Abstract
Members of the bone morphogenetic protein-1/mammalian tolloid (BMP-1/mTLD) family of proteases cleave diverse extracellular proteins, including the growth inhibitor myostatin. The purpose of this work was to examine the expression of BMP-1/mTLD, tolloid-like-1 and -2 (TLL1 and TLL2) in hindlimb muscles of the mouse in vivo and in C2C12 muscle cells in vitro. Quantitative real-time polymerase chain reaction revealed that neither BMP-1/mTLD nor TLL1 mRNA levels differed between the predominantly fast-twitch tibialis anterior (TA) and gastrocnemius (GAST) muscles and the more slow-twitch soleus (SOL) muscle; TLL2 mRNA levels were not detectable in any of the muscles examined. Interestingly, however, immunohistochemical analysis revealed that BMP-1 protein was expressed in type I and IIa but not in IIb fibers. TLL1 mRNA levels significantly increased in the TA but not the SOL with 3 days of hindlimb suspension and significantly decreased in both TA and SOL in response to 2 days of food deprivation. In contrast, BMP-1/mTLD mRNA levels were unaffected in either muscle by either condition. In addition, BMP-1/mTLD and TLL1 mRNA levels significantly decreased during C2C12 myoblast differentiation in vitro, and activity of a 1,200-bp mouse TLL1 promoter construct was significantly decreased in C2C12 myotubes by differentiation, by mutation of an nuclear factor kappa-beta (NF-kappaB) site, or deletion of a sma/mothers against decapentaplegic (SMAD) site. Together, these data demonstrate that TLL1 mRNA levels are altered by loading, energy status, and differentiation, and thus its expression may be regulated so as to modulate activity of myostatin or other extracellular substrates during these adaptive states.
Keywords: Myostatin, Fast-twitch, Slow-twitch, Food deprivation, Hindlimb suspension, C2C12
Introduction
The bone morphogenetic protein-1/mammalian tolloid/tolloid-like (BMP-1/mTLD/TLL) proteins are a family of secreted metalloproteinases that proteolytically process a number of extracellular substrates [1]. BMP-1/mTLD was originally identified along with six other BMPs as proteins having bone-forming capabilities [1]. However, BMPs 2-7 were shown to be related to members of the transforming growth factor-beta (TGF-β) super-family of secreted autocrine/paracrine factors, while BMP-1/mTLD was structurally different and was subsequently shown to be part of a small family of extracellular proteases. Four members of this family exist: BMP-1 and mTLD, which are alternatively spliced forms arising from the same gene [1], and tolloid-like-1 (TLL-1) and -2 [1]. Members of this family are responsible for the carboxy terminus processing of various fibrillar procollagens [1] and several other extracellular matrix proteins [1].
Moreover, BMP-1/mTLD/TLL family members also proteolytically process a select sub-group of TGF-β/BMP family members, including BMP-2, BMP-4, and chordin, which are critical regulators of dorsal–ventral patterning during development [2]. In addition, BMP-1/mTLD/TLL family members also cleave the muscle-specific growth inhibitor myostatin [3], and recent evidence suggests that perturbation of this cleavage can have profound effects on skeletal muscle growth. Transgenic muscle-specific over-expression of a protease-resistant form of myostatin produces profound increases in muscle mass, mimicking that observed in myostatin null mice [4], suggesting that altering the cleavage of myostatin by these regulatory proteases can greatly affect muscle growth.
The members of the BMP-1/mTLD/TLL family show differential spatiotemporal expression in mice. Specifically, both BMP-1 and mTLD are highly expressed during murine development, particularly in mesodermal tissue [5, 6], and in the adult are expressed in several tissues, including (in decreasing order of expression) lung, liver, heart, kidney, and skeletal muscle [6]. TLL1 is also highly expressed during murine development, peaking at day 17 post-coitum, and is also expressed in brain, kidney, lung and skeletal muscle in the adult mouse [7]. TLL2 is expressed predominantly in skeletal muscle in the developing prenatal mouse hindlimb [8] but its adult expression has not been characterized. Thus, all these proteases are expressed to some extent in skeletal muscle, but at present little is known regarding whether any of these proteases are differentially expressed in muscles containing different percentages of fiber types. Moreover, it is not known whether expression of these genes is altered during myogenic differentiation or in response to adult skeletal muscle adaptation.
The purpose of the present study was to examine the mRNA levels of these protease genes in different hindlimb skeletal muscles, in unloaded or food-deprived skeletal muscle, and in differentiating muscle cells. Our hypothesis was that changes in expression of these proteases may represent another node for regulating overall activity of myostatin and/or other TGF-β family members during skeletal muscle growth transitions. MRNA levels of BMP-1/mTLD and TLL1 did not differ between muscle types, but BMP-1 protein was localized in more oxidative fiber types such as I and IIa and was not expressed in type IIb fibers, a pattern complementary to that reported for one of its potential substrates, myostatin. In addition, levels of TLL1 but not BMP-1/mTLD mRNA were responsive to unloading and food deprivation. Both BMP-1/mTLD and TLL1 mRNA levels decreased significantly with myoblast differentiation in vitro, and the decrease in TLL1 mRNA levels during differentiation appeared to be at least in part transcriptionally driven. Finally, we identified two key upstream regulatory elements which may govern transcription of this gene. Together, these data are consistent with the hypothesis that alterations in expression of extracellular proteases such as TLL1 may help modulate the shifts in ECM protein processing and/or growth factor signaling occurring during conditions of muscle plasticity.
Methods
Experimental animals
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Colorado, Boulder, and complied with the guidelines of the American Physiological Society on the use of laboratory animals. Male wild-type C57/black6j mice were obtained from our breeding colony in the Department of Integrative Physiology at the University of Colorado, Boulder.
To examine expression of BMP-1/mTLD and TLL1 in different muscles, we isolated and froze the gastrocnemius (GAST), tibialis anterior (TA) and soleus (SOL) muscles from n = 5 wild-type C57 mice. For the hindlimb suspension studies, we used RNA isolated from TA and SOL from unsuspended control and 3 day hindlimb suspended mice from a study that is described in detail elsewhere (Hanson et al., in preparation). Briefly, mice (n = 7) were tail suspended for 3 days as previously described [9–11], and the TA and SOL were isolated, weighed, frozen, and stored at −70°C until use. Similarly, for the food deprivation studies, we used RNA isolated from TA and SOL muscles from fed, 2 days food-deprived, and 2 days food-deprived and refed mice from a previously published study (Allen et al., in press). Food deprivation was accomplished by removing the food from the cage top for 2 days starting in the morning inactive period (n = 4 mice). In addition, a subset of mice (n = 4) underwent food deprivation then were allowed ad libitum access to food for an additional 2 days. Control mice (n = 4) were allowed ad libitum access to food throughout the experiment. All mice had ad libitum access to drinking water throughout these experiments. At the end of the treatment period mice were sacrificed and the TA and SOL were isolated, frozen, and stored as described above.
Quantitative real-time RT-PCR
RNA was isolated from skeletal muscle and C2C12 myoblast and myotube samples with Trizol reagent (Invitrogen) using standard techniques. The reverse transcription (RT) reaction was carried out using 0.5 μg of RNA using the cDNA Archive kit (Advanced Biosystems) according to the manufacturers protocol. Primer and probe sets for BMP-1/mTLD, TLL1, TLL2, and β-actin were obtained from Applied Biosystems. The primers for BMP-1/mTLD recognize a sequence common to both BMP-1/mTLD and TOLL, which are formed by alternative splicing from the same gene [6]. All real-time PCR procedures were run in triplicate to correct for variances in loading. In addition, a standard curve ranging from 25 to 0.001 μg dilutions of mouse TA cDNA was run in duplicate for every assay and used for quantification. All values are expressed as the mean of the triplicate measure for the experimental (BMP-1/mTLD, TLL1, TLL2) divided by the mean of the triplicate measure of β-actin for each sample. BMP-1/mTLD was run in duplex with the β-actin primers and probes; TLL1 did not work in duplex so these reactions were run in singleplex.
Immunohistochemistry
For immunohistochemical analysis, calf (gastrocnemius, soleus, plantaris) muscle samples were attached to corkboard with optimal cutting temperature (OCT) media (Sakura Finetek, Torrance, CA, USA) and snap frozen in liquid nitrogen cooled isopentane. Ten-micrometer-thick serial sections were cut from the mid-belly of the muscle with a Cryostat Microtome Cryocut 1800 (Leica, Bannockburn, IL) and fixed to Superfrost® slides (VWR, West Chester, PA, USA) and stored at −20°C until use.
Muscle sections used for immunohistochemistry were air dried for 15 min and then incubated in a permeabilizing/blocking solution [0.1% BSA (Fisher Scientific, Pittsburgh, PA, USA), 0.1% nonfat dry milk (Kroger, Cincinnati, OH, USA) 0.1% Triton X-100 (Sigma, St. Louis, MO. USA) in PBS pH 7.4]. Permeabilization/blocking solution was applied on tissue sections for 1 h at room temperature in a humidifying chamber. Tissue sections were then rinsed three times in permeabilizing/blocking solution. Sections were then placed in a cocktail primary antibody solution of rabbit anti-laminin (Sigma; 1:100), Goat IgG BMP-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100), and mouse monoclonal IgG1 myosin heavy chain (MyHC) type IIa (SC-71, 1:3), or anti-laminin (Sigma; 1:100) and mouse monoclonal IgG1 MyHC type I (Novocastra, Newcastle upon Tyne, UK; 1:40), or anti-laminin and mouse monoclonal IgM MyHC type IIb (BF-F3; kindly provided by Dr. Leslie Leinwand) for 2 h at room temperature. After primary incubation, sections were rinsed three times in the permeabilization/blocking solution followed by three 5-min incubations in permeabilizing/blocking solution. Sections were then incubated with a cocktail of appropriate secondary antibodies for 1 h. Secondary antibodies used were donkey anti-mouse (H + L) IgG Alexa Fluor® 488 (Invitrogen, Carlsbad, CA, USA) at a dilution of 1:250 for MyHC type IIa and 1:500 for MyHC type I, donkey anti-goat (H + L) IgG Alexa Fluor® 568 (Invitrogen; 1:500), goat anti-mouse IgM FITC (Jackson Laboratories, West Grove, PA, USA; 1:250), and donkey anti-rabbit (H + L) IgG FITC (Jackson Laboratories; 1:200). Tissue sections were rinsed three times in PBS followed by three 5-min incubations in PBS. Sections were then mounted with a mounting medium of Gelvatol and DABCO (Sigma) and No. 1 coverslips (VWR) for subsequent imaging.
We used Metamorph V7.0x4 (Downingtown, PA) software alongside a Nikon (Tokyo, Japan) E600 microscope equipped with a CoolSnap Fx monochrome camera (Photometrics, Tucson, AZ, USA) and the following filter sets: B-2 E/C F (Nikon) and Y-2 E/C TR (Nikon) to acquire images. ImageJ (NIH Bethesda, MD, USA) and Photoshop (Adobe Systems, San Jose, CA, USA) were used for post-processing of images. Briefly, Metamorph images were opened in ImageJ as an image sequence and all similar color images were contrast adjusted. Composite images were then created, converted to 8-bit RGB images and aligned and blended together using Photoshop to create whole muscle photos.
Cloning and mutagenesis
Approximately 1300 bp of the mouse TLL1 promoter extending through the 5′ untranslated region to the translational start site was amplified from mouse genomic DNA using primers containing a MluI and NcoI site at the 5′ and 3′ end, respectively: 5′-TAGTGGACGCGTTAGTCGCATCTCCAGCCAAA-3′ and 5′-TAATAACTCGAGCCGTTATTTCTACGCCGCCAGACCTTAAAGC-3′. The resulting fragment was ligated into the pGL3 basic luciferase reporter plasmid.
In addition, mutagenesis was carried out using PCR and DpnI digestion as described previously for the myostatin promoter [12] using the following primers: for the forkhead box O (FoxO) site, 5′-GAAAGAAACACACATCGCCTAggATCTGGGGGTGGGGTGGGGC-3′; for the NF-kB site, 5′-TTGCTCTCCGGGCAGTCGaacaCTTCCCTAGCTTCGGCAG-3′; the putative binding site is underlined and the mutated nucleotides are in lower case. In addition, an extended repeat of ten CAGA sequences was deleted using inverse PCR with the primers 5′-TAGTGGACTAGTAGGATTCCGATATCCATGGCT-3′ and 5′-TAGTGGACTAGTTCTTTGCCTATCTGTCTCTCT-3′, which inserted an engineered SpeI site in the place of this sequence. All clones were positively identified using restriction analysis and/or sequencing at the University of Colorado, Boulder DNA Sequencing Facility.
Cell culture and transfection
C2C12 myoblasts were plated on 0.75% gelatin-coated 6-well plates in proliferation medium consisting of Dulbecco’s Modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin (pen/strep). Once cells reached confluence, they were either harvested for RNA isolation, or switched to differentiation medium consisting of DMEM + 1% horse serum (HS). After differentiating for 2 days, RNA was isolated from the myotubes using the Trizol method.
For transfection studies, C2C12 myoblasts were allowed to reach 90% confluence then were passaged onto 24-well plates. The following day, cells were transfected with TLL1 promoter constructs using Lipofectamine 2000 as previously described [12, 13]. Once myoblasts reached confluence, they were either harvested and lysed for quantification of myoblast luciferase activity or were differentiated into myotubes for 2 days prior to harvesting and lysing. All transfections were repeated three times with 4–8 wells per replication and the results were averaged.
Statistical analysis
Differences in expression between the three different control muscles or between fed, food-deprived, and refed TA or SOL muscle were determined using one-way analysis of variance (ANOVA) with Fisher’s post-hoc test; significance with an alpha level of 0.05 taken as significant. Differences between myoblast and myotube and control and hindlimb suspended mRNA values were determined by an independent t test, with an alpha level of 0.05 taken as significant.
Results
Expression of BMP-1/mTLD1, TLL1 and TLL2 in Hindlimb Muscles
Quantitative RT-PCR revealed that, relative to β-actin, mRNA levels of BMP-1/mTLD and TLL1 were not significantly different between the three muscles (Fig. 1). Moreover, we were unable to reliably detect TLL2 mRNA in either fast or slow skeletal muscle in consistently measurable amounts (data not shown).
Fig. 1.
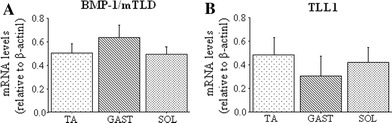
Bone morphogenetic protein-1/mammalian tolloid (BMP-1/mTLD) and tolloid-like-1 (TLL-1) gene expression in different muscle type. a BMP-1/mTLD mRNA expression relative to β-actin in the tibialis anterior (TA), gastrocnemius (GAST), and soleus (SOL). b TLL1 mRNA expression relative to β-actin in TA, GAST, and SOL. All results are reported as mean ± SEM of mRNA levels normalized to those of β-actin (n = 5 muscles/group). Neither mRNA showed any significant difference across the different muscles
Immunohistochemical localization of BMP-1
In addition, we used immunohistochemistry to examine the localization of BMP-1 protein within skeletal muscle tissue. Surprisingly, BMP-1 protein was found within a subset of muscle fibers in the calf musculature, particularly in the soleus and plantaris and deep portion of the gastrocnemius (Fig. 2a). BMP-1-positive fibers were predominantly clustered within these muscles in the deep portion of the calf and were rarely if ever observed in the superficial compartment of the GAST (Fig. 2b). Double staining with antibodies to type I MyHC showed that some but not all the BMP-1-positive fibers were also positive for type I MyHC (Fig. 2c). Similarly, double staining with an antibody against type IIa MyHC also showed that some but not all BMP-1-positive fibers were also positive for type IIa MyHC (Fig. 2d). Conversely, staining serial sections with antibodies to type IIb MyHC showed a complementary pattern to that of BMP-1, with few if any fibers staining with both antibodies (Fig. 2e, f). Attempts to immunohistochemically stain mouse hindlimb muscles with a commercially available mouse-derived monoclonal antibody against TLL1 failed to produce staining above background.
Fig. 2.
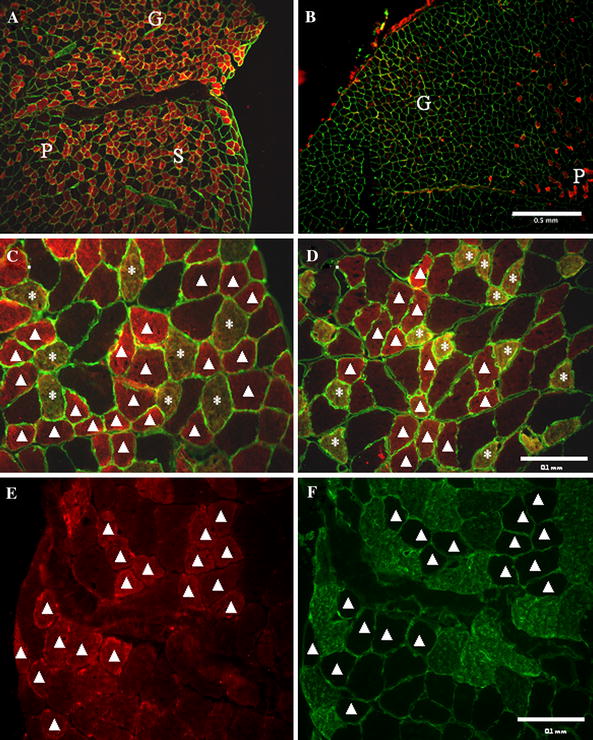
Immunohistochemical localization of BMP-1 in mouse calf muscles. a Low power image of BMP-1 (red) and laminin (green) immunofluorescence staining showing the relative lack of BMP-1-positive fibers in the superficial portion of the calf. b Low power image of BMP-1 (red) and laminin (green) immunofluorescence staining showing the fiber-specific pattern with more BMP-1-positive fibers in the deep portion of the calf. G, P, and S show the relative locations of the gastrocnemius, plantaris, and soleus muscles, respectively. Scale bar in (b) is 0.5 mm and is the same for (a). c High power image of BMP-1 (red) and type I MyHC and laminin (green) immunofluorescence staining of the SOL showing the co-localization of BMP-1 with MyHC type I. Asterisks indicate fibers positive for both BMP-1 and type I MyHC, arrowheads fibers positive for BMP-1 but negative for type I MyHC. d High power image of BMP-1 (red) and type IIa MyHC and laminin (green) immunofluorescence staining showing the co-localization of BMP-1 with MyHC type IIa in the SOL. Asterisk indicate fibers positive for both BMP-1 and type IIa MyHC, arrowheads fibers positive for BMP-1 but negative for type IIa MyHC. Scale bar in (d) is 0.1 mm and is the same for (c) and (d). e, f Serial sections with BMP-1 (red) and type IIb MyHC and laminin (green) immunofluorescence staining showing the lack of co-localization of BMP-1 and MyHC type IIb. Arrowheads indicate fibers positive for BMP-1 but negative for type IIb MyHC in (e). Scale bar in (f) is 0.1 mm and is the same for (e) and (f). See Fig. 1 for the abbreviations used (color figure online)
Expression of BMP-1/mTLD and TLL11 mRNA in response to unloading and food deprivation
As described in more detail elsewhere (Hanson et al., in preparation), 3 days of hindlimb suspension resulted in modest and non-significant decreases in TA and SOL muscle mass (45.8 ± 0.8 mg for control TA and 45.1 ± 1.8 mg for suspended TA; 8.70 ± 0.8 mg for control SOL and 8.0 ± 0.5 mg for suspended SOL). Three days of hindlimb suspension significantly increased absolute (data not shown) and relative to β-actin TLL1 mRNA levels compared to unsuspended controls (Fig. 3b), but had no effect on BMP-1/mTLD mRNA levels in the TA (Fig. 3a). In contrast, neither BMP-1/mTLD nor TLL1 mRNA levels were significantly altered by hindlimb suspension in the SOL (Fig. 3c, d).
Fig. 3.
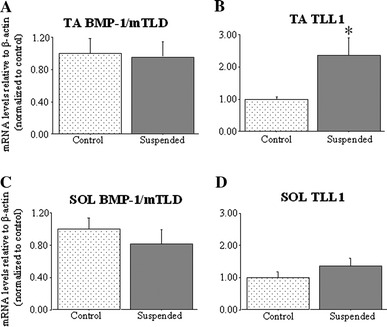
BMP-1/mTLD and TLL1 mRNA levels in the TA and SOL muscles in response to hindlimb suspension. a TA BMP-1/mTLD mRNA levels, b TA TLL1 mRNA levels, c SOL BMP-1/mTLD mRNA levels, d SOL TLL1 mRNA levels relative to β-actin in unsuspended (control) and 3-day hindlimb suspended (suspended) mice. Expression of TLL1 mRNA was significantly up-regulated in suspended TA but not SOL relative to unsuspended control values and BMP-1/mTLD was not affected in either muscle. All results are reported as mean ± SEM of mRNA levels normalized to those of β-actin and relative to the unsuspended control. Asterisk significantly different from unsuspended control, p < 0.05. See Fig. 1 for the abbreviations used
In addition, as reported elsewhere, 2 days of food deprivation resulted in a significant 25% decrease in TA but not SOL muscle mass (Allen et al., in press), and resulted in a significant decrease in TLL1 mRNA levels in both TA and SOL relative to levels in muscles from fed mice (Fig. 4b, d). Two days of ad libitum feeding increased TLL1 mRNA levels in the SOL such that they were not significantly different from those of fed mice, but in the TA TLL1 mRNA levels remained significantly decreased relative to fed mice (Fig. 4b). Finally, mRNA levels of BMP-1/mTLD were modestly and non-significantly decreased by food deprivation relative to levels in muscles from fed mice in both TA and SOL (Fig. 4a, c).
Fig. 4.
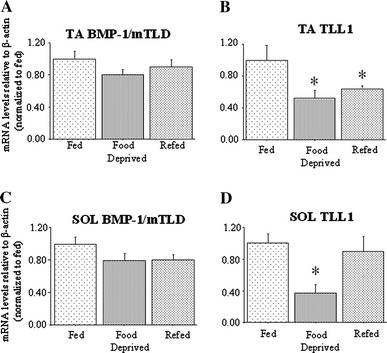
BMP-1/mTLD and TLL1 mRNA levels in the TA and SOL muscles in response to food deprivation. a TA BMP-1/mTLD mRNA levels, b TA TLL1 mRNA levels, c SOL BMP-1/mTLD mRNA levels, d SOL TLL1 mRNA levels relative to β-actin in control (fed), 2-day food deprived (food-deprived) and 2-day food-deprived then refed for 2 days (refed) mice. Expression of TLL1 mRNA was significantly decreased in TA and SOL of food deprived animals and remained significantly decreased in the TA, but not SOL, following 2 days of refeeding. All results are reported as mean ± SEM of mRNA levels normalized to those of β-actin and relative to the fed control. Asterisk significantly different from fed control, p < 0.05. See Fig. 1 for the abbreviations used
Expression of BMP-1/mTLD and TLL1 during in vitro myogenesis
Differentiation of C2C12 myoblasts into myotubes by 2 days exposure of confluent myoblasts to low serum differentiation medium significantly decreased both BMP-1/mTLD and TLL1 mRNA levels (Fig. 5a, b). Similarly, differentiation also significantly decreased the activity of a 1,200-bp mouse TLL1 promoter construct relative to its activity in confluent myoblasts (Fig. 6b).
Fig. 5.
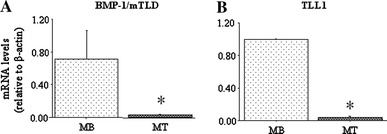
BMP-1/mTLD and TLL1 mRNA levels in myoblasts (MB) and myotubes (MT). a BMP-1/mTLD mRNA levels, b TLL1 mRNA levels. Expression of both BMP-1/mTLD and TLL1 mRNA was significantly decreased in differentiated myotubes relative to undifferentiated myoblasts. All results are reported as mean ± SEM of mRNA levels relative to β-actin. Asterisk significantly different from myoblasts, p < 0.05. See Fig. 1 for other abbreviations used
Fig. 6.
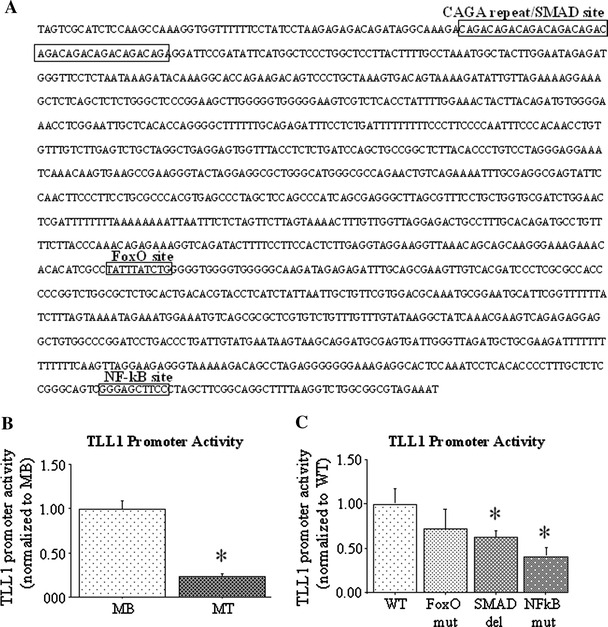
TLL1 promoter and mutagenesis. a Sequence of ~1,300 bp of the mouse TLL1 upstream promoter region. The 40-bp Serum response factor, Agamous, deficiens (SMAD)/CAGA repeat, the forkhead box O (FoxO)-like sequence (TATTTATCTG; on the minus strand), and the nuclear factor-kappa beta (NF-kappaB) consensus (GGGAGCTTCC). b TLL1 promoter-reporter activity in undifferentiated C2C12 myoblasts and 2-day differentiated C2C12 myotubes. Differentiation was associated with a significant decrease in TLL1 promoter activity. Asterisk significantly different from MB, p < 0.05. Results are reported as mean ± SEM of mRNA levels normalized to those of myoblasts. Asterisk significantly different from myoblasts, p < 0.05. c Wild-type (WT) and mutated/deleted TLL1 promoter–reporter activity in differentiated C2C12 myotubes. Deletion of the 40 bp CAGA repeat or mutation of the NF-kappaB site significantly decreased TLL1 promoter activity relative to wild-type. Results are reported as mean ± SEM of mRNA levels normalized to those of the wild type construct. Asterisk significantly different from wild-type, p < 0.05. See Fig. 1 for other abbreviations used
Using sequence analysis software, we identified three putative sequences that may regulate activity of this gene in skeletal muscle: a 10-repeat CAGA sequence that corresponds to the consensus for binding of the sma/mothers against decapentaplegic (SMAD) transcription factors at approximately −1,250 bp from the translation start site; a sequence matching the consensus for binding of the FoxO transcription factors at approximately −466 bp; and a sequence matching the consensus for binding of the transcription factor nuclear factor kappa-beta (NF-kappaB) at approximately −47 bp (Fig. 6a). We used mutagenesis to alter the FoxO and NF-kappaB sequences, and deletion to remove the 40 bp CAGA 10-mer repeat, and quantified luciferase activity of these constructs relative to a wild-type TLL1 promoter-reporter construct. As shown in Fig. 6c, mutation/deletion of any of the three sites resulted in a decrease in luciferase activity of the resulting promoter–reporter plasmid, but mutation of the NF-kappaB site or deletion of the SMAD repeat significantly decreased activity of the resulting promoter construct relative to the wild-type construct while mutation of the FoxO site did not.
Discussion
We explored the mRNA expression pattern of the BMP-1/mTLD/TLL genes in different skeletal muscles and under different conditions to better define their potential role in conditions of muscle plasticity and development. In addition, we explored the fiber-specific staining pattern of BMP-1 protein and found that it is predominantly expressed in more oxidative fibers. We hypothesized that differences in expression of these protease genes may represent another node for regulating activity of myostatin and other TGF-β family members, which are key regulators of muscle growth and differentiation. We also showed that the mRNA for TLL1 in particular is responsive to differentiation and to two different models of in vivo muscle adaptation.
We [12, 13] and others [14–16] have demonstrated that expression of myostatin shows a fiber- and/or muscle-type specificity, with greater expression in muscles containing predominantly fast-twitch, glycolytic fibers such as the TA or GAST compared to muscles containing more slow-twitch/oxidative fibers such as the SOL. We have further shown that expression of the myostatin receptor ActRIIb also shows muscle specificity, with higher expression in the TA than in the SOL [13]. Moreover, additional evidence suggests that other members of the TGF-β super-family are expressed in a fast/glycolytic muscle-preferential manner [17]. Our results demonstrate that, unlike these other members of the TGF-β/myostatin signaling cascade, neither BMP-1/mTLD nor TLL1 showed preferential mRNA expression in muscles containing a preponderance of one or the other muscle fiber types. Thus, differences in myostatin signaling between adult skeletal muscles containing different proportions of fiber types do not appear to be reflected in, or a consequence of, differential mRNA expression of its activating proteases.
However, when we examined the expression pattern of BMP-1 protein in the mouse calf musculature by immunohistochemistry, we observed a surprising fiber-specific pattern of expression. BMP-1-positive staining co-localized with type I and IIa MyHC staining and was not found in type IIb MyHC-positive fibers. Because we did not have access to an anti-type IId/x MyHC antibody, we were unable to determine whether BMP-1 was expressed in type IId/x fibers or not, though the fact that some BMP-1-positive fibers were observed in areas of the calf which did not contain type I or IIa fibers tends to suggest that IId/x MyHC fibers may also express BMP-1 (data not shown). Unfortunately, we were unable to detect any staining intensity above that seen with secondary antibody staining alone for the only currently commercially available antibody to TLL1, which most likely reflects the fact that this antibody was raised in mouse and thus identifying antibody staining beyond that of the secondary cross-reactivity with this same species was not feasible.
Nevertheless, the BMP-1 staining data suggest that BMP-1 is expressed predominantly in fibers containing a more oxidative phenotype. This observation is extremely interesting for two reasons. First, we observed no significant difference in BMP-1 mRNA levels across three different hindlimb muscles differing in their fiber type percentages, TA, SOL, and GAST (Fig. 1a). It might be expected that BMP-1 mRNA levels would be higher in the much more oxidative SOL than in either the TA or GAST. The fact that they were not might reflect a disconnect between mRNA expression and protein expression of BMP-1 in these muscles consistent with fiber-specific translational regulation of mRNA expression. Alternatively, if BMP-1 is indeed expressed in type IId/x MyHC-expressing fibers, these fibers are found in high levels in both TA and GAST, and thus expression might appear similar between the more IId/X-expressing TA and GAST than the highly I/IIa SOL.
In addition, the fact that BMP-1 immunostaining was rarely if ever observed in type IIb MyHC fibers is also in contrast to the expression pattern of one of its downstream substrates, myostatin, which tends to show much greater expression in type IIb fibers [14, 16]. This is an intriguing result as it suggests that muscle fibers not expressing myostatin are responsible for the expression of one of its activators, while fibers expressing myostatin are not. This implies a level of coordination and perhaps even communication between these two groups of muscle fibers in the regulation of overall myostatin activity. It is possible that, if the same fiber expressed both myostatin and its activator, it might produce too much active myostatin locally, which would then act back on those fibers in a positive feedback autocrine loop, resulting in excessive fiber growth. By separating myostatin expression and that of its activating protease into distinct subtypes of fibers, and moreover given the geographical separation of type IIb MyHC- and myostatin-expressing fibers in the superficial portion of the muscle and the expression of BMP-1 by more oxidative fibers found deeper in the muscle, local activity of myostatin may be modulated in a way that tends to avoid such an unwanted, out-of-control growth loop.
We were unable to reliably detect TLL2 mRNA in either the hindlimb muscles examined. Little is currently known regarding expression of TLL2 in adult tissues. In one of the only other studies to address expression of TLL2, Scott et al. [8] showed that TLL2 appeared to be expressed predominantly in developing muscles of day 15.5 post-coitum mouse by in situ hybridization [8]. Taken with the results of the present study, this suggests that TLL2 may be expressed during early pre- or post-natal development but that expression may decrease in the adult animal. In a recent paper, Lee demonstrated that mice in which the TLL2 gene is inactivated by homologous recombination present with modest (8–12%) increases in muscle mass which differ almost by an order of magnitude from the increases in muscle mass observed in mice harboring a protease-resistant form of myostatin [4]. It may be that the modest effects of inactivation of TLL2 reflect its minimal expression in adult animals as well as redundancy in the ability of the various BMP-1/mTLD/TLL family members to activate myostatin [3].
In the present study, expression of BMP-1/mTLD was not affected by either unloading or food deprivation in either muscle, but TLL1 mRNA levels were significantly altered in a muscle- and condition-specific manner. Specifically, TLL1 mRNA levels significantly increased in the TA but not the SOL in response to 3 days of hindlimb suspension and significantly decreased in both the TA and SOL of food-deprived mice (Fig. 4). These data raise three interesting points regarding expression of these proteases during muscle adaptation. Firstly, they do not appear to be regulated in concert with myostatin, particularly in the SOL. Myostatin expression is increased in both fast and slow muscles with hindlimb suspension [14] while TLL1 was increased in just the TA in the present study. Similarly, myostatin mRNA levels increase in the TA but not the SOL with food deprivation (Allen et al., in press) while TLL1 mRNA levels decreased in both the TA and the SOL in the present study. Taken together, these results suggest that expression of TLL1 is independent of, and may reflect the need for processing substrates other than, myostatin during these states.
Secondly, expression of TLL1 does not appear to be associated with the magnitude of muscle atrophy. Both TA and SOL experienced only modest and non-significant decreases in muscle mass with 3 days of hindlimb suspension that were nevertheless greater for the SOL (8% decrease in mass) than the TA (1.5% decrease in mass), while TLL1 mRNA levels increased significantly for TA but not SOL. Furthermore, TLL1 mRNA levels decreased in both TA and SOL, but only TA experienced a decrease in mass with food deprivation. The present data therefore suggest that TLL1 expression is not strictly dependent on muscle atrophy and may instead be independently regulated by activity levels, loading status, energy balance, and other factors impinging upon the muscle during different adaptive states.
Thirdly, changes in TLL1 mRNA levels in the TA were modality-dependent in that hindlimb suspension significantly increased while food deprivation significantly decreased TLL1 mRNA levels. Given the vast differences between these two models, it is perhaps not surprising that they evince such differential affects on TLL1 expression. But at the very least these data demonstrate that TLL1 is not an “atrogene” like the ubiquitin ligases muscle ring finger1 (MuRF1) or atrogin-1 whose expression is increased in a wide variety of atrophy models [18, 19].
The reason why TLL1 mRNA levels increased in the TA but not the SOL with hindlimb suspension was also not clear. One possibility is that these changes in TLL1 expression may occur in both muscles but with different kinetics which were not detected in the present study since we only evaluated a single post-suspension timepoint. Another possibility is that the greater increase in TLL1 mRNA levels in the TA with hindlimb suspension reflects a greater biological need for TLL1 in this muscle during the unloaded state. Previous work has suggested that fast-twitch muscles tend to show a greater increase in the expression of secreted factors such as TGF-β2 in response to inactivity [17]. Thus the greater increase in TLL1 mRNA in the TA muscle than in the SOL with hindlimb suspension observed in the present study may reflect a relatively greater need for processing of secreted signaling factors such as TGF-β2 in faster-twitch muscles in the unloaded state.
We also examined the expression of BMP1/mTLD and TLL1 mRNA in cultured C2C12 myoblasts and myotubes to determine whether their expression is responsive to differentiation. As shown in Fig. 5, expression of both BMP-1/mTLD and TLL1 mRNA was significantly decreased in myotubes compared to myoblasts. This decrease in TLL1 mRNA was mirrored by a similar significant decrease in activity of a ~1,300-bp mouse TLL1 promoter-luciferase reporter construct in myotubes compared to myoblasts (Fig. 6b), and suggests that this down-regulation in expression of TLL1 with differentiation is driven at least in part if not predominantly by a decrease in TLL1 gene transcription. This is in contrast to myostatin, as previous work has demonstrated that both myostatin mRNA levels [20, 21] and myostatin promoter activity [22] increase upon differentiation in vitro. The decrease in BMP-1/mTLD and TLL1 expression upon C2C12 differentiation may reflect an attempt to post-translationally attenuate the activity of this increased amount of myostatin by reducing expression of its activating proteases.
Finally, we examined the upstream promoter region of the TLL1 gene so as to identify putative transcriptional regulatory elements that may contribute to changes in expression of this gene during periods of muscle adaptation. We identified three elements within the proximal ~1,300 bp of the mouse TLL1 promoter sequence of particular relevance to atrophic signaling: a 40-bp-long CAGA repeat at approximately −1,250 from the translational start site that corresponds to the consensus sequence for SMAD binding [12]; a sequence (ATAAATA) similar to that of the consensus binding sequence ((C/G)(A/C)AAA(C/T)A; [12]) for the FoxO family of transcription factors; and finally, a sequence (GGGAGCTTC) matching that of the NF-kappaB consensus binding sequence (GGG(A/G)NN(C/T)(C/T)C; [23]). We used deletion and mutagenesis to alter these sequences in order to determine whether they affected transcription from the TLL1 promoter in C2C12 cells in vitro. As shown in Fig. 5c, deletion of the 40 bp SMAD repeat or mutation of the NF-kappaB site significantly attenuated TLL1 promoter activity relative to the wild-type construct; however, mutation of the FoxO-like site had no significant effect (Fig. 6c). Thus transcription of TLL1 may be regulated by SMAD and/or NF-kappaB signaling during periods of muscle adaptation. In a previous study, the TLL1 promoter was shown to contain consensus binding sites for the glucocorticoid receptor, as well as for the heart-specific transcription factor Nkx2-5 and the brain-specific transcription factor MyT1 [24], which may suggest that its expression is regulated by these elements in these other tissues.
Conclusion
In summary, we have examined the expression of two members of the BMP-1/mTLD/TLL family of extracellular proteases, BMP-1/mTLD and TLL1, in different hindlimb muscles, during two conditions of muscle adaptation, and during differentiation in vitro. These studies provide new insights into the pattern of expression of these critical regulatory proteins that may reflect their role(s) in muscle differentiation and adaptation.
Acknowledgments
This work was partially supported by K01 grant AR0505-01 from the National Institutes of Health, and by two University of Colorado Innovative Seed Grants.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s12576-010-0110-2
References
- 1.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge G, Greenspan DS. Developmental roles of the BMP1 metalloproteinases. Birth Defects Res C Embryo Today. 2006;78:47–68. doi: 10.1002/bdrc.20060. [DOI] [PubMed] [Google Scholar]
- 3.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ. Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS One. 2008;3:e1628. doi: 10.1371/journal.pone.0001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukagawa M, Suzuki N, Hogan BL, Jones CM. Embryonic expression of mouse bone morphogenetic protein-1 (BMP-1), which is related to the Drosophila dorsoventral gene tolloid and encodes a putative astacin metalloendopeptidase. Dev Biol. 1994;163:175–183. doi: 10.1006/dbio.1994.1133. [DOI] [PubMed] [Google Scholar]
- 6.Takahara K, Lyons GE, Greenspan DS. Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem. 1994;269:32572–32578. [PubMed] [Google Scholar]
- 7.Takahara K, Brevard R, Hoffman GG, Suzuki N, Greenspan DS. Characterization of a novel gene product (mammalian tolloid-like) with high sequence similarity to mammalian tolloid/bone morphogenetic protein-1. Genomics. 1996;34:157–165. doi: 10.1006/geno.1996.0260. [DOI] [PubMed] [Google Scholar]
- 8.Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, Greenspan DS. Mammalian BMP-1/tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 9.Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol. 1997;83:1857–1861. doi: 10.1152/jappl.1997.83.6.1857. [DOI] [PubMed] [Google Scholar]
- 10.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 11.Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, Morony S, Lacey DL, Hammond TG, Leinwand LL, Argraves WS, Bateman TA, Barth JL. Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol. 2009;106:582–595. doi: 10.1152/japplphysiol.90780.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol. 2007;292:C188–C199. doi: 10.1152/ajpcell.00542.2005. [DOI] [PubMed] [Google Scholar]
- 13.Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294:E918–E927. doi: 10.1152/ajpendo.00798.2007. [DOI] [PubMed] [Google Scholar]
- 14.Carlson CJ, Booth FW, Gordon SE. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol. 1999;277:R601–R606. doi: 10.1152/ajpregu.1999.277.2.r601. [DOI] [PubMed] [Google Scholar]
- 15.Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000;1497:77–88. doi: 10.1016/S0167-4889(00)00044-6. [DOI] [PubMed] [Google Scholar]
- 16.Salerno MS, Thomas M, Forbes D, Watson T, Kambadur R, Sharma M. Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am J Physiol Cell Physiol. 2004;287:C1031–C1040. doi: 10.1152/ajpcell.00492.2003. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma K, Watanabe K, Sano M, Kitajima S, Sakamoto K, Uramoto I, Totsuka T. The adaptive response of transforming growth factor-beta 2 and -beta RII in the overloaded, regenerating and denervated muscles of rats. Acta Neuropathol. 2000;299:177–185. doi: 10.1007/PL00007422. [DOI] [PubMed] [Google Scholar]
- 18.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 19.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 20.Ríos R, Carneiro I, Arce VM, Devesa J. Myostatin regulates cell survival during C2C12 myogenesis. Biochem Biophys Res Commun. 2001;280:561–566. doi: 10.1006/bbrc.2000.4159. [DOI] [PubMed] [Google Scholar]
- 21.Mendler L, Zádor E, Ver Heyen M, Dux L, Wuytack F. Myostatin levels in regenerating rat muscles and in myogenic cell cultures. J Muscle Res Cell Motil. 2000;21:551–563. doi: 10.1023/A:1026542303629. [DOI] [PubMed] [Google Scholar]
- 22.Ma K, Mallidis C, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab. 2001;281:E1128–E1136. doi: 10.1152/ajpendo.2001.281.6.E1128. [DOI] [PubMed] [Google Scholar]
- 23.Kaltschmidt B, Baeuerle PA, Kaltschmidt C. Potential involvement of the transcription factor NF-kappa B in neurological disorders. Mol Aspects Med. 1993;14:171–190. doi: 10.1016/0098-2997(93)90004-W. [DOI] [PubMed] [Google Scholar]
- 24.Tamura G, Olson D, Miron J, Clark TG. Tolloid-like 1 is negatively regulated by stress and glucocorticoids. Brain Res Mol Brain Res. 2005;142:81–90. doi: 10.1016/j.molbrainres.2005.09.016. [DOI] [PubMed] [Google Scholar]


