Abstract
The putative human tumor suppressor gene FHIT (fragile histidine triad) (M. Ohta et al., Cell 84:587–597, 1996) encodes a protein behaving in vitro as a dinucleoside 5′,5′′′-P1,P3-triphosphate (Ap3A) hydrolase. In this report, we show that the Saccharomyces cerevisiae APH1 gene product, which resembles human Fhit protein, also hydrolyzes dinucleoside 5′,5′-polyphosphates, with Ap3A being the preferred substrate. Accordingly, disruption of the APH1 gene produced viable S. cerevisiae cells containing reduced Ap3A-hydrolyzing activity and a 30-fold-elevated Ap3N concentration.
Aberrant transcripts of the human FHIT (fragile histidine triad) gene have been detected in nearly 50% of esophageal, stomach, and colon carcinomas (26). Accordingly, deletions in the FHIT gene, which is located at chromosome region 3p14.2, were found associated with many types of common human cancers, including lung (7, 37), skin (36), head and neck (22, 38), breast (20, 23), cervical (9), and colorectal (10) cancers. Actually, the FHIT gene encompasses FRA3B, the most highly inducible fragile site in the human genome (13, 26). The FRA3B breakpoints, including the renal carcinoma-associated one, t(3;8), fall within introns 3, 4, and 5 of the FHIT gene and are likely to be at the origin of the cancer-associated deletions (39). However, although the strong correlation between FHIT alterations and cancer development may indicate a suppressor function of the FHIT gene product, this function has not yet been proven (for reviews, see references 13, 16, and 27).
A marked similarity between the protein product of FHIT and the amino acid sequence of a Schizosaccharomyces pombe enzyme described as a diadenosine 5′,5′′′-P1,P4-tetraphosphate (Ap4A) asymmetrical hydrolase (12, 33) was noted early (26). Ap4A belongs to an unusual family of ubiquitous dinucleoside polyphosphates, the possible functions of which are still debated. At one time, these nucleotides were suspected of being involved in the proliferation activity of animal cells (for a review, see reference 8). However, this hypothesis did not receive support from further studies (for a review, see reference 32). Because their intracellular concentration increases in response to oxidative stresses (for reviews, see references 15 and 28), dinucleoside polyphosphates were also proposed to participate to cellular adaptation. Finally, extracellular functions have been envisaged. For instance, dinucleoside polyphosphates were reported to interfere with cardiovascular and neurotransmission activities (for reviews, see references 2 and 24).
The Fhit protein could be produced from cDNA in Escherichia coli. In agreement with its resemblance to an Ap4A hydrolase, this protein accelerated the in vitro hydrolysis of various dinucleoside polyphosphates, including Ap4A (1). The most efficient substrate was diadenosine 5′,5′′′-P1,P3-triphosphate (Ap3A). In light of the putative cancer suppression function of the FHIT product, the observations described above supported the idea of some role of dinucleoside polyphosphates in tumor development. Whether dinucleoside triphosphates rather than dinucleoside tetraphosphates would be involved is still an open question.
In Saccharomyces cerevisiae, Ap4A is transformed into ATP plus ADP by two Ap4A phosphorylases encoded by two distinct genes, APA1 and APA2 (30, 31). To recycle Ap3A, which is fully resistant to the two Ap4A phosphorylases, the yeast cell produces an Ap3A hydrolase capable of converting Ap3A into ADP plus AMP (3). The enzyme also slowly hydrolyzes Ap4A into ATP plus AMP.
The S. cerevisiae genome sequence data indicates a gene (APH1) product highly similar to the Ap4A hydrolase of S. pombe (12, 26). To determine whether this protein product behaves like the S. pombe Ap4A hydrolase or whether it corresponds to the previously described S. cerevisiae Ap3A hydrolase, we have undertaken its expression in E. coli. In vitro characterization of the obtained protein strongly indicates that it corresponds to the already isolated Ap3A hydrolase, therefore resembling the Fhit protein. In addition, gene disruption experiments unambiguously show that the APH1 product is the main actor in S. cerevisiae Ap3N (N = A, C, G, or U) catabolism. This conclusion emphasizes the putative predominance of dinucleoside triphosphates in any FHIT-mediated function.
MATERIALS AND METHODS
Yeasts, bacteria, and plasmids.
The strains used are listed in Table 1. Bacterial and yeast transformations were performed by electroporation and by the lithium acetate method of Ito et al. (14), respectively. YPD and minimal sporulation media were as described before (34). Plasmid pBluescript SK(−) was from Stratagene (San Diego, Calif.).
TABLE 1.
Bacterial and yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| E. coli JM101TR | Δ(lac-pro) supE thi recA56 srl-300::Tn10 (F′ traD36 proAB lacIqlacZΔM15) | 11 |
| S. cerevisiae | ||
| CMY214 | trp1-Δ1/trp1-Δ1 his3Δ200/his3Δ200 ura3-52/ura3-52 ade2-101/ade2-101 lys2-801/lys2-801 can1/CAN1 | 21 |
| YPALHU | CMY214 APA1/apa1Δ::HIS3 APA2/apa2Δ::URA3 | 30 |
| YPALHUT | YPALHU APH1/aph1Δ::TRP1 | This work |
| YPALS | trp1-Δ1 his3Δ200 ura3-52 ade2-101 lys2-801 can1 | 30 |
| YPALSH | YPALS apa1Δ::HIS3 | 30 |
| YPALSU | YPALS apa2Δ::URA3 | 30 |
| YPALSHU | YPALS apa1Δ::HIS3 apa2Δ::URA3 | 30 |
| YPALST | YPALS aph1Δ::TRP1 | This work |
| YPALSHT | YPALS apa1Δ::HIS3 aph1Δ::TRP1 | This work |
| YPALSUT | YPALS apa2Δ::URA3 aph1Δ::TRP1 | This work |
| YPALSHUT | YPALS apa1Δ::HIS3 apa2Δ::URA3 aph1Δ::TRP1 | This work |
Nucleotides.
Ap4C and Ap4G were extracted from E. coli PAL2103D (18) as described previously (30). Ap3C was enzymatically synthesized with purified E. coli lysyl-tRNA synthetase (29). Other nucleotides were from Boehringer (Ap4A and Ap5A), Sigma (Ap3A), or Pharmacia (Ap3G, Gp3G, and Gp4G). [3H]Ap4A (reference batch TRQ.4405, 159 GBq/mmol, radiochemical purity of >98%) was from Amersham.
Preparation of S. cerevisiae crude extracts.
For the preparation of S. cerevisiae crude extracts, cells were grown until the optical density at 650 nm of the culture reached 2 ± 0.5. After centrifugation at 12,000 × g for 15 min, the cell pellet was suspended in 50 mM Tris-HCl buffer (pH 7.8) containing 0.1 mM EDTA, 10 mM 2-mercaptoethanol, and 1 mM phenylmethylsulfonide fluoride (PMSF). Cells were sonicated twice for 5 min at 0°C, and cell debris was removed by centrifugation at 17,000 × g for 15 min. The total amount of protein in the supernatant was determined by using the Bio-Rad protein assay reagent.
Enzymatic assays.
Hydrolysis of [3H]Ap4A was used to monitor enzyme activity; the reaction mixture (100 μl) contained 50 mM Tris-HCl (pH 7.8), 5 mM MgCl2, 0.1 mM EDTA, 50 μM [3H]Ap4A (2 GBq/mmol), and a 140-U/ml concentration of alkaline phosphatase from calf intestine (2,000 U/mg; Boehringer). After an incubation ranging from 10 to 90 min at 37°C, the [3H]adenosine produced was counted as described previously (31).
When Ap3A hydrolysis was used to monitor enzyme activity, the same reaction mixture was made, except that unlabeled 50 μM Ap3A was the substrate. After a 10- to 120-min incubation at 37°C, the reaction was stopped by the addition of perchloric acid (10%, wt/vol) and the mixture was centrifuged at 17,000 × g for 15 min. The supernatant was neutralized by K2CO3 and further centrifuged for 5 min at 17,000 × g. The supernatant was diluted 20-fold in Tris-HCl buffer (20 mM, pH 7.8) containing 0.1 mM ZnCl2 and 1 mM MgCl2. The concentration of the Ap3A remaining at the end of the reaction was determined with the help of the luminescence assay described below.
To compare the activity of the APH1 gene product in response to various substrates, the incubation mixture (105 μl) contained 40 mM Tris-HCl (pH 7.8), 0.5 mM MgCl2, 20 μM EDTA, 200 μg of bovine serum albumin per ml, and a 20 μM concentration of the substrate under study. After a 10-min incubation at 25°C, the reaction was stopped by freezing the samples in liquid nitrogen. The intact substrate remaining was quantitated after separation from the reaction products on a high-pressure liquid chromatography column (0.46 by 20 cm) packed with Lichrosorb RP18. The column was isocratically eluted at a flow rate of 1.5 ml/min with 50 mM potassium phosphate (pH 5.3). The concentrations of the nucleotides under study were deduced from their absorbancy in the column effluent at 254 nm.
Determination of Ap4N and Ap3N concentrations in S. cerevisiae crude extracts.
Nucleotides were extracted with perchloric acid from 30-ml aliquots of the cultures, as described previously (31). The sample was then neutralized by K2CO3 and diluted fivefold in a Tris-HCl buffer (20 mM, pH 7.8) containing 0.1 mM ZnCl2 and 1 mM MgCl2. After digestion of ATP with alkaline phosphatase from calf intestine (3,000 U/mg, enzyme immunoassay grade; Boehringer), two successive luminescence assays were performed (4). In the first one, a 10-μl extract sample was mixed with 100 μl of the ATP-bioluminescence HS mixture from Boehringer. After extinction of the luminescence background due to ATP contamination, snake venom phosphodiesterase (Boehringer) was added at a final concentration of 5 μg/ml and the luminescence was recorded. In this assay, only Ap4N nucleotides contribute to the signal. In the second assay, 10 μl of sample was mixed with 100 μl of the luminescence mixture containing, in addition, 0.25 mM phosphoenolpyruvate (from Sigma) and 35 U of rabbit muscle pyruvate kinase (Boehringer) per ml. After extinction of the signal caused by contaminating ATP and ADP, phosphodiesterase was added at a final concentration of 5 μg/ml and the luminescence was recorded. Under these conditions, both the Ap3N and Ap4N compounds contributed to the light signal. The Ap3N concentration was deduced from the difference between the two assays (4, 25).
Cellular concentrations of Ap4N and Ap3N were calculated by assuming that, in the yeast culture, one optical density unit at 650 nm corresponds to 0.4 μl of intracellular volume (6).
Cloning of the S. cerevisiae APH1 gene.
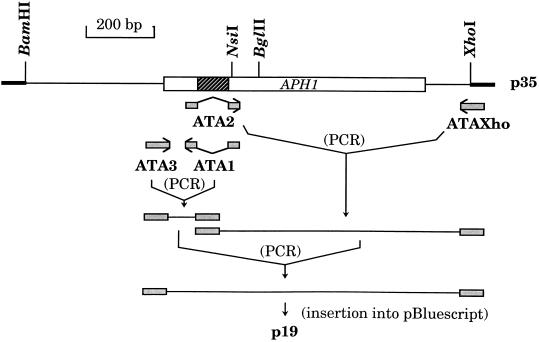
The APH1 gene of S. cerevisiae was amplified by PCR using 25 pmol of the oligonucleotides ATABam (5′-CTTAGAATGCAGCGGATCCTTGGGATTAGC-3′) and ATAXho (5′-TTTACTGTTGAGTCTCCTCGAGGAAAGTAG-3′) and 3.4 μg of genomic DNA from strain CMY214 (30 cycles; 95°C for 1 min, 40°C for 1 min, 72°C for 2 min). The sequences of the two primers encompass the BamHI and XhoI sites flanking the APH1 gene (Fig. 1). The amplified DNA fragment of 1.3 kbp was purified with the Qiagen Plasmid Mini Kit 100. After digestion by BamHI and XhoI, the purified DNA fragment was inserted into the corresponding sites of plasmid pBluescript SK(−) to give plasmid p35.
FIG. 1.
Construction of plasmid p19. The p35-inserted APH1 gene is represented by a rectangle with the intron shaded. Two PCR amplifications were performed with plasmid p35 as the template and either ATA3 plus ATA1, or ATA2 plus ATAXho, as the primer pair. Primers ATA1 and ATA2 are complementary to the exonic sequences on both sides of the intron. The two amplified DNA fragments were mixed and further subjected to PCR with ATA3 and ATAXho as the primers. The resulting DNA was cut with BamHI and XhoI, the sites of which are present in ATA3 and ATAXho, respectively, and inserted into plasmid pBluescript SK(−), to give plasmid p19.
Disruption of the APH1 gene in S. cerevisiae.
To inactivate the APH1 gene, a 75-bp NsiI-BglII fragment internal to the gene was deleted and substituted by the selectable marker TRP1. For this purpose, the TRP1 marker carried by plasmid pRS314 (35) was amplified by PCR with primers TRPNsi (5′-GAGTGATGCATAAACGACATTACTATATATA-3′) and TRPBgl (5′-GTCACAGATCTGGCAAGTGCACAAACAATA-3′) (30 cycles; 95°C for 1 min, 45°C for 1 min, 72°C for 2 min). The resulting DNA was digested by NsiI and BglII before insertion into p35.
The resulting plasmid was digested by BamHI and XhoI and used to transform the diploid yeast strain YPALHU. PCR amplifications using either ATABam and ATAXho, or ATABam and TRPBgl, as primers were performed to verify that the resulting transformed strain, YPALHUT, actually harbored both a wild-type and a disrupted copy of the APH1 gene.
Recombination of the APH1 gene for overexpression in E. coli cells.
Deletion of the intron harbored by the APH1 gene and insertion of a canonical Shine-Dalgarno sequence upstream of this gene was performed as described in the legend to Fig. 1. Briefly, two PCR amplifications were performed with plasmid p35 as the template and either ATA3 (5′-GCGGGATCCCTAGAAAGGAGGTACGATCATGAATAAGCCAATATATTTCAGCA-3′) plus ATA1 (5′-ATATTTTGACTTATAGAAAACTTGTTCAGTTACAAG-3′) or ATA2 (5′-CAAGTTTTCTATAAGTCAAAATATACGTATGCATTG-3′) plus ATAXho as the primer pair (30 cycles; 95°C for 1 min, 50°C for 1 min, 72°C for 2 min). After Qiagen purification, the two resulting DNA fragments were mixed and further subjected to 30 cycles of amplification using ATA3 and ATAXho as primers. The final purified DNA was cut by BamHI and XhoI and inserted into the corresponding sites of plasmid pBluescript SK(−), to give plasmid p19. The nucleotide sequence of the insert was verified.
Purification of the APH1 gene product.
JM101TR cells harboring plasmid p19 were grown in 125 ml of LB medium containing 100 μg of ampicillin per ml and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). When the optical density at 650 nm reached 2.0, the cells were harvested by a 30-min centrifugation at 12,000 × g. The cell pellet, resuspended in 8 ml of a 50 mM Tris-HCl buffer (pH 7.8) containing 10% glycerol and 0.5 mM PMSF, was sonicated for 4 min at 0°C. After removal of cellular debris by centrifugation (30 min, 12,000 × g), nucleic acids were precipitated by the addition of streptomycin sulfate (3% [wt/vol] final concentration) and centrifuged (30 min, 12,000 × g). The supernatant (7.5 ml) was dialyzed against 1 liter of 20 mM potassium phosphate (pH 7.0)–10% glycerol–0.1 mM PMSF and applied to a DEAE Sephadex column (1 by 5 cm) equilibrated in 20 mM potassium phosphate (pH 7.0). The column was eluted by use of 20 to 200 mM linear gradient of potassium phosphate (80 ml, pH 7.0) at a flow rate of 6 ml/h. During the purification, the activity of the APH1 gene product was monitored through hydrolysis of either Ap4A or Ap3A. The fractions containing activity were pooled and concentrated by ammonium sulfate precipitation. At the end of this step, the specific Ap3A degradation activity of the sample had increased ∼16-fold in comparison to that of the crude extract.
RESULTS AND DISCUSSION
Expression of the S. cerevisiae APH1 gene in E. coli.
In 1996, the sequencing of the FHIT cDNA revealed homologies between the Fhit protein and two yeast proteins, an Ap4A hydrolase produced by the S. pombe aph1 gene and the product of an S. cerevisiae gene (26). The latter gene was named APH1 because of its homology to the S. pombe aph1 gene. It was also designated HNT2 by other authors (see accession no. U28374 in the EMBL and GenBank databases) and received the name YDR305c in the systematic genome sequence designation. Two possible in-frame initiator codons are found in the 5′ region of the S. cerevisiae APH1 gene; they correspond to either MILSK or MNKPI N-terminal sequences. The second one is likely to be used as the translational start because it corresponds to the initiator methionine in S. pombe. The coding sequence of the APH1 gene is interrupted by a single intron of 89 bp. The 5′ boundary of this intron is located 56 bp downstream of the putative translational start. The protein encoded by the APH1 gene is expected to contain 206 amino acids (Mr = 23,541).
To compare the S. cerevisiae APH1 gene product and the previously isolated Ap3A hydrolase (3), chromosomal DNA of strain CMY214 was used to amplify the APH1 DNA by PCR. The resulting DNA was inserted into plasmid pBluescript under control of the Plac promoter. The intron of the gene was then deleted, and the sequence upstream of the initiation codon was replaced by a powerful Shine-Dalgarno sequence (5, 17), yielding plasmid p19 (Fig. 1).
Upon transformation by plasmid p19, E. coli JM101TR clearly showed a new protein band on sodium dodecyl sulfate-polyacrylamide gels. This band migrated with an apparent Mr of 26,000 ± 2,000 (data not shown). This molecular ratio fits with that expected for the APH1 product. Moreover, a crude extract of the p19-transformed E. coli strain contained 500- and 20,000-fold-larger amounts of Ap4A- and Ap3A-hydrolyzing activities, respectively, than an extract of the control strain containing pBluescript. Upon chromatography through a DEAE-Sephadex column, both the Ap3A- and Ap4A-hydrolyzing activities comigrated with the 26-kDa polypeptide described above. However, lability of activity precluded further purification of the enzyme. Such a behavior resembles that of the previously isolated Ap3A hydrolase, the activity of which also vanished during purification (3). The partially purified APH1 product could be stored after ammonium sulfate precipitation, and various dinucleotides could be assayed as substrates (Table 2). Strikingly, the relative rates of hydrolysis by APH1 of the assayed substrates were nearly identical to those already reported in the case of Ap3A hydrolase from S. cerevisiae (3). In particular, the dinucleoside triphosphates Ap3A, Ap3G, and Ap3C were the most efficient substrates.
TABLE 2.
Comparison of the substrate specificities of the APH1 gene product and of the S. cerevisiae Ap3A hydrolase
| Substrate | Relative rate of hydrolysis (%)
|
|
|---|---|---|
| APH1 gene producta | Ap3A hydrolaseb | |
| Ap3A | 100 | 100 |
| Ap4A | 11 ± 1.6 | 13 |
| Ap5A | 6 ± 1.1 | 4 |
| Gp3G | 80 ± 3 | 74 |
| Gp4G | 51 ± 2 | 61 |
| Ap3C | 94 ± 6 | 94 |
| Ap3G | 92 ± 7 | 95 |
| Ap4G | 73 ± 9 | 76 |
| Ap4C | 7.8 ± 1.3 | 4.3 |
The reaction mixture (105 μl) contained 40 mM Tris-HCl (pH 7.8), 0.5 mM MgCl2, 20 μM EDTA, 200 μg of bovine serum albumin per ml, a 20 μM concentration of the substrate under study, and catalytic amounts of enzyme. Initial rates of hydrolysis are expressed as percentages (means ± standard deviations) of the rate measured with Ap3A. Each measurement was done in duplicate.
Data from reference 3.
Disruption of the APH1 gene.
In S. cerevisiae, Ap4A catabolism is mainly sustained by two Ap4A phosphorylases produced by the APA1 and APA2 genes (30, 31). Strains fully devoid of Ap4A phosphorylase activity have been described. In crude extracts of such a strain, a weak Ap4A-hydrolyzing activity was measurable (3). This hydrolysis could be assigned to an Ap3A hydrolase having a rather broad specificity. To examine whether the weak Ap4A hydrolysis described above was associated with the APH1 gene, we have undertaken the knockout of this gene in an apa1 apa2 context and measured dinucleoside tri- and tetraphosphate-hydrolyzing activities in cell extracts.
In the diploid apa1/APA1 apa2/APA2 strain YPALHU, one copy of each of the APA1 and APA2 genes are inactivated by insertion of a HIS3 and a URA3 cassette, respectively. To achieve disruption of APH1, a 75-bp fragment of this gene was deleted on plasmid p35 and replaced by the selectable marker TRP1 (35). The corresponding plasmid was linearized and used to transform strain YPALHU. Resulting diploid cells were left to sporulate, and haploids were selected as canavanine-resistant clones. Among the canr cells obtained, 14% proved to be his− ura− trp− (APA1 APA2 APH1), 12% were his+ ura− trp− (apa1), 8% were his− ura+ trp− (apa2), 17% were his− ura− trp+ (aph1), 9% were his+ ura+ trp− (apa1 apa2), 16% were his+ ura− trp+ (apa1 aph1), 11% were his− ura+ trp+ (apa2 aph1), and 12% were his+ ura+ trp+ (apa1 apa2 aph1). These numbers show that the APH1 product is not essential to cell viability, even in the absence of Ap4A phosphorylase activity. Whereas the growth of the cells in YPG solid or liquid medium was not sensitive to inactivation of one or two of the three genes APA1, APA2, and APH1, it was slightly but significantly impaired by simultaneous inactivation of all three genes. Generation times of strains YPALS, YPALST, and YPALSHU in liquid medium were 101 ± 4 (n = 4), 103 ± 4 (n = 4), and 102 ± 3 (n = 4) min, respectively. In contrast, the generation time of strain YPALSHUT was 150 ± 5 min (n = 4).
In crude extracts of strain YPALST (aph1) and YPALSHUT (apa1 apa2 aph1), the specific Ap3A hydrolase activity was reduced more than 20-fold, as compared to that of YPALS, the control strain. In addition, the Ap4A hydrolase activity of a crude extract of strain YPALSHUT (apa1 apa2 aph1) was reduced nearly fourfold, as compared to that of strain YPALSHU (apa1 apa2). These measurements establish that APH1 actually directs hydrolytic activity towards Ap3A as well as Ap4A. They also show that APH1 is responsible for the major part of the weak Ap4A-hydrolyzing activity detected in a YPALSHU extract.
Ap3N and Ap4N concentrations in various aph1 mutants.
In the control strain YPALS (APA1 APA2 APH1), Ap3N and Ap4N concentrations are equal to 0.4 μM. These concentrations were increased 30- and 2.5-fold, respectively, upon disruption of the APH1 gene only (Table 3). The variation of Ap4N may appear relatively small in view of the broad specificity of the APH1 product. However, in the YPALS and YPALST strains, two Ap4A phosphorylases which are likely to control Ap4N concentration occur, thereby counterbalancing the effect of the absence of Ap3A hydrolase activity on the cellular pool of these nucleotides.
TABLE 3.
Degradation activities and dinucleotide concentrations in various S. cerevisiae strainsa
| Strain | Relative degradation activityb
|
Relative dinucleotide concentrationc
|
||
|---|---|---|---|---|
| Ap4A | Ap3A | Ap4N | Ap3N | |
| YPALS (control) | 1 | 1 | 1 | 1 |
| YPALST (aph1) | 0.8 ± 0.07 | <0.05 | 2.5 ± 0.2 | 31 ± 1 |
| YPALSHU (apa1 apa2) | 0.011 ± 0.007 | 1 ± 0.1 | 50 ± 3 | NMd |
| YPALSHUT (apa1 apa2 aph1) | 0.003 ± 0.001 | <0.05 | 750 ± 50 | NM |
Yeast cells were grown in rich YPD medium. When the optical density of the culture reached 2 ± 0.5 at 650 nm, two samples of the culture were removed for either enzymatic or dinucleotide measurements (100 and 30 ml, respectively). Each measurement was done in duplicate. Ap4A and Ap3A degradation activities were measured in crude extracts obtained by sonication. The reaction mixtures contained 50 mM Tris-HCl (pH 7.8), 5 mM MgCl2, 0.1 mM EDTA, 140 U of alkaline phosphatase per ml, and a 50 μM concentration of the substrate under study. Ap4N and Ap4N plus Ap3N concentrations were assayed by bioluminescence as described in Materials and Methods. Values are given as means ± standard deviations.
With the control strain YPALS, Ap3A degradation activity was 0.8 μmol/min/g of protein. Ap4A degradation activities were 6.9 μmol/min/g in the absence of added phosphate and 14.0 μmol/min/g in the presence of 1 mM phosphate. Comparison of the latter values indicates that Ap4A phosphorylases in the extracts from YPALS and YPALST mainly contribute to the measured Ap4A degradation activities. The values in the table correspond to the activities in the absence of phosphate.
In the control strain YPALS, Ap4N and Ap3N concentrations amounted to 0.4 μM each.
NM, not measurable.
The APH1 gene was also disrupted in a strain lacking the two Ap4A phosphorylase genes. This time, the lack of Ap3A hydrolase activity raised the Ap4N pool 15-fold. Ap3N concentrations could not be reliably measured in extracts of these strains (YPALSHU and YPALSHUT) because of an excessive contribution of Ap4N to the pyruvate kinase-containing luminescence assay.
The combination of these results with the above finding that the growth rate of YPALST (aph1) is indistinguishable from that of YPALS (APH1) suggests that the S. cerevisiae cell cycle is not under the control of the Ap3N pool. In the case of YPALSHUT (apa1 apa2 aph1), the observed reduction of the growth rate can be simply related to the nucleotide unbalance caused by the ∼300 μM cellular Ap4N concentration.
Conclusions.
Altogether, the present results establish that APH1, an analog of FHIT in S. cerevisiae, encodes a protein product involved mainly in Ap3N catabolism. This product corresponds to the Ap3A hydrolase already characterized (3). The similarity between the in vitro functions of the APH1- and FHIT-encoded polypeptide products sustains the view that Ap3N concentration in human cells also might be deregulated by FHIT inactivation.
Regarding the putative role of FHIT in tumor suppression, an abnormally high Ap3N cellular concentration resulting from inactivation of one FHIT allele in the diploid human cell can be hypothesized to directly or indirectly impair growth control or induce tumorigenesis. In this context, involvement of the Fhit protein as well as of the APH1 product in an as-yet-unrecognized nucleotidyl transferase reaction at the expense of the Ap3N substrate (19) must not be neglected. The present study, however, has not made it possible to relate APH1 inactivation to a growth defect in S. cerevisiae.
REFERENCES
- 1.Barnes L D, Garrison P N, Siprashvili Z, Guranowski A, Robinson A K, Ingram S W, Croce C M, Ohta M, Huebner K. Fhit, a putative tumor suppressor in humans, is a dinucleoside 5′,5′′′-P1,P3-triphosphate hydrolase. Biochemistry. 1996;35:11529–11535. doi: 10.1021/bi961415t. [DOI] [PubMed] [Google Scholar]
- 2.Baxi M D, Vishwanatha J K. Diadenosine polyphosphates: their biological and pharmacological significance. J Pharmacol Toxicol Methods. 1995;33:121–128. doi: 10.1016/1056-8719(94)00127-p. [DOI] [PubMed] [Google Scholar]
- 3.Brevet A, Chen J, Fromant M, Blanquet S, Plateau P. Isolation and characterization of a dinucleoside triphosphatase from Saccharomyces cerevisiae. J Bacteriol. 1991;173:5275–5279. doi: 10.1128/jb.173.17.5275-5279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brevet A, Plateau P, Best-Belpomme M, Blanquet S. Variation of Ap4A and other dinucleoside polyphosphates in stressed Drosophila cells. J Biol Chem. 1985;260:15566–15570. [PubMed] [Google Scholar]
- 5.Chen J, Brevet A, Lapadat-Tapolsky M, Blanquet S, Plateau P. Properties of the lysyl-tRNA synthetase gene and product from the extreme thermophile Thermus thermophilus. J Bacteriol. 1994;176:2699–2705. doi: 10.1128/jb.176.9.2699-2705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coste H, Brevet A, Plateau P, Blanquet S. Nonadenylylated bis(5′-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J Biol Chem. 1987;262:12096–12103. [PubMed] [Google Scholar]
- 7.Fong K M, Biesterveld E J, Virmani A, Wistuba I, Sekido Y, Bader S A, Ahmadian M, Ong S T, Rassool F V, Zimmerman P V, Giaccone G, Gazdar A F, Minna J D. FHIT and FRA3B 3p14.2 allele loss are common in lung cancer and preneoplastic bronchial lesions and are associated with cancer-related FHIT cDNA splicing aberrations. Cancer Res. 1997;57:2256–2267. [PubMed] [Google Scholar]
- 8.Grummt F. Diadenosine tetraphosphate as a putative intracellular signal of eukaryotic cell cycle control. Mod Cell Biol. 1988;6:29–64. [Google Scholar]
- 9.Hendricks D T, Taylor R, Reed M, Birrer M J. FHIT gene expression in human ovarian, endometrial, and cervical cancer cell lines. Cancer Res. 1997;57:2112–2115. [PubMed] [Google Scholar]
- 10.Hibi K, Taguchi M, Nakamura H, Hirai A, Fujikake Y, Matsui T, Kasai Y, Akiyama S, Ito K, Takagi H. Alternative splicing of the FHIT gene in colorectal cancers. Jpn J Cancer Res. 1997;88:385–388. doi: 10.1111/j.1349-7006.1997.tb00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirel P H, Lévêque F, Mellot P, Dardel F, Panvert M, Mechulam Y, Fayat G. Genetic engineering of methionyl-tRNA synthetase: in vitro regeneration of an active synthetase by proteolytic cleavage of a methionyl-tRNA synthetase-β-galactosidase chimeric protein. Biochimie. 1988;70:773–782. doi: 10.1016/0300-9084(88)90107-1. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Garrison P N, Barnes L D. Cloning of the Schizosaccharomyces pombe gene encoding diadenosine 5′,5′′′-P1,P4-tetraphosphate (Ap4A) asymmetrical hydrolase: sequence similarity with the histidine triad (HIT) protein family. Biochem J. 1995;312:925–932. doi: 10.1042/bj3120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebner K, Hadaczek P, Siprashvili Z, Druck T, Croce C M. The FHIT gene, a multiple tumor suppressor gene encompassing the carcinogen sensitive chromosome fragile site, FRA3B. Biochim Biophys Acta. 1997;1332:M65–M70. doi: 10.1016/s0304-419x(97)00009-7. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzler J W, Farr S B, Ames B N. Intracellular functions of ApnN: prokaryotes. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 135–149. [Google Scholar]
- 16.Kok K, Naylor S L, Buys C H C M. Deletions of the short arm of chromosome 3 in solid tumors and the search for suppressor genes. Adv Cancer Res. 1997;71:27–92. doi: 10.1016/s0065-230x(08)60096-2. [DOI] [PubMed] [Google Scholar]
- 17.Lauer G, Rudd E A, McKay D L, Ally A, Ally D, Backman K C. Cloning, nucleotide sequence, and engineered expression of Thermus thermophilus DNA ligase, a homolog of Escherichia coli DNA ligase. J Bacteriol. 1991;173:5047–5053. doi: 10.1128/jb.173.16.5047-5053.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lévêque F, Blanchin-Roland S, Fayat G, Plateau P, Blanquet S. Design and characterization of Escherichia coli mutants devoid of Ap4N-hydrolase activity. J Mol Biol. 1990;212:319–329. doi: 10.1016/0022-2836(90)90127-8. [DOI] [PubMed] [Google Scholar]
- 19.Lima C D, D’Amico K L, Naday I, Rosenbaum G, Westbrook E M, Hendrickson W A. MAD analysis of FHIT, a putative human tumor suppressor from the HIT protein family. Structure. 1997;5:763–774. doi: 10.1016/s0969-2126(97)00231-1. [DOI] [PubMed] [Google Scholar]
- 20.Man S, Ellis I O, Sibbering M, Blamey R W, Brook J D. High levels of allele loss at the FHIT and ATM genes in non-comedo ductal carcinoma in situ and grade I tubular invasive breast cancers. Cancer Res. 1996;56:5484–5489. [PubMed] [Google Scholar]
- 21.Mann C, Buhler J-M, Treich I, Santenac A. RPC40, a unique gene for a subunit shared between yeast RNA polymerases A and C. Cell. 1987;48:627–637. doi: 10.1016/0092-8674(87)90241-8. [DOI] [PubMed] [Google Scholar]
- 22.Mao L, Fan Y-H, Lotan R, Hong W K. Frequent abnormalities of FHIT, a candidate tumor suppressor gene, in head and neck cancer cell lines. Cancer Res. 1996;56:5128–5131. [PubMed] [Google Scholar]
- 23.Negrini M, Monaco C, Vorechovsky I, Ohta M, Druck T, Baffa R, Huebner K, Croce C M. The FHIT gene at 3p14.2 is abnormal in breast carcinomas. Cancer Res. 1996;56:3173–3179. [PubMed] [Google Scholar]
- 24.Ogilvie A, Bläsius R, Schulze-Lohoff E, Sterzel R B. Adenine dinucleotides: a novel class of signalling molecules. J Auton Pharmacol. 1996;16:325–328. doi: 10.1111/j.1474-8673.1996.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogilvie A, Jakob P. Diadenosine 5′,5′′′-P1,P3-triphosphate in eukaryotic cells: identification and quantitation. Anal Biochem. 1983;134:382–392. doi: 10.1016/0003-2697(83)90313-5. [DOI] [PubMed] [Google Scholar]
- 26.Ohta M, Inoue H, Cotticelli M G, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce C M, Huebner K. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 27.Pennisi E. New gene forges link between fragile site and many cancers. Science. 1996;272:649. doi: 10.1126/science.272.5262.649. [DOI] [PubMed] [Google Scholar]
- 28.Plateau P, Blanquet S. Dinucleoside oligophosphates in micro-organisms. Adv Microb Physiol. 1994;36:81–109. doi: 10.1016/s0065-2911(08)60177-0. [DOI] [PubMed] [Google Scholar]
- 29.Plateau P, Fromant M, Brevet A, Gesquière A, Blanquet S. Catabolism of bis(5′-nucleosidyl) oligophosphates in Escherichia coli: metal requirements and substrate specificity of homogeneous diadenosine 5′,5′′′-P1,P4-tetraphosphate pyrophosphohydrolase. Biochemistry. 1985;24:914–922. doi: 10.1021/bi00325a016. [DOI] [PubMed] [Google Scholar]
- 30.Plateau P, Fromant M, Schmitter J M, Blanquet S. Catabolism of bis(5′-nucleosidyl) tetraphosphates in Saccharomyces cerevisiae. J Bacteriol. 1990;172:6892–6899. doi: 10.1128/jb.172.12.6892-6899.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plateau P, Fromant M, Schmitter J M, Buhler J M, Blanquet S. Isolation, characterization, and inactivation of the APA1 gene encoding yeast diadenosine 5′,5′′′-P1,P4-tetraphosphate phosphorylase. J Bacteriol. 1989;171:6437–6445. doi: 10.1128/jb.171.12.6437-6445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remy P. Intracellular functions of ApnN: eukaryotes. In: McLennan A G, editor. Ap4A and other dinucleoside polyphosphates. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 151–204. [Google Scholar]
- 33.Robinson A K, de la Peña C E, Barnes L D. Isolation and characterization of diadenosine tetraphosphate (Ap4A) hydrolase from Schizosaccharomyces pombe. Biochim Biophys Acta. 1993;1161:139–148. doi: 10.1016/0167-4838(93)90207-8. [DOI] [PubMed] [Google Scholar]
- 34.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 35.Sikorski R S, Hieter P. A system for shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sozzi G, Alder H, Tornielli S, Corletto V, Baffa R, Veronese M L, Negrini M, Pilotti S, Pierotti M A, Huebner K, Croce C M. Aberrant FHIT transcripts in Merkel cell carcinoma. Cancer Res. 1996;56:2472–2474. [PubMed] [Google Scholar]
- 37.Sozzi G, Veronese M L, Negrini M, Baffa R, Cotticelli M G, Inoue H, Tornielli S, Pilotti S, De Gregorio L, Pastorino U, Pierotti M A, Ohta M, Huebner K, Croce C M. The FHIT gene at 3p14.2 is abnormal in lung cancer. Cell. 1996;85:17–26. doi: 10.1016/s0092-8674(00)81078-8. [DOI] [PubMed] [Google Scholar]
- 38.Virgilio L, Shuster M, Gollin S M, Veronese M L, Ohta M, Huebner K, Croce C M. FHIT gene alterations in head and neck squamous cell carcinomas. Proc Natl Acad Sci USA. 1996;93:9770–9775. doi: 10.1073/pnas.93.18.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimonjic D B, Druck T, Ohta M, Kastury K, Croce C M, Popescu N C, Huebner K. Positions of chromosome 3p14.2 fragile sites (FRA3B) within the FHIT gene. Cancer Res. 1997;57:1166–1170. [PubMed] [Google Scholar]