preserve baroreflex gain against pulsatile pressure. Am J Physiol Heart Circ Physiol. 2002;282:H1149–H1156. doi: 10.1152/ajpheart.00750.2001. [DOI] [PubMed] [Google Scholar]
- 8.Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol Endocrinol Metab. 1992;262:E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 9.Sanderford MG, Bishop VS. Angiotensin II acutely attenuates range of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;279:H1804–H1812. doi: 10.1152/ajpheart.2000.279.4.H1804. [DOI] [PubMed] [Google Scholar]
- 10.McMullan S, Goodchild AK, Pilowsky PM. Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurons in the rat. J Physiol. 2007;582:711–722. doi: 10.1113/jphysiol.2007.128983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai K, Reid IA. Angiotensin II exerts differential actions on renal nerve activity and heart rate. Hypertension. 1994;24:451–456. doi: 10.1161/01.hyp.24.4.451. [DOI] [PubMed] [Google Scholar]
- 12.Tan PS, Killinger S, Horiuchi J, Dampney RA. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–R2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
- 13.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 14.Shoukas AA, Callahan CA, Lash JM, Haase EB. New technique to completely isolate carotid sinus baroreceptor regions in rats. Am J Physiol Heart Circ Physiol. 1991;260:H300–H303. doi: 10.1152/ajpheart.1991.260.1.H300. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Kawada T, Miyano H, Shishido T, Inagaki M, Yoshimura R, Tatewaki T, Sugimachi M, Alexander J, Jr, Sunagawa K. New simple methods for isolating baroreceptor regions of carotid sinus and aortic depressor nerves in rats. Am J Physiol Heart Circ Physiol. 1999;276:H326–H332. doi: 10.1152/ajpheart.1999.276.1.H326. [DOI] [PubMed] [Google Scholar]
- 16.Grant TL, McGrath JC. Interactions between angiotensin II, sympathetic nerve-mediated pressor response and cyclo-oxygenase products in the pithed rat. Br J Pharmacol. 1988;95:1220–1228. doi: 10.1111/j.1476-5381.1988.tb11759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haywood JR, Fink GD, Buggy J, Phillips MI, Brody MJ. The area postrema plays no role in the pressor action of angiotensin in the rat. Am J Physiol Heart Circ Physiol. 1980;239:H108–H113. doi: 10.1152/ajpheart.1980.239.1.H108. [DOI] [PubMed] [Google Scholar]
- 18.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- 19.Glantz SA. Primer of biostatistics. 5. New York: McGraw-Hill; 2002. [Google Scholar]
- 20.Mohrman DE, Heller LJ. Cardiovascular physiology. 6. New York: McGraw Hill; 2006. pp. 172–177. [Google Scholar]
- 21.Sato T, Kawada T, Inagaki M, Shishido T, Takaki H, Sugimachi M, Sunagawa K. New analytic framework for understanding sympathetic baroreflex control of arterial pressure. Am J Physiol Heart Circ Physiol. 1999;276:H2251–H2261. doi: 10.1152/ajpheart.1999.276.6.H2251. [DOI] [PubMed] [Google Scholar]
- 22.Kawada T, Shishido T, Inagaki M, Zheng C, Yanagiya Y, Uemura K, Sugimachi M, Sunagawa K. Estimation of baroreflex gain using a baroreflex equilibrium diagram. Jpn J Physiol. 2002;52:21–29. doi: 10.2170/jjphysiol.52.21. [DOI] [PubMed] [Google Scholar]
- 23.Kashihara K, Takahashi Y, Chatani K, Kawada T, Zheng C, Li M, Sugimachi M, Sunagawa K. Intravenous angiotensin II does not affect dynamic baroreflex characteristics of the neural or peripheral arc. Jpn J Physiol. 2003;53:135–143. doi: 10.2170/jjphysiol.53.135. [DOI] [PubMed] [Google Scholar]
- 24.Sanderford MG, Bishop VS. Central mechanisms of acute ANG II modulation of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2002;282:H1592–H1602. doi: 10.1152/ajpheart.00222.2001. [DOI] [PubMed] [Google Scholar]
- 25.Fukiyama K. Central action of angiotensin and hypertension—increased central vasomotor outflow by angiotensin. Jpn Circ J. 1972;36:599–602. doi: 10.1253/jcj.36.599. [DOI] [PubMed] [Google Scholar]
- 26.Guo GB, Abboud FM. Angiotensin II attenuates baroreflex control of heart rate and sympathetic activity. Am J Physiol Heart Circ Physiol. 1984;246:H80–H89. doi: 10.1152/ajpheart.1984.246.1.H80. [DOI] [PubMed] [Google Scholar]
- 27.Kamiya A, Kawada T, Yamamoto K, Michikami D, Ariumi H, Uemura K, Zheng C, Shimizu S, Aiba T, Miyamoto T, Sugimachi M, Sunagawa K. Resetting of the arterial baroreflex increases orthostatic sympathetic activation and prevents postural hypotension in rabbits. J Physiol. 2005;566:237–246. doi: 10.1113/jphysiol.2005.086512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Kawada T, Kamiya A, Takaki H, Miyamoto T, Sugimachi M, Sunagawa K. Muscle mechanoreflex induces the pressor response by resetting the arterial baroreflex neural arc. Am J Physiol Heart Circ Physiol. 2004;286:H1382–H1388. doi: 10.1152/ajpheart.00801.2003. [DOI] [PubMed] [Google Scholar]
- 29.Lumbers ER, McCloskey DI, Potter EK. Inhibition by angiotensin II of baroreceptor-evoked activity in cardiac vagal efferent nerves in the dog. J Physiol. 1979;294:69–80. doi: 10.1113/jphysiol.1979.sp012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes J, Roth RH. Evidence that angiotensin enhances transmitter release during sympathetic nerve stimulation. Br J Phrmacol. 1971;41:239–255. doi: 10.1111/j.1476-5381.1971.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmerman BG, Gomer SK, Liao JC. Action of angiotensin on vascular adrenergic nerve endings: facilitation of norepinephrine release. Federation Proc. 1972;31:1344–1350. [PubMed] [Google Scholar]
- 32.Reid IA, Chou L. Analysis of the action of angiotensin II on the baroreflex control of heart rate in conscious rabbits. Endocrinology. 1990;126:2749–2756. doi: 10.1210/endo-126-5-2749. [DOI] [PubMed] [Google Scholar]
- 33.Brooks VL. Chronic infusion of angiotensin II resets baroreflex control of heart rate by an arterial pressure-independent mechanism. Hypertension. 1995;26:420–424. doi: 10.1161/01.hyp.26.3.420. [DOI] [PubMed] [Google Scholar]
- 34.Schunkert H, Tang SS, Litwin SE, Diamant D, Riegger G, Dzau VJ, Ingelfinger JR. Regulation of intrarenal and circulating renin–angiotensin systems in severe heart failure in the rat. Cardiovasc Res. 1993;27:731–735. doi: 10.1093/cvr/27.5.731. [DOI] [PubMed] [Google Scholar]
- 35.Brown AJ, Casals-Stenzel J, Gofford S, Lever AF, Morton JJ. Comparison of fast and slow pressor effects of angiotensin II in the conscious rat. Am J Physiol Heart Circ Physiol. 1981;241:H381–H388. doi: 10.1152/ajpheart.1981.241.3.H381. [DOI] [PubMed] [Google Scholar]


