Abstract
The flavonoid quercetin has recently been reported to have neuroprotective effects, and the role of the gamma-aminobutyric acid A alpha 5 subunit (GABAA α5) receptor has been determined in some nervous system disorders. The aim of this study was to identify the molecular mechanism of the effect of quercetin administered at anticonvulsive doses on the expression of the GABAA α5 receptor gene in kainic acid (KA)-induced seizures in mice. The experimental animals were divided into four groups: control, KA, and KA + quercetin at 50 or 100 mg/kg, respectively. The results showed a dose-dependent reduction in the behavioral seizure score with quercetin pre-treatment in the KA mouse model. Two hours after the end of the 7-day treatment regimen, expression of the GABAA α5 receptor gene in the hippocampus was found to be increased in the KA group, but this increase was reduced in the KA + quercetin 50 or 100 mg/kg treatment groups. These results suggest that expression of the GABAA α5 receptor could be a mechanism for reducing seizure severity or may be a marker of seizure severity. Further studies are necessary to clarify quercetin’s mechanism of action and the relation of GABAA α5 receptor gene expression to seizure severity.
Keywords: Quercetin, Seizure, GABAA α5 subunit, Gene expression
Introduction
Epilepsy is one of the most common neurological disorders and affects approximately 1 % of the general population [1]. Most of the antiepileptic drugs currently available either control or reduce the occurrence of seizure. However, about one-third of patients with epilepsy have a refractory form of the disease. Temporal lobe epilepsy (TLE) is a form of partial epilepsy in adults [2]. Kainic acid (KA) is used to induce TLE in model systems and causes neuropathological and electroencephalographic manifestations that are observed in patients with TLE [3]. KA is a potent excitotoxin [4], and its effects are mediated through changes in the expression of the gamma-aminobutyric acid A alpha 5 subunit (GABAA α5) receptor in the hippocampus [5].
γ-Aminobutyric acid (GABA) is one of the major inhibitory neurotransmitters in the central nervous system. It acts on receptors coupled to chloride channels [6], controlling neuronal excitability by activating GABAA receptors on neurons by two major modes—phasic and tonic. Phasic inhibition is mediated by synaptic receptors, and tonic inhibition is mediated by extrasynaptic receptors [7]. Tonic inhibitory conductance is predominantly mediated by the GABAA α5 receptor [8], which is expressed in a number of areas of the brain but expressed at a higher level in the dendritic membrane of the principal cells of the hippocampus [9].
Quercetin is a flavonoid found in vegetables and fruits that has several biological effects [10], including antioxidative [11] and anti-inflammatory [12, 13] activities. Various studies have shown that quercetin has neuroprotective properties in central nervous system disorders, including memory impairment [14–16], seizure [17, 18], Huntington [19], and Parkinson’s disease [20]. In an earlier study we showed that quercetin has anticonvulsant activity in acute and chronic models of chemical kindling induced by pentylenetetrazole (PTZ) [15, 18]. More recently, Schipper et al. suggested that tonic GABAA receptors are a potential target for the treatment of TLE [21].
The aim of this study was to determine the molecular mechanism of the antiseizure effects of quercetin on the expression of the GABAA α5 receptor gene in a KA model of epilepsy in mice.
Materials and methods
A total of 48 male BALB/c mice (body weight 20–25 g) were obtained from the Razi Institute (Karaj, Iran) and housed under standard laboratory conditions. The mice were maintained at constant room temperature (21 ± 2 °C) under a 12:12 h light:dark cycle with free access to food and water. All animal experiments were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) to minimize the number of animals used and their suffering.
Quercetin and KA were purchased from Sigma (St. Louis, MO). Other drugs used in the study included xylazine (Loughrea Co., Galway, Ireland) and ketamine (Rotexmedica GmbH, Trittau, Germany). Quercetin was dissolved in Tween 80 (0.8 % v/v) and KA was dissolved in saline.
The mice were divided into four groups of 12 animals each. The control group was given an intraperitoneal (i.p.) injection of saline + Tween 80 (10 ml/kg) daily for 7 days; on the last day, saline was injected 30 min after the administration of saline + Tween 80. The KA group was given an i.p. injection of saline (10 ml/kg) daily for 7 days; on the last day, KA (10 mg/kg, i.p.) was injected 30 min after the administration of saline. In the two treatment groups, the mice were given an i.p. injection of quercetin at either 50 and 100 mg/kg daily for 7 days; on the last day, KA (10 mg/kg, i.p.) was injected 30 min after the administration of quercetin.
Following the administration of KA, mice were observed for behavioral changes over a period of 2 h. The behavioral scores were as follows: 0, no response; 1, immobility; 2, rigid posture; 3, scratching/circling/head bobbing; 4, forelimb clonus/rearing/falling; 5, repetitive pattern of 4; 6, severe tonic–clonic seizures [22]. Two hours after the administration of KA, all animals were anesthetized with an i.p. injection of ketamine (60 mg/ml)/xylazine (6 mg/kg) and sacrificed. The hippocampus each animal was immediately removed, cleaned with chilled saline, and frozen until used for the molecular analysis.
In the molecular analysis, frozen hippocampus tissues were homogenized and total RNA was extracted using the Total RNA Extraction kit of Jena Bioscience GmbH (Jena, Germany). The extracted RNA was then reverse transcribed using the Revert Aid First Strand cDNA Synthesis kit (Thermo Scientific–Fermentas, Waltham, MA).
The primers used were GABRA5 (F: AGTTGGAGGCAAGAACAGTT; R: AAGGAGGGTTTGGGTCATG) for the target gene and β-actin (F: TTACTGAGCTGCGTTTTACAC; R: ACAAAGCCATGCCAATGTTG) for the β-actin gene (internal control). All primers were designed using Gene Runner software (version 3.05). Quantitative reverse transcription (RT)-PCR was used to detect GABAA α5 RNA content in hippocampal tissues. A multiplex real-time PCR assay using SYBR Green I was performed in final reaction volumes of 20 μl containing 10 μl of SYBR Green I Master Mix (Bioneer, Korea), 10 pmol of forward and reverse primers, and 20 ng total RNA-derived cDNAs. Thermal cycling was performed using the ABI-7500 Sequence Detection System (Applied Biosystems, Foster, CA) with cycling conditions of 10 min at 95 °C for the first denaturation step, followed by 40 cycles at 95 °C for 20 s and 58 °C for 45 s; dissociation was run at 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s. The 2−ΔΔCt method was used to quantify data [23].
Data were expressed as the mean ± standard error of the mean. The data reported were analyzed by using a one-way analysis of variance accompanied by post hoc Turkey test for multiple comparisons. The analysis was completed using GraphPad Prism software (v5.04; GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered to represent a statistically significant difference.
Results and discussion
In the mice model used in our study, KA administered at a dose of 10 mg/kg caused seizures. Quercetin administered at 50 and 100 mg/kg, respectively, reduced the seizure score in animals in a dose-dependent manner compared to the KA group (P < 0.05 and P < 0.001, respectively) (Fig. 1).
Fig. 1.
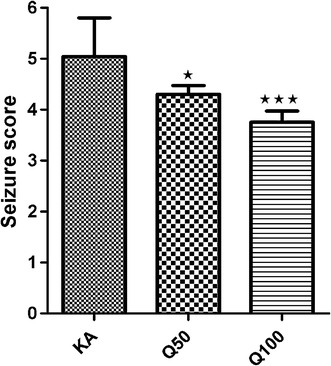
The effects of quercetin (doses 50 and 100 mg/kg) on seizure scores in mice with kainic acid (KA)-induced seizures. The KA group was administered an intraperitoneal (i.p.) dose of KA for 7 consecutive days, and on the last day, KA (10 mg/kg, i.p.) was injected 30 min after the administration of saline. The treatment groups were administered an i.p. dose of quercetin, either 50 (Q50) or 100 (Q100) mg/kg, for 7 days, and on the last day KA (10 mg/kg, i.p.) was injected 30 min after the administration of quercetin. Data are expressed as the mean ± standard error of the mean (SEM). Asterisks indicate a significant difference (*P < 0.05, ***P < 0.001) compared to the KA group according to the Tukey–Kramer test. n = 12 mice in each group
Expression of the GABAA α5 receptor gene was increased in the KA group compared to the control group at 2 h after the administration of KA (P < 0.001). The administration of quercetin doses at both 50 and 100 mg/kg significantly decreased the expression of the GABAA α5 receptor gene compared to the KA group (P < 0.001). The difference in expression of the GABAA α5 receptor gene in the quercetin and control groups was not significant (Fig. 2).
Fig. 2.
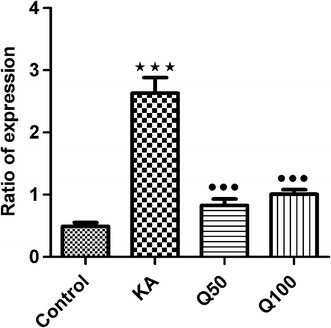
Effect of quercetin on the mRNA ratio of expression of the GABAA α5 receptor gene in a KA model of seizure in the hippocampus of mice. Data are expressed as the mean ± SEM. ***Significant difference at P < 0.001 compared to controls, •••significant different at P < 0.001 compared to the KA group according to the Tukey–Kramer test. n = 12 mice in each group
The results of our study show a dose-dependent reduction in the behavioral seizure score with quercetin pre-treatment in a KA mouse model of epilepsy, as well as a reduction in the expression of the GABAA α5 gene. In a previous study, we found that quercetin administered at anti-convulsive doses provided protection against memory impairment caused by PTZ-induced chemical kindling [17]. Other studies have also reported an increase in GABAA α5 expression in KA and pilocarpine models [24–26].
Increased expression of GABA receptors has been reported in epilepsy, suggesting that compensatory mechanisms are involved in disease pathogenesis [26]. Long-lasting decreases in the mRNA levels of the α5 and α2 GABAA receptor subunits in the CA1 area of the hippocampus and increases in α5 in the dentate granule cell layer have been observed in a pilocarpine model of epilepsy [27].
In a model of febrile seizures, the administration of lipopolysaccharide/KA to rat pups increased the protein levels of the GABAA α5 receptor concomitant with increasing interictal-like hippocampal activity and excitation of the Shaffer collateral–CA1 pathway in adult rats [28]. The authors of this study suggested that increased levels might be related to a compensatory mechanism [28]. It has also been shown that status epilepticus (SE) induced by KA causes early pyramidal neuron loss in the hippocampus with a transient increase in GABAA/central benzodiazepine (cBZR) density. These results suggest that an increase in the GABA/cBZR expression for each neuron might be a neuroprotective mechanism against the excitotoxic effects of SE to protect cells against further seizures [29]. Pretreatment with quercetin (100 mg/kg) has been found to have a modulatory effect on the expression of the GABAA subunits β1 and β3 receptor genes in a KA model of epilepsy [30]. However, flavonoids have also been shown to have an effect on ionotropic GABA receptors by acting as a positive, neutralizing allosteric modulator, suggesting that they could act on a variety of modulatory receptors [31]. Quercetin administered at a dose of 30 μM inhibited α1β1γ2 GABAA and ρ1 GABAc receptors in Xenopus laevis oocytes [32].
In conclusion, the expression of GABAA α5 may be a mechanism for reducing seizure severity or may, as a response to seizure activity, be a marker of seizure severity. A better understanding of the time course of changes in the expression of the GABAA α5 gene and quantification of seizure severity (e.g., by electroencephalography) are necessary to clarify quercetin’s mechanism of action and the relation of α5 gene expression to seizure severity.
Acknowledgments
The authors are grateful to the Vice Chancellor of Research, Qazvin University of Medical Sciences, for financial support.
Compliance with ethical standards
Funding
This study was funded by Qazvin University of Medical Sciences (Grant No. 28.20.8918).
Ethical approval
All applicable international national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
All of the authors declare that they have no conflicts of interest.
Footnotes
S. Moghbelinejad and S. Alizadeh contributed equally to this work.
References
- 1.Sankaraneni R, Lachhwani D. Antiepileptic drugs—a review. Pediatr Ann. 2015;1(44):e36–e42. doi: 10.3928/00904481-20150203-10. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lévesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37:2887–2899. doi: 10.1016/j.neubiorev.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- 5.Sperk G, Schwarzer C, Tsunashima K, Kandlhofer S. Expression of GABA(A) receptor subunits in the hippocampus of the rat after kainic acid-induced seizures. Epilepsy Res. 1998;32:129–139. doi: 10.1016/S0920-1211(98)00046-1. [DOI] [PubMed] [Google Scholar]
- 6.Ito S. GABA and glycine in the developing brain. J Physiol Sci. 2016;66:375–379. doi: 10.1007/s12576-016-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 8.Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, Rosahl TW, Maubach K, Buhl EH, Whittington MA. Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol. 2004;559:721–728. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabata K, Mukai R, Ishisaka A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food Funct. 2015;6(5):1399–1417. doi: 10.1039/C4FO01178C. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary S, Ganjoo P, Raiusddin S, Parvez S. Nephroprotective activities of quercetin with potential relevance to oxidative stress induced by valproic acid. Protoplasma. 2015;252:209–217. doi: 10.1007/s00709-014-0670-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Park W (2016) Anti-inflammatory effect of quercetin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Molecules 21(4):450 [DOI] [PMC free article] [PubMed]
- 13.Zheng J, Wu J, Chen J, Liu J, Lu Y, Huang C, Hu G, Wang X, Zeng Y. Therapeutic effects of quercetin on early inflammation in hypertriglyceridemia-related acute pancreatitis and its mechanism. Pancreatology. 2016;16:200–210. doi: 10.1016/j.pan.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Hu J, Zhong L, Wang N, Yang L, Liu CC, Li H, Wang X, Zhou Y, Zhang Y, Xu H, Bu G, Zhuang J. Quercetin stabilizes apolipoprotein E and reduces brain Aβ levels in amyloid model mice. Neuropharmacology. 2016;108:179–192. doi: 10.1016/j.neuropharm.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Nassiri-Asl M, Moghbelinejad S, Abbasi E, Yonesi F, Haghighi MR, Lotfizadeh M, Bazahang P. Effects of quercetin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav. 2013;28:151–155. doi: 10.1016/j.yebeh.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Abdalla FH, Schmatz R, Cardoso AM, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Gonçalves Nunes MA, Rubin MA, da Cruz IB, Barbisan F, Dressler VL, Pereira LB, Schetinger MR, Morsch VM, Gonçalves JF, Mazzanti CM. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na(+), K(+)-ATPase activities. Physiol Behav. 2014;135:152–167. doi: 10.1016/j.physbeh.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Nieoczym D, Socała K, Raszewski G, Wlaź P. Effect of quercetin and rutin in some acute seizure models in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:50–58. doi: 10.1016/j.pnpbp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Nassiri-Asl M, Hajiali F, Taghiloo M, Abbasi E, Mohseni F, Yousefi F. Comparison between the effects of quercetin on seizure threshold in acute and chronic seizure models. Toxicol Ind Health. 2016;32:936–944. doi: 10.1177/0748233713518603. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s Disease. CNS Neurosci Ther. 2014;20:10–19. doi: 10.1111/cns.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez del Rio MA, Sánchez-Reus MI, Iglesias I, Pozo MA, García-Arencibia M, Fernández-Ruiz J, García-García L, Delgado M, Benedí J. Neuroprotective properties of standardized extracts of Hypericum perforatum on Rotenone model of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2013;12:665–679. doi: 10.2174/1871527311312050013. [DOI] [PubMed] [Google Scholar]
- 21.Schipper S, Aalbers MW, Rijkers K, Swijsen A, Rigo JM, Hoogland G, Vles JS. Tonic GABAA receptors as potential target for the treatment of temporal lobe epilepsy. Mol Neurobiol. 2016;53:5252–5265. doi: 10.1007/s12035-015-9423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16(4):1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Schwarzer C, Tsunashima K, Wanzenböck C, Fuchs K, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/S0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- 25.Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM. Early loss of interneurons and delayed subunit-specific changes in GABA(A)-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–445. doi: 10.1016/S0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 27.Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA, DeLorenzo RJ. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc Natl Acad Sci USA. 1996;93:9665–9669. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid AY, Riazi K, Campbell Teskey G, Pittman QJ. Increased excitability and molecular changes in adult rats after a febrile seizure. Epilepsia. 2013;54:e45–e48. doi: 10.1111/epi.12061. [DOI] [PubMed] [Google Scholar]
- 29.Vivash L, Tostevin A, Liu DS, Dalic L, Dedeurwaerdere S, Hicks RJ, Williams DA, Myers DE, O’Brien TJ. Changes in hippocampal GABAA/cBZR density during limbic epileptogenesis: relationship to cell loss and mossy fibre sprouting. Neurobiol Dis. 2011;41:227–236. doi: 10.1016/j.nbd.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Moghbelinejad S, Rashvand Z, Khodabandehloo F, Mohammadi G, Nassiri-Asl M. Modulation of the expression of the GABAA receptor β1 and β3 subunits by pretreatment with quercetin in the KA model of epilepsy in mice. J Pharmacopuncture. 2016;9:163–166. doi: 10.3831/KPI.2016.19.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanrahan JR, Chebib M, Johnston GAR (2015) Interactions of flavonoids with ionotropic GABA receptors. In: Rudolph U (ed) Advances in pharmacology, vol 72. Elsevier, Amsterdam, pp 190–199 [DOI] [PubMed]
- 32.Goutman JD, Waxemberg MD, Doñate-Oliver F, Pomata PE, Calvo DJ. Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur J Pharmacol. 2003;461:79–87. doi: 10.1016/S0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]


