Abstract
Orotate is a precursor of pyrimidine synthesis. The kidney uses exogenous orotate for the synthesis of uridine diphosphosugars, which are used in the glycosylation of collagen in glomerular and tubular basement membranes. Orotate uptake occurs in the liver and kidney, but its molecular mechanism is largely unknown. Since orotate has been shown to be a substrate of the renal urate/anion exchanger in brush border membrane vesicle studies, we investigated whether human URAT1 (hURAT1) mediates the transport of orotate using HEK293 cells expressing hURAT1 (HEK-hURAT1). hURAT1 mediated a time- and dose-dependent uptake of orotate (K m 5.2 μM). hURAT1-mediated [3H]orotate transport was inhibited strongly by non-labeled (cold) orotate and the uricouric agent benzbromarone, and moderately inhibited by urate, nicotinate, and another uricouric agent, probenecid. This is the first report demonstrating that hURAT1 mediates the transport of orotate. hURAT1 may function as one of the entrance pathways in renal proximal tubular cells.
Keywords: Orotic acid, Pyrimidine, Transporter
Introduction
Orotate (orotic acid, 6-carboxyuracil), a normal intermediate in pyrimidine metabolism, is converted to uridine 5′-triphosphate (UTP), which is utilized for the synthesis of RNA and for the synthesis of uridine 5′-diphosphohexoses and uridine 5′-diphosphohexosamines (UDP sugars) [1–3]. Since UDP sugars are used in the glycosylation of the basement membrane collagen, increased bioavailability of these compounds is involved in the pathogenesis of renal hypertrophy with concomitant thickening of the glomerular and tubular basement membranes observed in human diabetes mellitus [4, 5]. Therefore, as a precursor of uridine nucleotides, exogenous orotate incorporation is important for the synthesis of basement membrane material. Orotate uptake is observed in the normal liver and kidney [6], but its molecular mechanism for transmembrane permeation is largely unknown.
The findings that orotate trans-stimulates the uptake of urate via the urate/anion exchanger in brush-border membrane vesicles in humans [7] and that it inhibits cloned human renal urate transporter hURAT1-mediated urate uptake in Xenopus oocytes [8] prompted us to investigate whether orotate is a true transport substrate for hURAT1. The purpose of this study was to elucidate the molecular mechanism underlying the membrane transport of orotate using HEK293 cells stably expressing hURAT1 (HEK-hURAT1).
Materials and methods
Materials
[3H]Orotate (20 Ci/mmol) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO). Nonlabeled orotate, urate, nicotinate, lactate, probenecid, pyrazinoate (PZA) and para-aminohippurate (PAH) were obtained from Sigma Chemical Co. (St. Louis, MO). Fetal bovine serum, trypsin and geneticin were obtained from Invitrogen (Carlsbad, CA).
Cell culture
HEK-hURAT1 cells were established as previously described [9]. HEK-hURAT1 cells were obtained by transfecting HEK293 cells with the full-length cDNA of hURAT1 subcloned into pcDNA 3.1 (Invitrogen, Carlsbad, CA) using Lipofectamine 2000 (Invitrogen). HEK293 cells transfected with pcDNA3.1 lacking an insert were used as a control (HEK-mock cells). These cells were grown in a humidified incubator at 37°C and in 5% CO2 using minimum essential medium containing 10% fetal bovine serum and 400 μg/ml geneticin, and were used for 15–25 passages.
Uptake experiments
Uptake experiments were performed as previously described [10]. The cells were seeded in 24-well tissue culture plates at a density of 1.6 × 105 cells/well. After the cells had been cultured for 2 days, they were washed three times with serum- and chloride-free Hanks’ balanced salt solution (HBSS) containing the following in mM: 125 Na gluconate, 4.8 K gluconate, 1.2 KH2PO4, 1.2 MgSO4, 1.3 Ca gluconate, 5.6 glucose and 25 HEPES, pH 7.4. After preincubation in the same solution in a water bath at 37°C for 10 min, the cells were incubated in a solution containing 50 nM [3H]orotate at 37°C for the indicated time. [3H]Orotate uptake was stopped by adding ice-cold HBSS, and the cells were washed three times with the same solution. The cells in each well were lysed with 0.5 ml of 0.1 N sodium hydroxide and transferred to 2.5 ml of aquasol-2, and then radioactivity was determined using a β-scintillation counter (LSC-3100, Aloka, Tokyo, Japan).
Inhibition study
To evaluate the inhibitory effects of various substrates on urate uptake mediated by hURAT1, HEK-hURAT1 cells were incubated in a solution containing 50 nM [3H]orotate for 2 min in the absence or presence of various of inhibitors (100 μM) at 37°C. Drugs such as probenecid and benzbromarone were dissolved in dimethylsulfoxide. The final concentration of dimethylsulfoxide in the incubation medium was adjusted to <0.5%.
Transport study in Xenopus oocyte
Trans-stimulation experiments were performed using Xenopus oocytes injected with or without hURAT1 cRNA as described previously [8]. Briefly, defolliculated Xenopus oocytes (stage IV and V) were injected with 20 ng of capped hURAT1 cRNA and incubated at 18°C in a modified Barth’s solution (88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO3)2, 0.4 mM CaCl2, 0.8 mM MgSO4, 2.4 mM NaHCO3, and 10 mM HEPES) containing gentamicin (50 μg/ml). After incubation for 2–3 days, uptake experiments were performed at room temperature in ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH 7.4). Control and hURAT1-expressing oocytes were injected with 50 nl of water or unlabeled anions using fine-tipped micropipettes, and the uptake experiment was initiated by replacing the ND96 solution with that containing radiolabeled 50 nM [3H]orotate and was terminated by adding ice-cold ND96 solution after 60 min of incubation. Oocytes were washed five times with ice-cold ND96 solution, solubilized with 5% SDS, and the radioactivity content was determined.
Statistical analysis
Data are expressed as means ± SEM. Statistical differences were determined using one-way analysis of variance with Dunnett’s post hoc test or Student’s t test. Differences were considered significant at P < 0.05.
Results
We showed that hURAT1 mediates the uptake of orotate. hURAT1 exhibited a time-dependent uptake of orotate up to 15 min (Fig. 1a). To elucidate the property of orotate transport via hURAT1, we performed kinetic analysis of orotate uptake. hURAT1 mediated a dose-dependent uptake of orotate (Fig. 1b), and Eadie-Hofstee analysis revealed that the K m value for hURAT1-mediated orotate uptake was 5.2 ± 0.4 μM. To examine whether hURAT1 mediates orotate/anion exchange, we compared the effect of Cl− for hURAT1-mediated orotate uptake. As shown in Fig. 1c, removal of outside Cl− enhanced orotate uptake via hURAT1. To compare the orotate transport property via hURAT1 with its property for urate transport, we examine the trans-stimulatory effects of anions on hURAT1-mediated orotate transport using Xenopus oocyte expression system. Similar to hURAT1-mediated urate transport, orotate uptake via hURAT1 was strongly stimulated by intracellularly loaded pyrazinoate (25 mM) and nicotinate (25 mM), although its effect was not comparable for lactate (100 mM) (Fig. 1d).
Fig. 1.
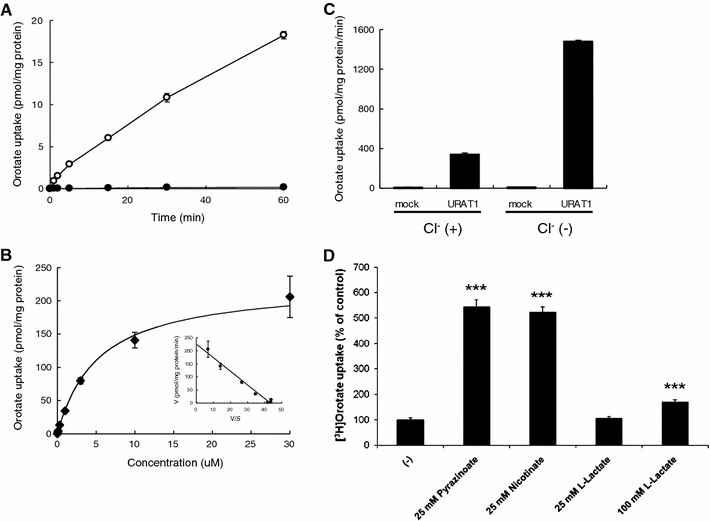
Orotate uptake by hURAT1. a Time course of orotate uptake in HEK-hURAT1 cells. hURAT1 and mock cells were incubated in Cl−-free HBSS solution containing 50 nM [3H]orotate for 2 min at 37°C. Open circles represent uptake into hURAT1-expressing cells. Closed circles represent uptake into mock cells (mean ± SEM; n = 4). b Concentration-dependent uptake of orotate in HEK-hURAT1 cells. Uptake of 50 nM [3H]orotate by control or hURAT1-expressing cells was measured for 2 min at various concentrations (0.03, 0.1, 0.3, 1, 3, 10, 30 μM) (mean ± SEM; n = 4). Inset, Eadie-Hofstee plot. V, velocity; V/S, velocity per concentration of orotate. c Orotate uptake via hURAT1 was stimulated by removal of Cl− from the bath. Transport rates of [3H]orotate (50 nM) in HEK-mock and HEK-hURAT1 cells were measured for 2 min in the presence or absence of extracellular Cl−. Each value represents the mean ± SE of four monolayers from two separate experiments. d Trans-stimulatory effect of test anions on the uptake of [3H]orotate via hURAT1. Control and hURAT1-expressing oocytes were injected with several unlabeled anions indicated, or water and incubated for 15 min at RT. Then, the oocytes were incubated with [3H]orotate (50 nM) and its uptake for 1 h was determined. The data are mean ± SEM with n = 6–8. ***P < 0.001 versus the uptake of water injected oocytes
In order to further investigate the substrate selectivity of hURAT1-mediated orotate uptake and orotate uptake via hURAT1, we examined the inhibitory effects of probenecid, benzbromarone, PZA, and PAH. Probenecid, benzbromarone, PZA, and PAH inhibited orotate uptake mediated by hURAT1 (Fig. 2).
Fig. 2.
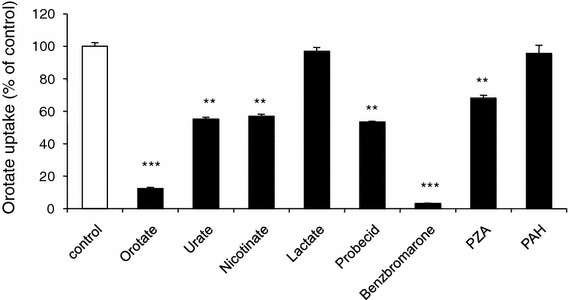
Effects of various substrates on orotate uptake via hURAT1. HEK-URAT1 cells were incubated in a solution containing 50 nM [3H]orotate at 37°C for 2 min in the absence (open column) or presence (closed column) of several urate-related compounds (100 μM). Each value represents the mean ± SEM of four determinations. **P < 0.01; ***P < 0.001 versus control
Discussion
In this study, we investigated whether orotate is a true transport substrate for hURAT1 using HEK293 cells stably expressing hURAT1 (HEK-hURAT1) and tried to elucidate the molecular mechanism underlying the membrane transport of orotate in renal tubules.
Orotate is formed de novo in the liver and is released into blood circulation [11]. It is excreted mainly from the kidneys and is present in the urine of humans [12]. Since the tubular excretion of orotate was inhibited by probenecid, it is speculated that the renal organic anion transporter system mediates the membrane permeation of this compound [12]. In 2008, we examined orotate uptake by human organic anion transporters OATs (OAT1, OAT3, OAT4, and OAT7) using proximal tubular cells stably expressing hOATs (S2-hOAT1, S2-hOAT3, S2-hOAT4, and S2-hOAT7) [13, 14], and we found that only OAT4 showed significant uptake of orotate with low affinity (K m 922 μM) [15]. This value is larger than the orotate concentration in the plasma (~1.8 μM) and final urine (~3.2 μM) [16]. We then tried to check the transport of orotate by another OAT family member, URAT1, a urate/anion exchanger that is expressed only in the kidney [8].
As we demonstrated in this study, orotate (K m 5.2 μM) is a better transport substrate for URAT1 than is urate (K m 371 μM) (Fig. 1b). The Cl− exchange property (Fig. 1c) and the inhibitory profile for hURAT1-mediated orotate uptake (Fig. 2) are the same as its urate uptake [8]. In addition, the profile of trans-stimulatory effects (strong stimulation by pyrazinoate and nicotinate, but weak by lactate) for hURAT1-mediated orotate uptake (Fig. 1d) is similar to urate uptake via hURAT1 as previously reported [7]. Since defects in the hURAT1 gene (SLC22A12) lead to idiopathic renal hypouricaemia (MIM number, 220150), hURAT1 is thought to participate in tubular urate reabsorption and to regulate blood urate levels in humans [17–19]. There is no doubt about the role of URAT1 in humans, but, so far, we have no idea about the role of this gene in other mammals in which serum urate levels are not as high as those in humans because of the normal function of hepatic urate oxidase that metabolizes urate into allantoin. Since genetic loss of this enzyme occurred in the Miocene epoch, only humans and higher apes have elevated serum urate levels [20]. Thus, this gene can function as a urate reabsorptive transporter in such species and, for other mammals, it may function as an orotate reabsorptive transporter, taking the existence of the outwardly directed lactate gradient in the tubular cells created by sodium-coupled monocarboxylate transporters (Smcts) into account.
As we mentioned in the Introduction, orotate uptake is observed in the liver and kidney [6], and orotate is incorporated not only in the tubules, but also in the glomeruli as a uridine nucleotide precursor necessary for the glycosylation of basement membrane collagen [4, 5]. We demonstrated that URAT1 seems to contribute to orotate uptake in renal tubules, but its molecular identity in glomeruli and its exit pathway from hepatocytes are still unknown. Our results may provide a clue for identifying the proteins responsible for membrane permeation of orotate in these tissues.
In conclusion, human URAT1 mediated the transport of orotate, which may be the molecular mechanism underlying the membrane permeation of this nucleic acid precursor in renal tubular cells.
Acknowledgments
The authors thank Sirinun Nilwarangkoon, Rie Noshiro-Kofuji, Naoko Ohtsu, and Ai Tsukada for the technical assistance. This work was supported in part by grants from the Japan Society for the Promotion of Science (JSPS KAKENHI 21390073, 21659216), Gout Research Foundation of Japan, the Shimabara Science Promotion Foundation, the Nakatomi Foundation, and Kyorin University School of Medicine (Kyorin Medical Research Award 2006).
References
- 1.Cortes P, Levin NW, Martin PR. Ribonucleic acid synthesis in the renal cortex at the initiation of compensatory growth. Biochem J. 1976;158:457–470. doi: 10.1042/bj1580457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JS, Malamud D, Caulfield JA, Malt RA. Differential labeling with orotic acid and uridine in compensatory renal hypertrophy. Am J Physiol. 1975;229:952–954. doi: 10.1152/ajplegacy.1975.229.4.952. [DOI] [PubMed] [Google Scholar]
- 3.Lewan L, Petersen I, Yngner T. Incorporation of orotic acid into nucleotides and RNA in mouse organs during 60 minutes. Hoppe Seylers Z Physiol Chem. 1975;356:425–429. doi: 10.1515/bchm2.1975.356.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Dumler F, Cortes P, Levin NW, Spargo BH, Rubenstein AH, Verghese CP. Effects of orotate administration on the normal rat kidney: similarity to changes observed in diabetes mellitus. Diabetes. 1979;28:680–685. doi: 10.2337/diab.28.7.680. [DOI] [PubMed] [Google Scholar]
- 5.Cortes P, Dumler F, Goldman J, Levin NW. Relationship between renal function and metabolic alterations in early streptozocin-induced diabetes in rats. Diabetes. 1987;36:80–87. doi: 10.2337/diabetes.36.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Lea MA, Oliphant V, Luke A. Orotate uptake and metabolism in normal and neoplastic tissues. Comp Biochem Physiol B. 1987;86:581–586. doi: 10.1016/0305-0491(87)90452-4. [DOI] [PubMed] [Google Scholar]
- 7.Roch-Ramel F, Guisan B, Jaeger P, Diezi J. Transport of urate and other organic anions by anion exchange in human renal brush-border membrane vesicles. Cell Physiol Biochem. 1996;6:60–71. doi: 10.1159/000154795. [DOI] [Google Scholar]
- 8.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 9.Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin HJ, Enomoto A, Sakamoto S, Hirata T, Tomita K, Kanai Y, Endou H. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem. 2004;279:45942–45950. doi: 10.1074/jbc.M406724200. [DOI] [PubMed] [Google Scholar]
- 10.Shin HJ, Takeda M, Enomoto A, Fujimura M, Miyazaki H, Anzai N, Endou H. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology. 2011;16:156–162. doi: 10.1111/j.1440-1797.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 11.Tax WJ, Veerkamp JH, Schretlen ED. The urinary excretion of orotic acid and orotidine, measured by an isotope dilution assay. Clin Chim Acta. 1978;90:217–223. doi: 10.1016/0009-8981(78)90260-7. [DOI] [PubMed] [Google Scholar]
- 12.Volle RL, Green RE, Peters L, Handschumacher RE, Welch AD. Renal tubular excretion studies with pyrimidine derivatives and analogs. J Pharmacol Exp Ther. 1962;136:353–360. [PubMed] [Google Scholar]
- 13.Babu E, Takeda M, Nishida R, Noshiro-Kofuji R, Yoshida M, Ueda S, Fukutomi T, Anzai N, Endou H. Interactions of human organic anion transporters with aristolochic acids. J Pharmacol Sci. 2010;113:192–196. doi: 10.1254/jphs.09339SC. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, Anzai N, Enomoto A, He X, Kim K, Endou H, Kanai Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45:1046–1055. doi: 10.1002/hep.21596. [DOI] [PubMed] [Google Scholar]
- 15.Anzai N, Miura D, Endou H. Orotic acid transport via organic anion transporters OATs. Gout Nucleic Acid Metab. 2008;32:141–146. [Google Scholar]
- 16.Calvo L, Rodríguez J, Vinagre F, Sánchez A. Determination of orotic acid (vitamin B13) in human serum and urine by differential-pulse polarography. Analyst. 1988;113:321–323. doi: 10.1039/an9881300321. [DOI] [PubMed] [Google Scholar]
- 17.Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19:151–157. doi: 10.1097/BOR.0b013e328032781a. [DOI] [PubMed] [Google Scholar]
- 18.Anzai N, Jutabha P, Endou H. Renal solute transporters and their relevance to serum urate disorder. Curr Hypertens Rev. 2010;6:148–154. doi: 10.2174/157340210791936732. [DOI] [Google Scholar]
- 19.Anzai N, Jutabha P, Kimura T, Fukutomi T (2011) Urate transport: regulators of serum urate levels in humans. Curr Rheumatol Rev (in press)
- 20.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.HYP.0000028589.66335.AA. [DOI] [PubMed] [Google Scholar]


