Abstract
In response to atherogenic stimuli, blood monocytes transmigrate across the vascular endothelium not only through endothelial cell–cell junctions (para-cellular) but also through endothelial cells themselves (trans-cellular). The molecular mechanism of the latter is mostly unknown, because it rarely happens, especially in vitro. Although many reports have recognized trans-cellular migration from snapshot images of leukocytes halfway across the endothelium at non-junctional locations, it often produces a false-positive result, because some leukocytes that initiate trans-cellular migration withdraw and return to the apical endothelial surface. Thus, analyzing the entire process is essential. In this study, complete monocyte trans-cellular migration was successfully captured for live cells, with simultaneous visualization of endothelial PECAM-1. We suggest the possible existence of both PECAM-1-related migration at peri-junctional sites and PECAM-1-unrelated migration at sites remote from junctions. This is the first report to describe the entire process of monocyte trans-cellular migration for live cells and its relationship with endothelial PECAM-1.
Electronic supplementary material
The online version of this article (doi:10.1007/s12576-011-0181-8) contains supplementary material, which is available to authorized users.
Keywords: Atherosclerosis, Leukocyte, Endothelium, Trans-cellular migration, PECAM-1
Introduction
One of the key events in early atherosclerosis is the recruitment of blood leukocytes, especially monocytes, to proatherogenic vascular regions [1] and their subsequent transmigration across endothelial cells (EC). Interactions between leukocytes and ECs involve multi-step processes including rolling, adhesion, locomotion, and transmigration [2, 3]. Previously we have shown that ECs relax mechanically upon monocyte adhesion [4], with reduced stress fibers and focal adhesion kinase [5]. During transmigration, leukocytes invade endothelium predominantly through intercellular junctions (para-cellular) and partially through the body of a single EC (trans-cellular). A large number of molecules at endothelial junctions have been implicated in the former, including platelet-endothelial cell adhesion molecule-1 (PECAM-1) [6–10], vascular endothelial cadherin (VE-cadherin) [7], and junctional adhesion molecules (JAMs) [11]. We have previously described a live-cell imaging system for monocyte trans-endothelial migration [12], and demonstrated that oxidized LDL [7] or monocyte para-cellular migration [13] alters endothelial junctional organization, increasing PECAM-1 and reducing VE-cadherin, which results in enhanced transmigratory activity.
Although the molecular mechanisms of trans-cellular migration are largely unknown, emerging evidence suggests that leukocytes probe, by use of podosome-like protrusions [14, 15], the endothelial surface, where caveolae marker caveolin-1, vesicles, vesiculo-vacuolar organelle (VVO), and fusogenic proteins are enriched on the endothelial side [14, 16], and the leukocytes are partially embraced by the intermediate filament-dependent, intercellular adhesion molecule-1 (ICAM-1)-enriched and vascular cell adhesion molecule-1 (VCAM-1)-enriched cup-like structures of ECs [17, 18].
The quantitative contribution of trans-cellular migration to overall transmigration (including both para-cellular and trans-cellular) is typically low (~5–10%) in well-characterized in-vitro models of human umbilical vein endothelial cells (HUVECs) [15]. However in-vivo studies with serial-section electron microscopy have shown predominant use of the trans-cellular route [15, 19], suggesting that this route is involved in the pathophysiology of atherogenesis. In addition to differences in endothelial junctional integrity and surrounding milieu between in-vitro and in-vivo models, one of the reasons the contribution of trans-cellular migration is so different in the two models is ascribed to a technical limitation. Trans-cellular events have been significantly underestimated in the light microscopic analysis that has frequently been used in in-vitro models, because most of the events occur in close proximity of, often within 100-200 nm, lateral junctions, which can be accurately distinguished by the electron microscopy often used in in-vivo studies, but not by light microscopy [15]. Thus in an appreciable number of in-vitro studies, including that reported here, only those events that occurred relatively distant (usually at least several micrometers) from junctions were scored as trans-cellular [15].
Although the two transmigration processes (para-cellular and trans-cellular) have long been regarded as different, it has recently been suggested that PECAM-1, a junctional molecule, is involved in trans-cellular migration [14], and a more recent study surprisingly reported the possibility that the two are regulated by basically the same mechanism [20]. In ECs, there is a network of interconnected membrane vesicles just below the plasma membrane called the lateral border recycling compartment (LBRC) that is connected to the cell surface at lateral junctions and contains junctional proteins, for example PECAM-1, CD99, and JAM-A, but not VE-cadherin [9, 10, 20]. The LBRC membrane is targeted at sites of both para-cellular [9, 10] and trans-cellular [20] migration in a kinesin-mediated, microtubule-dependent manner [10], in which fusogenic events of LBRC vesicles among junctions and luminal/abluminal plasma membranes may generate a transmigration channel for both routes. Trafficking of PECAM-1 into and out of LBRC requires tyrosine 663 in its cytoplasmic domain [6]. However many of these studies have recognized trans-cellular migration from the snapshot image of leukocytes halfway across the endothelium at non-junctional locations, which is insufficient, because leukocytes that initiate trans-cellular migration often withdraw and return to the apical surface of the endothelium, leading to false-positive results. Therefore analyzing the entire process is essential. In addition, it still remains unclear how PECAM-1, a key component of LBRC, is actually trafficked with transmigrating leukocytes in live samples. In this study, by use of our live-cell imaging techniques and confocal laser scanning microscopy (CLSM), the entire process of monocyte trans-cellular migration across HUVECs was captured for the first time for live cells, with simultaneous visualization of endothelial PECAM-1. In general, ECs from atherosclerosis-associated large-sized vessels, for example HUVECs, have relatively loose junctions compared with microvascular ECs and thus favor para-cellular migration. Despite this, in this study, rare trans-cellular events (3 out of 149 total transmigration events from a large number of time-consuming live-cell observations) were successfully captured.
Materials and methods
Cells
HUVECs were purchased from Kurabo (Japan), and cultured in HuMedia-EG2 complete medium (Kurabo) supplemented with primocin (Amaxa, Germany) to protect against microbial contaminants including mycoplasma. For experiments, cells at passage 3 were used. Human CD14+ monocytes were freshly isolated from peripheral blood of young, healthy volunteers as described elsewhere [12]. Human monocytic cell line THP-1 was purchased from DS Pharma Biomedical (Japan), and maintained in RPMI 1640 (Sigma, MO, USA) supplemented with 10% FBS and 5 × 10−5 M 2-mercaptoethanol. All procedures involving human subjects were approved by an institutional review committee. Informed consent was obtained from all participants.
Molecular imaging of PECAM-1 during monocyte trans-cellular migration
A PECAM-1-GFP vector, in which Aequorea coerulescens-derived GFP (AcGFP1) was fused with the intracellular C-terminus of PECAM-1, was constructed, and the proper expression and intracellular location of the fusion protein was confirmed as reported elsewhere [8, 13]. Transient transfection of PECAM-1-GFP into HUVECs was performed by use of nucleofector (Lonza, Switzerland), and the transfectant was seeded on to 35-mm glass-bottomed dishes (cover glass ϕ = 12 mm; Asahi glass, Japan) coated with fibronectin (4 μg/cm2). Cells were stimulated with interleukin (IL)-1β (5 ng/mL, R&D systems, MN, USA) on day 1 or 2 posttransfection, and used for experiments on the next day. Monocytes labeled with CellTracker orange-CMRA (2–3 × 10−7 M; Invitrogen, CA, USA) were added to HUVECs (approximately 0.7–1.5 × 105 cells/dish). Live imaging was performed on a microscopic stage equipped with a mini-CO2 incubator (TK-INCO2; Tokken, Japan) at 37°C, with CLSM (TCS-NT; Leica, Germany) with a 40 × 1.25 numerical aperture oil-immersion objective as described elsewhere [7, 8, 12, 13]. Acquired images were processed by CLSM software (Leica), Image-J (National Institute of Health, MD, USA), and Photoshop (Adobe systems, CA, USA). Transmigration events were judged as trans-cellular only when the site of transmigration was clearly non-junctional in the light microscopic observation, and so events occurring close to junctions were excluded from the analysis, leading to underestimation as mentioned in the “Introduction”. The location of cell–cell junctions (cell borders) was determined by PECAM-1 staining. In some experiments, THP-1 was used as a substitute for primary monocytes with a slight modification as follows. Before adding to HUVECs, some populations of THP-1 were pre-stimulated with monocyte chemotactic protein-1 (MCP-1, R&D systems) at 50 ng/mL or chemotactic peptide fMLP (Sigma) at 10−6 M for 30 min. In some settings, after 10 min incubation of THP-1 with HUVECs, samples were washed twice with HuMedia-EG2 to remove unbound THP-1 cells, and then served for live imaging, where time 0 was set at the time of initiation of live imaging, not at the time of addition of THP-1. Confocal images were processed by Image-J. For clear visualization of the images in Figs. 1 and 2, color balance and levels were adjusted by use of Photoshop.
Fig. 1.
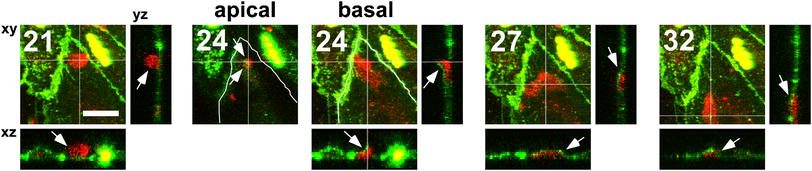
PECAM-1-related trans-cellular migration at peri-junctional sites. During trans-cellular migration at a peri-junctional site, endothelial PECAM-1 was observed around the transmigrating monocyte. In this case, the PECAM-1 signal was seen at the lower left-hand edge of the transmigrating monocyte (arrows at 24 min, apical and basal including xz and yz images). Red monocytes. Green PECAM-1-GFP in ECs. The location of endothelial cell borders was delineated as curved white lines at identical locations on both the apical and basal sections at 24 min. Time after monocyte addition in minutes is shown in the upper-left corner. Maximum-projected xy-images toward the z-axis are shown, with xz/yz orthogonal images cut with the specified planes that are indicated as white lines on xy-images. At 24 min, z-stacks were optically separated into “apical” and “basal” sections. Bar 20 μm
Fig. 2.
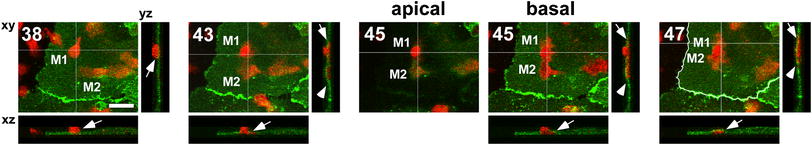
PECAM-1-unrelated trans-cellular migration at sites remote from junctions. Monocyte M1 underwent trans-cellular migration at a site relatively remote (>20 μm) from the nearest junction (arrows at 38–47 min), but the related PECAM-1 was not observed. A movie clip for the entire time series, in which its dynamic process can easily be recognized, is available as supplemental movie 1. M1 seemed to be in contact with another monocyte M2 located beneath endothelium that had previously transmigrated (arrowheads at 43–47 min), and the two monocytes seemed tightly stuck to each other at 47 min (arrow and arrowhead). Red monocytes. Green PECAM-1-GFP in ECs. The location of endothelial cell borders was delineated as curved white lines at 47 min. Time after monocyte addition in minutes is shown in the upper-left corner. Maximum-projected xy-images toward the z-axis are shown, with xz/yz orthogonal images cut with the specified planes that are indicated as white lines on xy-images. At 45 min, z-stacks were optically separated into “apical” and “basal” sections. Bar 20 μm
Results
Trans-cellular migration of monocytes
In our in-vitro settings using HUVECs, ~55% of adherent monocytes proceeded to transmigration (including both para-cellular and trans-cellular migration) at 30 min. Of those transmigrations, complete trans-cellular events without the withdrawal of monocytes were extremely rare, probably because of the relatively loose junctional integrity of HUVECs, as stated in the “Introduction”. Thus we conducted a large number of time-consuming live-cell observations, and successfully captured three complete trans-cellular events for live cells from 149 total events. Of those three events, two occurred at peri-junctional sites (defined as the site that was non-junctional and <15 μm from the nearest junction), and the remaining event occurred at a site relatively remote from junctions (defined as the site that was non-junctional and >20 μm from the nearest junction). The boundary between the two categories was set to be equivalent to the diameter of one monocyte (10–20 μm).
PECAM-1-related trans-cellular migration at peri-junctional sites
In Fig. 1, a monocyte invaded the endothelium at a peri-junctional, but clearly a non-junctional, location (arrows at 24 min, apical and basal). For clarity, endothelial cell borders were delineated on the basis of PECAM-1 staining in curved white lines at identical locations on both the apical and basal sections. The trans-cellular pore, through which the monocyte intruded itself, was indicated as an intersection point of horizontal and vertical white lines at the identical location on both sections, demonstrating that the pore is clearly in the cytoplasm (i.e. trans-cellular). In our models, transmigrating monocytes beneath ECs cannot migrate deep in vertical direction, because there is insufficient space between the basal plane of ECs and the bottom of the culture dish. Therefore, those monocytes spread thinly and leave the ECs by migrating in a horizontal direction beneath the ECs (24–32 min; clearly shown in xz/yz orthogonal images).
Importantly, endothelial PECAM-1, which is normally located at intercelluar junctions, was observed around the transmigrating monocyte, suggesting that PECAM-1 is involved in monocyte trans-cellular migration at peri-junctional sites. In this case, the PECAM-1 signal was seen at the lower left-hand edge of the transmigrating monocyte (arrows at 24 min, apical and basal including xz and yz images). This relationship of PECAM-1 with transmigrating monocytes was seen in all cases (2 out of 2) of peri-junctional trans-cellular migration, and was similarly seen in most para-cellular events as we have recently reported [13].
PECAM-unrelated trans-cellular migration at sites remote from junctions
In a trans-cellular event occurring at a site relatively remote from junctions (Fig. 2), monocyte M1 underwent trans-cellular migration (arrows at 38–47 min), but the related PECAM-1 was not observed, which is in striking contrast with the trans-cellular event at a peri-junctional site (Fig. 1). A movie clip for the entire time series, in which its dynamic process is readily apparent, is available as supplemental movie 1. In this event (Fig. 2), M1 seemed to be in contact with another monocyte M2 located beneath the endothelium that had previously transmigrated (arrowheads at 43–47 min), and the two monocytes seemed tightly stuck to each other at 47 min (arrow and arrowhead). These contacts between transmigrated monocytes beneath the endothelium and newly trans-cellularly migrating monocytes were seen in 2 out of 3 trans-cellular events, irrespective of their relationship with PECAM-1 (1 out of 2 events at peri-junctional sites, and 1 out of 1 event at a site relatively remote from junctions).
Involvement of PECAM-1 in monocyte adhesion at peri-junctional sites
To overcome the problem of the limited number of trans-cellular events with primary monocytes, we next used monocytic cell line THP-1, which adheres to endothelium, but never proceeds to transmigration. Adhesion of THP-1 cells to ECs at peri-junctional sites induced a clear cluster of endothelial PECAM-1 at the adhesion site (Fig. 3, arrows), suggesting that PECAM-1 is involved in the monocyte adhesion step at peri-junctional sites. This cluster was observed in almost all cases (8 out of 9) of THP-1 adhesion.
Fig. 3.
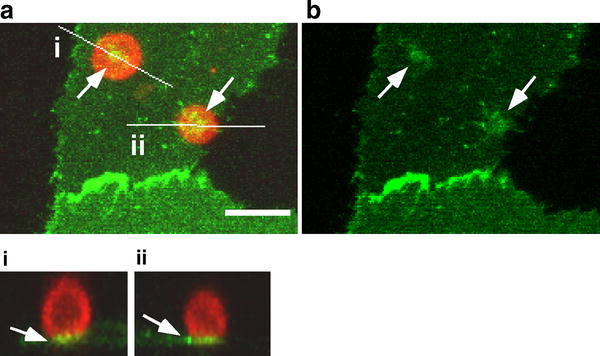
Involvement of PECAM-1 in monocyte adhesion at peri-junctional sites. Adhesion of THP-1 cells to ECs at peri-junctional sites induced a clear cluster of endothelial PECAM-1 at the adhesion site (arrows). Red THP-1 monocytes. Green PECAM-1-GFP in ECs. a The merged image of red and green channels. b The green channel. Maximum-projected xy-images toward the z-axis are shown with orthogonal images (i and ii) cut with the specified planes that are indicated as white lines on xy-images. Bar 20 μm
Discussion
In this study, using our PECAM-1-GFP-based imaging techniques, the entire process of monocyte trans-cellular migration was visualized for the first time in live cells; this is rare and difficult to capture especially in in-vitro settings using ECs from atherosclerosis-associated large-sized vessels, for example HUVECs. This system could potentially overcome the problem of false-positive results observed in fixed-cell systems that is derived from the withdrawal of leukocytes on the way to transmigration. The normal behavior of the expressed protein PECAM-1-GFP, including intracellular location, was confirmed in this and previous work [8, 13]. Our results also suggest the possible existence of both PECAM-1-related trans-cellular migration at peri-junctional sites and PECAM-1-unrelated trans-cellular migration at a site remote from junctions.
Currently, the intracellular trafficking mechanism of PECAM-1 is largely unknown. although believed to be a junctional molecule, PECAM-1 has been demonstrated, by ultrastructural observation in mouse blood vessels, to be distributed over the entire EC surface, including luminal/abluminal plasma membranes and in vesicular networks called VVOs [21]. This raises the possibility that PECAM-1 can be dynamically trafficked among lateral junctions, luminal/abluminal plasma membranes, and membrane networks such as LBRC and VVO, to support the formation of transmigration channels for both routes. In this sense, it is not surprising that such PECAM-1-containing membranes participate not only in para-cellular migration [9, 10], but also in monocyte adhesion (Fig. 3) and trans-cellular migration (Fig. 1) at peri-junctional sites. This PECAM-1-containing membrane may be one constituent of cup-like structures reported to be formed during trans-cellular migration which are enriched with ICAM-1 and VCAM-1. In contrast, trans-cellular migration at sites relatively remote from junctions seems unrelated to PECAM-1 (Fig. 2), possibly because of the difficulty of trafficking PECAM-1 over a relatively long distance. These observations, together with the fact that most trans-cellular events occur in close proximity to lateral junctions, led us to speculate that the underlying mechanism(s) of trans-cellular migration at peri-junctional sites is fundamentally the same as that of para-cellular migration (i.e. PECAM-1-dependent), because PECAM-1-containing membranes can be recruited to the site of transmigration in these regions. In contrast, trans-cellular migration at sites remote from junctions may be mediated by different unknown mechanism(s), and this can be regarded as “true” trans-cellular migration. The underlying mechanisms of these processes must be thoroughly investigated at cellular and molecular levels.
Irrespective of the relationship with PECAM-1, we observed the novel phenomenon in which transmigrated monocytes beneath endothelium seem to be in contact with newly trans-cellularly migrating monocytes. The mechanism(s) underlying this process is/are currently unclear. It may be possible that these contacts are mediated by previously-described podosome-like protrusions [14, 15], and facilitate the process of trans-cellular migration. Recently we have reported a possible positive feedback mechanism in which monocyte para-cellular migration alters endothelial junctional organization, resulting in augmentation of subsequent transmigratory activity [13]. It is interesting to speculate that in trans-cellular mode, this positive feedback can be mediated by these contacts to support efficient trans-cellular migration.
In this study, we could not sufficiently evaluate the results quantitatively, because of the low occurrence of trans-cellular migration as previously reported [14, 16, 17], which is a limitation of our study. Although 149 transmigration events were analyzed from a large number of time-consuming live-cell observations, only 3 events (2%) were clearly scored as trans-cellular in our in-vitro settings using primary human monocytes and HUVECs. As mentioned in the “Introduction”, this is attributable to the relatively loose junctional integrity of HUVECs. In addition, use of light microscopy to examine the detailed molecular dynamics of PECAM-1 might lead to underestimation of trans-cellular events. Investigating experimental conditions that induce abundant trans-cellular events (e.g., cell culture conditions, use of ECs from different vascular beds, or use of different inflammatory stimulus besides IL-1β) and in-vivo analyses will be necessary for large-scale quantitative analysis. However, we believe that our results provide novel important information as the first report to describe the entire process of monocyte trans-cellular migration of live cells and its relationship with endothelial PECAM-1, which could never be achieved with the fixed-cell assay. In Fig. 3, we used THP-1 cells as a substitute for primary human monocytes. The advantage of THP-1 cells is the convenient supply of a large number of cells without donor-dependency. They express PECAM-1, and have long been used for studying interactions with ECs [6, 9]. In our system, THP-1 cells adhered to ECs, and started but never completed transmigration, as reported elsewhere [6, 9]; this enabled us to intensely examine the step from adhesion to initiation of transmigration. However, we cannot exclude the possibility that the results obtained by use of THP-1 were because of their specific differences from primary monocytes.
In summary, this is the first report to describe the dynamics of the entire process of monocyte trans-cellular migration of live cells with simultaneous visualization of endothelial PECAM-1. The results suggest the possible existence of both a PECAM-1-related mode at peri-junctional sites and a PECAM-1-unrelated mode at a site remote from junctions. By use of these live-cell-based systems, molecular regulation during trans-cellular migration can be examined closely, which is never achieved with fixed-cell systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental movie 1. PECAM-1-unrelated trans-cellular migration at sites remote from junctions. Monocyte M1 (red) underwent trans-cellular migration at a site relatively remote (>20 μm) from the nearest junction (arrows at 38–47 min), but related endothelial PECAM-1 (green, GFP-tagged) was not observed. Beneath the endothelium, M1 seemed to be in contact with another monocyte M2 that had previously transmigrated (arrowheads at 43–47 min), and the two monocytes seemed tightly stuck each other at 47 min (arrow and arrowhead). The movie clip was integrated from sequential images in Fig. 2, together with other images from the same sample. Time after monocyte addition in minutes is shown in the lower-right corner. Maximum-projected xy-images toward the z-axis are shown, with xz/yz orthogonal images cut with the specified planes that are indicated as white lines on xy-images. Bar, 20 μm.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research in Japan (18300156 and 19700391), and grants from Kawasaki Medical School (Project-Research 18-306T and 19-314Y). Ken Hashimoto is also supported by grants from Takeda Science Foundation (Japan) for research on monocyte trans-endothelial migration.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka N, Iwaki K, Hashimoto K, Mochizuki S, Ogasawara Y, Sato M, Tsujioka K, Kajiya F. Measurements of endothelial cell-to-cell and cell-to-substrate gaps and micromechanical properties of endothelial cells during monocyte adhesion. Proc Natl Acad Sci USA. 2002;99:15638–15643. doi: 10.1073/pnas.242590799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwaki K, Ohashi K, Ikeda M, Tsujioka K, Kajiya F, Kurimoto M. Decrease in the amount of focal adhesion kinase (p125(FAK)) in interleukin-1beta-stimulated human umbilical vein endothelial cells by binding of human monocytic cell lines. J Biol Chem. 1997;272:20665–20670. doi: 10.1074/jbc.272.33.20665. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta B, Dufour E, Mamdouh Z, Muller WA. A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J Immunol. 2009;182:5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto K, Kataoka N, Nakamura E, Tsujioka K, Kajiya F. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis. 2007;194:e9–e17. doi: 10.1016/j.atherosclerosis.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Kataoka N, Nakamura E, Okamoto T, Kanouchi H, Minatogawa Y, Mohri S, Tsujioka K, Kajiya F. An experimental model for studying molecular behavior of platelet-endothelial cell adhesion molecule-1 during mechanical interactions between monocytes and vascular endothelial cells. J Biomech Sci Eng. 2010;5:281–290. doi: 10.1299/jbse.5.281. [DOI] [Google Scholar]
- 9.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 10.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradfield PF, Scheiermann C, Nourshargh S, Ody C, Luscinskas FW, Rainger GE, Nash GB, Miljkovic-Licina M, Aurrand-Lions M, Imhof BA. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–2555. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto K, Kataoka N, Nakamura E, Asahara H, Ogasawara Y, Tsujioka K, Kajiya F. Direct observation and quantitative analysis of spatiotemporal dynamics of individual living monocytes during transendothelial migration. Atherosclerosis. 2004;177:19–27. doi: 10.1016/j.atherosclerosis.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto K, Kataoka N, Nakamura E, Hagihara K, Hatano M, Okamoto T, Kanouchi H, Minatogawa Y, Mohri S, Tsujioka K, Kajiya F. Monocyte trans-endothelial migration augments subsequent transmigratory activity with increased PECAM-1 and decreased VE-cadherin at endothelial junctions. Int J Cardiol. 2011;149:232–239. doi: 10.1016/j.ijcard.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carman CV, Springer TA. Trans-cellular migration: cell–cell contacts get intimate. Curr Opin Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millán J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 17.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 19.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 20.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J Histochem Cytochem. 2004;52:87–101. doi: 10.1177/002215540405200109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


