Abstract
Myokines are skeletal muscle-derived hormones. In this study, using a C2C12 myotube contraction system, we sought to determine whether the skeletal muscle secreted thioredoxin (TRX) and related redox proteins. Redox proteins such as TRXs, peroxiredoxins, and glutaredoxins were detected in the C2C12 myotube culture medium in the absence of any stimulation. The amounts of TRXs, peroxiredoxins, and glutaredoxins secreted by the C2C12 myotubes were not affected by the contraction, unless the myotubes were injured. Because TRX-1 was known to be a secreted protein that lacks a signal peptide, we examined whether this protein was secreted via exosome vesicles. The results indicated that TRX-1 was not secreted via exosome vesicles. We concluded that TRX-1 and related redox proteins are myokines that are constitutively secreted by the skeletal muscle cells. Although the mechanism of TRX-1 secretion remains unclear, our findings suggest that the skeletal muscle is an endocrine organ and the redox proteins that are constitutively secreted from the skeletal muscle may exert antioxidant and systemic health-promoting effects.
Electronic supplementary material
The online version of this article (doi:10.1007/s12576-014-0334-7) contains supplementary material, which is available to authorized users.
Keywords: Thioredoxin, Myokines, Skeletal muscle, Secretion
Introduction
Myokines produced and released by the muscle fibers are considered to exert either paracrine or endocrine effects, thereby regulating metabolism [1]. In particular, interleukin (IL)-6 has been extensively studied as a myokine [2]. Nedachi et al. [3] found that the contraction of C2C12 myotubes triggered the release of IL-6. However, the precise role of skeletal muscle-derived IL-6 is yet to be ascertained. This is particularly because both positive (increased glucose uptake in myocytes [4] and increased insulin-stimulated glycogen synthesis in skeletal muscle [5]) and negative (reduced insulin sensitivity in skeletal muscle [6], adipocytes [7], and hepatocytes [8]) roles have been reported. Irisin, a recently identified myokine, has drawn considerable attention because of its potential to increase the energy expenditure and improve insulin resistance [9]. Although it is unclear whether the muscle contraction triggers the secretion of irisin, recent studies have found increased levels of plasma irisin in mice after 3 weeks of exercise and in human subjects after 10 weeks of exercise [9]. To date, over 20 myokines have been identified [10]. However, the mechanism of their secretion, the sites of their action, and their physiological roles are poorly understood.
We have reported that macrophage migration inhibitory factor (MIF) is constitutively secreted by the skeletal muscle [11]. We found that the contraction of the skeletal muscle had an inhibitory effect on the secretion of MIF. Because MIF inhibits an AMP-activated protein kinase activator (AICAR)- or insulin-stimulated glucose transport in the skeletal muscle, reduced secretion of MIF in response to muscle contraction might contribute to enhanced glucose transport in the skeletal muscle [11].
MIF is a thioredoxin (TRX) family protein and contains the conserved redox active sequence motif (-C-X-X-C-motif) [12]. Both of the TRX isoforms, TRX-1 and TRX-2, possess oxidoreductase activity and mainly regulate the intracellular redox status. Recent studies have revealed that TRX-1 is secreted by various cell types, including the activated lymphocytes [13], neoplastic cells [14], liver cells [15], gastric mucosal cells [16], and monocytes [17]. It is believed that extracellular TRX-1 takes part in a variety of processes, including cell proliferation [15], redox regulation [18], cytokine-like activities [19], and apoptosis [20].
Physical exercise induces the production of reactive oxygen species (ROS) in the skeletal muscle, causing oxidative damages to many cells [21]. In contrast, ROS generated as a result of physical exercise act as signaling molecules that stimulate the expression of genes involved in antioxidant and DNA repair pathways [22, 23]. It has been reported that oxidative stress induced by exercise augments TRX expression in peripheral blood mononuclear cells [24]. Considering that TRX-1 is a secreted protein and exercise stimulates the antioxidant system, it is likely that TRX-1 is secreted from the skeletal muscle.
In the present study, we investigated whether TRX-1 and related redox proteins are secreted by C2C12 myotubes. Here we show that TRX-1 and related redox proteins are constitutively secreted by myotubes. The amount of TRX-1 secreted does not appear to be affected by the muscle contraction evoked by electrical stimulation, unless the myotubes are injured during the contraction.
Materials and methods
Cell culture
The mouse skeletal muscle-derived cells, C2C12 myoblasts (American Type Culture Collection, Manassas, VA, USA), were seeded in four-well rectangular plates (Thermo Scientific, Waltham, MA, USA) at a density of 2 × 105 cells/well in 3 ml of growth medium comprising Dulbecco’s modified Eagle’s medium (DMEM, 25 mM glucose; Life Technologies, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (Bio West, Nuaillé, France) and 1 % penicillin-streptomycin. The cultures were maintained in an incubator at 37 °C under an atmosphere of 5 % CO2. Upon reaching confluence, the medium was switched to a differentiation medium comprising a 1:1 mixture of Dulbecco’s modified Eagle's medium and nutrient F-12 1:1 mixture (DMEM/F-12; Life Technologies) containing 1 % penicillin-streptomycin, 10 μg/ml insulin (Life technologies), and 1× l-glutamine (Life Technologies) (day 0). The medium was changed once every 24 h. After 5 days of differentiation, the cells were used for the experiments.
Cell contraction
Immediately before the experiments, the medium was changed to 2 ml of fresh DMEM/F-12 (without any supplement and phenol red). The four-well plates were connected to an electrical stimulation apparatus, a four-well C-Dish (Ion Optix Corp., Milton, MA, USA). The C2C12 myotubes were stimulated with or without electric pulses of defined voltage at 1 Hz for 3 ms at intervals of 997 ms for a desired period in an incubator maintained at 37 °C [25]. Subsequently, the culture media (supernatant) and the myotubes were collected, and the volume of the recovered culture medium (R1) was measured. Then, the medium was centrifuged at 2,000×g for 20 min, followed by a second centrifugation at 12,000×g for 35 min. The supernatant was filtered through a Millex-GV Filter (0.22 μm, low protein binding, Millipore, Billerica, MA, USA). Following this, 1.2 ml of the filtered medium was concentrated using up to an approximately five-fold amount of Vivaspin 20 (Sartorius, Goettingen, Germany), and the volume of the concentrated medium recovered (R2) was measured. Although the volume of the filtered medium subjected to concentration was maintained constant, the concentration rate varied. Therefore, the expected recovery volume (R3) was calculated using the following expression: (R3) = (R2) × (1.2 ml/R1). In immunoblotting experiments, the expected recovered volume was used for volume-corrected sample loading. The myotubes were lysed with 300 μl of ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM beta-glycerophosphate, 5 mM sodium pyrophosphate tetrabasic, 1 mM sodium orthovanadate, 1 mM ethylenediaminetetraacetic acid (pH 8.0), 1 % Nonidet P-40, 150 mM sodium chloride, 10 mM sodium fluoride, 10 mg/l leupeptin, 3 mM benzamidine, 5 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride. The lysate was sonicated and centrifuged at 13,000×g for 15 min at 4 °C, and the supernatant was used for immunoblotting. Protein concentration was determined by the Bradford method.
For culture experiments involving the use of protease inhibitors, a protease inhibitor cocktail (P1860, Sigma, St. Louis, MO, USA) was added to the culture medium at a final concentration of 0.75 %. For control cultures, a medium containing DMSO at a final concentration of 0.75 % was used.
Immunoblotting
We used tricine-SDS PAGE for the detection of low-molecular-weight proteins [26]. It was unclear whether the electric stimulation increased or limited the amount of secreted proteins. A secreted housekeeping protein has not been reported. Therefore, we used the volume ratio of the recovered medium for the loading. The proteins present in the cell lysate or in the concentrated recovered culture medium were separated by SDS-PAGE (16 % polyacrylamide) and transferred onto a polyvinylidene difluoride membrane. The membrane was then blocked for 1 h at room temperature with Tris-buffered saline (10 mM Tris, 150 mM NaCl) containing 0.1 % Tween 20 (TBS-T, pH 7.8) and either 5 % nonfat dry milk or 5 % BSA. Following this, the membranes were incubated in TBS-T containing 5 % nonfat dry milk and the desired antibody overnight at 4 °C. Membranes were then probed with HRP-conjugated secondary antibodies (1:1,000) in TBS-T containing 5 % nonfat dry milk for 1 h at room temperature, and the specific immunocomplexes were visualized using the enhanced chemiluminescence system (PerkinElmer Life Sciences, Boston, MA). Bands were scanned and quantitated with Image J (http://rsbweb.nih.gov/ij/).
Fractionation of exosome
Exosomes were prepared according to reported methods [27] with minor modifications (Fig. 5a). In brief, the cell culture medium was centrifuged for 15 min at 2,000×g to remove debris. Following this, the medium was centrifuged at 12,000×g for 35 min. Both the supernatant and the pellet were used for subsequent experiments. The supernatant was filtered through a Millex-GV Filter (0.22 μm), and the filtrate was ultra-centrifuged at 110,000×g for 70 min at 4 °C. The supernatant was concentrated with the help of Vivaspin 20 by centrifuging at 3,000×g for 110 min. The pellet was washed with 600 μl PBS and centrifuged at 110,000×g for 70 min at 4 °C. The pellet was mixed with 50 μl of sample buffer for SDS-PAGE followed by immunoblotting.
Fig. 5.
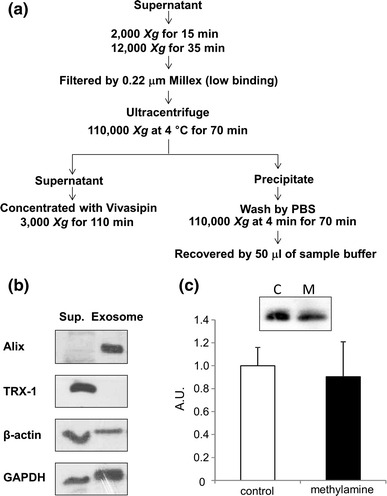
Mechanism of secretion of TRX-1. a Flow chart of the exosome fractionation of the C2C12 myotube culture medium. b Immunoblotting of the supernatant and exosome fraction. From each fraction, a 50-μl sample was loaded. Alix was used as the exosome marker. TRX-1 was detected in the supernatant. c TRX-1 secreted in the presence of methylamine. The C2C12 myotubes were treated with 1 mM methylamine for 1 h. Each sample was loaded according to the volume-corrected method (Materials and methods). Representative blots showing TRX-1 (C represents control; M represents methylamine treatment) and the calculated graph are presented. Values in the vertical axis are in arbitrary units (AU). Secretion of TRX-1 was unaffected by methylamine treatment (n = 4)
Lactate dehydrogenase activity assay
To determine the lactate dehydrogenase (LDH) activity, the LDH released into the culture medium from the cytosol of damaged cells was assayed using an LDH assay kit (Roche, Basel, Switzerland) as per the manufacturer’s instructions.
Reagents and antibodies
Anti-thioredoxin-1, Alix (3A9) mouse mAb, and anti-β-actin antibodies were purchased from Cell Signaling Technology (Boston, MA, USA). Anti-myoglobin was purchased from epitomics (Abcam, Cambridge, MA, USA). Anti-glutaredoxin-1 and anti-thioredoxin-2 antibodies were obtained from R&D Systems (Minneapolis, MN, USA). The anti-peroxiredoxin-1 antibody was obtained from Thermo Scientific (Rochester, NY, USA). Anti-peroxiredoxin-3 antibody was purchased from AbFrontier (Seoul, Korea). Anti-peroxiredoxin-6 was purchased from Sigma. Anti-glutaredoxin 2 antibody was obtained from the ProteinTech Group (Chicago, IL, USA). Anti-glutaredoxin 3 was purchased from Abnova (Taipei, Taiwan). The HRP-conjugated anti-mouse secondary antibody was obtained from Thermo Scientific, the HRP-conjugated anti-rabbit secondary antibody was obtained from Cell Signaling Technology, and the HRP-conjugated anti-goat antibody was purchased from Millipore (Temecula, CA, USA). All other reagents used in this study were purchased from Sigma or Wako Pure Chemical Industries (Osaka, Japan).
Statistics
Data are shown as mean ± SEM. The unpaired Student’s t test was performed to evaluate the statistical differences between the two groups. Statistical significance among multiple groups was determined by performing two-way ANOVA, followed by Tukey’s test. P < 0.05 was considered statistically significant.
Results
TRX-1 in the cell culture medium
We first examined whether unstimulated C2C12 myotubes secreted TRX-1 into the culture medium. TRX-1 was detected in the culture medium after 1, 3, or 6 h of incubation (Fig. 1). Furthermore, the amount of TRX-1 in the culture medium increased in a time-dependent manner, suggesting that TRX-1 is constitutively secreted by C2C12 myotubes (Fig. 1). We also examined the presence of other redox proteins, including TRX-2, glutaredoxin-1 (GLRX, a member of the thioredoxin family), GLRX-2, GLRX-3, peroxiredoxin-1 (PRX, a member of the thiol-redox peroxidase family), PRX-3, and PRX-6. These proteins were also detected in the cell culture medium and their abundance increased in a time-dependent manner (Fig. 1), suggesting that these redox proteins were also constitutively secreted by C2C12 myotubes.
Fig. 1.
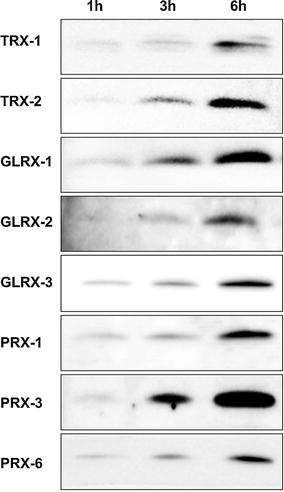
Immunoblotting of the TRX-1 and related redox proteins present in the C2C12 myotube culture medium. C2C12 myotubes were incubated in fresh serum-free medium for 1, 3, or 6 h. The supernatant from the cultures were then concentrated as described in the “Materials and methods” section and was used for immunoblotting. Each sample was loaded according to the volume-corrected method (Materials and methods). TRX-1 and -2; glutaredoxin-1, -2, and -3; and peroxiredoxin-1, -3, and -6 were detected in the culture medium in a time-dependent manner
Secretion of TRX-1 is not regulated by myotube contraction
It was reported that muscle contraction regulates the secretion of some myokines [2]. We examined whether the secretion of TRX-1 was regulated by muscle contraction. A cultured myotube contraction system that mimicked the in vivo skeletal muscle contraction was used for this purpose [25]. Because cultured C2C12 cells form a monolayer of myotubes and are more vulnerable to physical stress (such as contraction or extension) compared to muscle tissues isolated from mice or rats, LDH activity, a marker of cell injury in the myotube culture medium was also measured. The C2C12 myotubes were stimulated to contract with the help of electric pulses of various strengths for desired periods. The amount of TRX-1 secreted in response to contraction induced by electric pulses of 10 V for 1 h, 10 V for 3 h, 30 V for 3 h, and 12 V for 24 h was similar to that of the controls (Fig. 2). By examination under a microscope, we confirmed that the C2C12 myotubes contracted in response to electric pulses of 10 and 30 V (Supplemental Video 1 and 2).
Fig. 2.
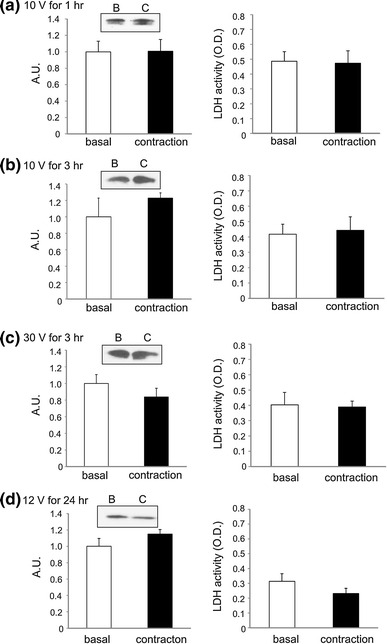
Immunoblotting of the TRX-1 present in the culture supernatant and the LDH activity in unstimulated (basal) and contracted C2C12 myotubes. The C2C12 myotubes were stimulated by electric pulses of a 10 V for 1 h, b 10 V for 3 h, c 30 V for 3 h, or d 12 V for 24 h at 1 Hz for 3 ms duration at intervals of 997 ms. The medium collected was concentrated and was subjected to immunoblotting for TRX-1, and the LDH activity was measured. Left column shows the representative blots and calculated graph based on the intensity of the bands. Values in the vertical axis are in arbitrary units (AU). Right column shows LDH activity under various conditions of stimulation (n = 4 and 5)
We noticed that under high voltage (50 V at 1 Hz for 3 ms at 997-ms intervals for 6 h), which induced severe contraction, the amount of TRX-1 in the culture medium was significantly greater (Fig. 3a). Further, the LDH activity, a marker of cell injury, in the culture medium of contracting myotubes was significantly higher than that in the control medium (Fig. 3b). These observations suggested that under conditions that induced extreme contraction, the amount of TRX-1 in the culture medium increased, likely because of a leakage from the intracellular sites of the injured C2C12 myotubes. Under these conditions, the amount of other redox proteins examined also increased in the medium (Fig. 3c). Thus, adequate regulation of the strength of contraction (or level of electrical stimulation) is necessary to avoid muscle injury and false results regarding the modulation of myokine secretion by muscle contraction.
Fig. 3.
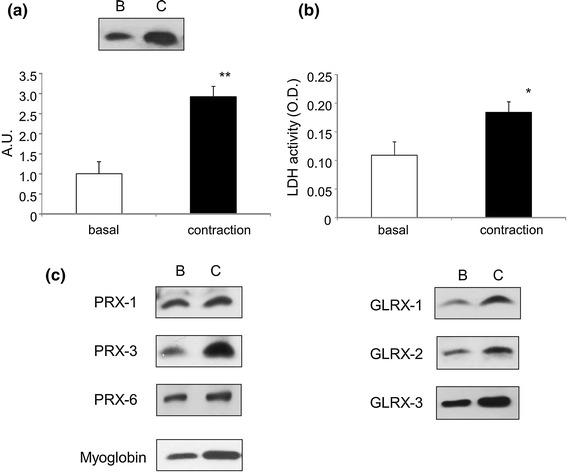
Immunoblotting of TRX-1-related proteins and LDH activity in the culture medium of unstimulated (basal) and contracted C2C12 myotubes. The C2C12 myotubes were stimulated by electric pulses of 50 V at 1 Hz for 3 ms at intervals of 997-ms for 6 h. The media collected were concentrated and subjected to immunoblotting and LDH activity measurement. a Representative immunoblots (TRX-1) and calculated graph based on the intensity of the bands. Values in the vertical axis are in arbitrary units (AU). The amount of TRX-1 was significantly higher under this condition (**P < 0.01, n = 4). b LDH activity in medium. LDH activity was significantly higher (*P < 0.05, n = 4). c Representative immunoblots showing peroxiredoxin-1, -3, and -6; glutaredoxin-1, -2, and -3; and myoglobin. Strenuous contraction augmented the abundance of all proteins investigated
Effect of protease inhibition on TRX-1 secretion
It has been reported that cell types such as cancer cells [28], mesenchymal stem cells [29], glial cells [30], and cardiomyocytes [31] release proteases. To exclude the possibility that the secreted TRX-1 is degraded by secreted proteases, the C2C12 myotubes were stimulated with electrical pulses of 50 V for 1 h to contract in the presence or absence of protease inhibitors in the culture medium. The amount of TRX-1 accumulated in the culture medium treated with protease inhibitors was significantly higher than that detected in the absence of the protease inhibitors, suggesting that TRX-1 is cleaved by secreted proteases present in the culture medium. In contrast, there was no difference in the TRX-1 levels between the control and contracting myotube cultures, suggesting that the secretion of the proteases was not contraction dependent (Fig. 4). This result further confirmed that muscle contraction does not stimulate TRX-1 secretion from C2C12 myotubes.
Fig. 4.
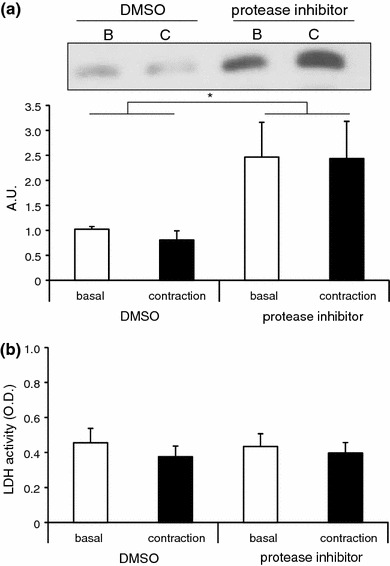
Effect of protease inhibitor on the abundance of secreted thioredoxin-1 (TRX-1) in the culture medium. a Immunoblotting of TRX-1 present in the myotube culture medium produced in the presence or absence of the protease inhibitor cocktail. C2C12 myotubes were stimulated by electric pulses of 50 V at 1 Hz for 3 ms for 1 h. Values in the vertical axis are in arbitrary units (AU). The amount of secreted TRX-1 present in the myotube culture medium was higher in the presence of protease inhibitor, but the secretion was not regulated by the contraction (n = 9–11). Two-way analysis showed a significant effect for the inhibitor. The amount of TRX-1 in the culture medium treated with protease inhibitor was significantly higher than that in the absence of the protease inhibitor [two-way ANOVA, F(1, 29) = 6.715, P < 0.05]. b LDH activity in C2C12 myotubes treated with protease inhibitor. Treatment with protease inhibitor or that combined with electrical stimulation did not result in an increase in the LDH activity in the medium (n = 9–11)
Exosome fractionation
Proteins secreted via the classical pathway possess an N-terminal signal peptide, which is necessary for the entry into the endoplasmic reticulum-Golgi system, where the proteins are modified, folded, sorted, and transported to the extracellular space [32]. Recently, a non-classical secretory pathway, in which the secretion does not depend on the signal peptide sequence, has been described [32]. This pathway involves various protein secretion routes, including protein translocation across the plasma membrane, lysosome-dependent secretion, and exosome-mediated secretion [32]. Although TRX-1 was categorized as a non-classical secreted protein [14], the detailed mechanism of its secretion is poorly understood. It has been reported that the skeletal muscle secretes exosomes, which are microvesicles originating from multivesicular endosomes [27]. It has also been reported that TRX exists in exosomes [33]. Therefore, we examined whether TRX-1 was secreted via exosome vesicles. We fractionated exosomes by ultracentrifugation as shown in Fig. 5a. Alix, a marker protein of exosome, was used to verify the accuracy of fractionation. As shown in Fig. 5b, we were able to successfully isolate the exosomes. TRX-1 was detected in the supernatant, but not in the exosomal fraction. This result suggested that TRX-1 is secreted via exosome-independent pathways. It has been reported that methylamine inhibits the secretion of interleukin-1β, a protein without a signal peptide sequence, in activated T cells via an unknown mechanism [14]. We examined the effect of methylamine on the secretion of TRX-1 and found that the constitutive secretion of TRX-1 was not inhibited by methylamine (Fig. 5c).
Discussion
The results of this study showed that redox proteins such as TRX-1 are secreted by C2C12 myotubes. We found that in the absence of any stimulation, these secreted proteins accumulated in the culture medium in a time-dependent manner, suggesting that these proteins were constitutively secreted as myokines. Further, except when the myotubes were injured, the TRX-1 secretion was found to be contraction independent. These results suggested that redox-related proteins are not myokines secreted in response to muscle contraction. Our results clearly show that myotube injuries caused by severe contraction or electrical stimulation may produce false results in the studies regarding the secretion of myokines. Previous reports showed that a 12-week walking program increased plasma TRX-1 levels in human subjects [34]. Although muscle contraction does not stimulate acute TRX-1 secretion, it might be possible that sustained (or intermittent) exercise training increases TRX-1 expression by de novo synthesis, which contributes to enhanced constitutive secretion of this protein. A previous study found enhanced expression of TRX-1 protein in the peripheral blood mononuclear cells of mice after 12 and 24 h of swimming [24]. Similarly, increased expression of TRX-1 was found in HeLa cells subjected to oxidative stress for 24 or 48 h [35]. Taken together, enhanced expression of TRX-1 in the skeletal muscle induced by exercise may be accompanied by an increase in its constitutive secretion.
TRX-1 is a secreted protein that lacks a signal peptide sequence. Many proteins lacking a signal peptide sequence are known to be secreted through exosome-mediated pathways [32]. Therefore, to understand the mechanism of secretion of TRX-1, we examined whether TRX-1 was secreted through an exosome-mediated pathway. Contrary to our expectations, we found that TRX-1 was present in the supernatant fraction, but not in the exosome fraction. Although an earlier study found that methylamine inhibited the secretion of TRX-1 in activated T cells [14], such an effect of methylamine on the secretion of TRX-1 from myotubes was not observed in this study. Therefore, the precise mechanism by which TRX-1 is secreted from the skeletal muscle remains unknown. Similar to TRX-1, IL-1β, a secretory protein, also lacks a signal peptide sequence. Although a number of studies have attempted to elucidate the mechanism of secretion of IL-1β, the pathway remains unclear. A recent review discussed five potential pathways of IL-1β secretion. These pathways include secretory lysosomes, secretory autophagy, exosome, microvesicle shedding, and/or membrane translocation [36]. TRX-1 may be secreted via exosome-independent mechanisms.
The role of the secreted TRX-1 remains unknown. One possibility is that TRX-1 is involved in redox regulation. Since TRX expression is induced by oxidative stress [35, 37], ROS generated as a result of muscle contraction could induce TRX-1 expression. Further, it has been shown that endurance training limits the oxidative damage in mice [38], likely because of increased expression of antioxidant genes and enzymes such as a manganese superoxide, dismutase, and catalase [22, 39, 40]. Therefore, TRX-1 induced by exercise may contribute to reduce oxidative stress as one of the defense mechanisms. Extracellular TRX-1 plays a role as a redox regulator, a cell growth regulator [41], and it has chemotactic effects [19]. Extracellular TRX-1 is rapidly oxidized [42]. However, the oxidized TRX-1 has an inhibitory effect on the expression and secretion of IL-1β, an inflammatory cytokine in macrophages [42]. MIF, a TRX family member, is a constitutively secreted myokine that appears to regulate glucose metabolism [11]. It has been reported that muscle contraction suppresses the secretion of MIF, which is thought to enhance glucose transport in the skeletal muscle after exercise [11]. Although these findings suggest the roles of secreted TRX-1 in a variety of processes, the precise effects of TRX-1 secreted from skeletal muscle remain to be determined.
Increased levels of TRX-1 were found in the culture medium under strenuous contraction that was accompanied by an increase in LDH activity in the medium, an indicator of cellular damage. The skeletal muscle was injured by strenuous exercise, but was promptly repaired, and the muscle mass increased via the process of regeneration. This finding suggested that some of the proteins released from the damaged skeletal muscle might act as myokines. Studies have shown that fibroblast growth factor-2 is released following injury and is involved in skeletal muscle hypertrophy [43]. Whether this type of release of TRX-1 into the medium is included as one of the secretion mechanisms remains controversial. However, the increased TRX-1 release in response to strenuous contraction is more likely a result of leakage and not secretion. We observed an increase in IL-6 in the medium following the contraction of intact C2C12 myotubes (unpublished data). Therefore, it is likely that mechanisms exist that regulate contraction-stimulated secretion in muscle cells.
In conclusion, our results showed that the secretion of TRX-1 from C2C12 myotubes was not regulated by contraction. Thus, TRX-1 and other related redox proteins are constitutively secreted from C2C12 myotubes in the absence of any stimulation. The precise mechanism by which TRX-1 is secreted from the skeletal muscle remains unclear. These redox proteins that are constitutively secreted from the skeletal muscle may exert antioxidant and systemic health-promoting effects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by Grant-in-Aid for Young Scientists B 24700700 (to YM) and the Funding Program for World-Leading Innovative R&D on Science and Technology by the Council for Science and Technology Policy LS102 (to NLF).
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 3.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1191–E1204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- 4.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 5.Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab. 2005;289:E251–E257. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- 6.Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57:3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311:372–379. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 9.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manabe Y, Miyatake S, Takagi M. Myokines: do they really exist? J Phys Fit Sports Med. 2013;1:51–58. doi: 10.7600/jpfsm.1.51. [DOI] [Google Scholar]
- 11.Miyatake S, Manabe Y, Inagaki A, Furuichi Y, Takagi M, Taoka M, Isobe T, Hirota K, Fujii NL. Macrophage migration inhibitory factor diminishes muscle glucose transport induced by insulin and AICAR in a muscle type-dependent manner. Biochem Biophys Res Commun. 2014;444:496–501. doi: 10.1016/j.bbrc.2014.01.089. [DOI] [PubMed] [Google Scholar]
- 12.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 13.Ericson ML, Horling J, Wendel-Hansen V, Holmgren A, Rosen A. Secretion of thioredoxin after in vitro activation of human B cells. Lymphokine Cytokine Res. 1992;11:201–207. [PubMed] [Google Scholar]
- 14.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 15.Rubartelli A, Bonifaci N, Sitia R. High rates of thioredoxin secretion correlate with growth arrest in hepatoma cells. Cancer Res. 1995;55:675–680. [PubMed] [Google Scholar]
- 16.Dekigai H, Nakamura H, Bai J, Tanito M, Masutani H, Hirota K, Matsui H, Murakami M, Yodoi J. Geranylgeranylacetone promotes induction and secretion of thioredoxin in gastric mucosal cells and peripheral blood lymphocytes. Free Radic Res. 2001;35:23–30. doi: 10.1080/10715760100300561. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto M, Inoue T, Takeshita K, Nakamura H, Yodoi J. Effects of a new anti-rheumatic drug KE-298 and its active metabolite: KE-758 on secretion of thioredoxin and on the level of intracellular glutathione in human monocytes and T cells. Mol Immunol. 2002;38:793–799. doi: 10.1016/S0161-5890(01)00116-X. [DOI] [PubMed] [Google Scholar]
- 18.Schwertassek U, Balmer Y, Gutscher M, Weingarten L, Preuss M, Engelhard J, Winkler M, Dick TP. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertini R, Howard OM, Dong HF, Oppenheim JJ, Bizzarri C, Sergi R, Caselli G, Pagliei S, Romines B, Wilshire JA, Mengozzi M, Nakamura H, Yodoi J, Pekkari K, Gurunath R, Holmgren A, Herzenberg LA, Ghezzi P. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Cederbaum A. The mode of cisplatin-induced cell death in CYP2E1-overexpressing HepG2 cells: modulation by ERK, ROS, glutathione, and thioredoxin. Free Radic Biol Med. 2007;43:1061–1075. doi: 10.1016/j.freeradbiomed.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Radak Z, Apor P, Pucsok J, Berkes I, Ogonovszky H, Pavlik G, Nakamoto H, Goto S. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72:1627–1633. doi: 10.1016/S0024-3205(02)02476-1. [DOI] [PubMed] [Google Scholar]
- 24.Sumida S, Nakamura H, Yodoi J. Thioredoxin induction of peripheral blood mononuclear cells in mice in response to a single bout of swimming exercise. Gen Physiol Biophys. 2004;23:241–249. [PubMed] [Google Scholar]
- 25.Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS One. 2012;7:e52592. doi: 10.1371/journal.pone.0052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 27.Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316:1977–1984. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 29.Tiaden AN, Breiden M, Mirsaidi A, Weber FA, Bahrenberg G, Glanz S, Cinelli P, Ehrmann M, Richards PJ. Human serine protease HTRA1 positively regulates osteogenesis of human bone marrow-derived mesenchymal stem cells and mineralization of differentiating bone-forming cells through the modulation of extracellular matrix protein. Stem Cells. 2012;30:2271–2282. doi: 10.1002/stem.1190. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi H. Microglial functions and proteases. Mol Neurobiol. 2003;27:163–176. doi: 10.1385/MN:27:2:163. [DOI] [PubMed] [Google Scholar]
- 31.Xie L, Terrand J, Xu B, Tsaprailis G, Boyer J, Chen QM. Cystatin C increases in cardiac injury: a role in extracellular matrix protein modulation. Cardiovasc Res. 2010;87:628–635. doi: 10.1093/cvr/cvq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 33.http://exocarta.org/index.html. Accessed 8 Sep 2014
- 34.Takahashi M, Miyashita M, Kawanishi N, Park JH, Hayashida H, Kim HS, Nakamura Y, Sakamoto S, Suzuki K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur J Appl Physiol. 2013;113:1117–1126. doi: 10.1007/s00421-012-2531-5. [DOI] [PubMed] [Google Scholar]
- 35.Makino Y, Okamoto K, Yoshikawa N, Aoshima M, Hirota K, Yodoi J, Umesono K, Makino I, Tanaka H. Thioredoxin: a redox-regulating cellular cofactor for glucocorticoid hormone action Cross talk between endocrine control of stress response and cellular antioxidant defense system . J Clin Invest. 1996;98:2469–2477. doi: 10.1172/JCI119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccioli P, Rubartelli A. The secretion of IL-1beta and options for release. Semin Immunol. 2013;25:425–429. doi: 10.1016/j.smim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, Matsuda M, Furuke K, Kitaoka Y, Iwata S, Toda K, Inamoto T, Yamaoka Y, Ozawa K,Yodoi J (1994) Adult T cell leukemia-derived factor/human thioredoxin protects endothelial F-2 cell injury caused by activated neutrophils or hydrogen peroxide. Immunol Lett 42: 75-80 [DOI] [PubMed]
- 38.Salminen A, Vihko V. Lipid peroxidation in exercise myopathy. Exp Mol Pathol. 1983;38:380–388. doi: 10.1016/0014-4800(83)90077-1. [DOI] [PubMed] [Google Scholar]
- 39.Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. doi: 10.1155/2012/756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 41.Powis G, Oblong JE, Gasdaska PY, Berggren M, Hill SR, Kirkpatrick DL. The thioredoxin/thioredoxin reductase redox system and control of cell growth. Oncol Res. 1994;6:539–544. [PubMed] [Google Scholar]
- 42.Billiet L, Furman C, Larigauderie G, Copin C, Brand K, Fruchart JC, Rouis M. Extracellular human thioredoxin-1 inhibits lipopolysaccharide-induced interleukin-1beta expression in human monocyte-derived macrophages. J Biol Chem. 2005;280:40310–40318. doi: 10.1074/jbc.M503644200. [DOI] [PubMed] [Google Scholar]
- 43.Clarke MS, Feeback DL. Mechanical load induces sarcoplasmic wounding and FGF release in differentiated human skeletal muscle cultures. FASEB J. 1996;10:502–509. doi: 10.1096/fasebj.10.4.8647349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


