Abstract
After weaning, piglets are often fed diets supplemented with high concentrations of zinc (Zn) to decrease post-weaning diarrhea. The aim of this study was to elucidate the regulation of Zn homeostasis within intestinal epithelial cells during excessive Zn exposure. High Zn concentrations elevated the intracellular Zn level in IPEC-J2 and Caco-2 cells which was influenced by differentiation status and time of exposure. With increasing Zn concentrations, mRNA and protein levels of metallothionein (MT) and zinc transporter 1 (ZnT1) were upregulated, whereas zinc transporter 4 (ZIP4) expression was downregulated. Metal-regulatory transcription factor-1 (MTF1) mRNA expression was upregulated at high Zn concentrations in IPEC-J2 cells, which corresponded to higher intracellular Zn concentrations. Based on these results, we suggest that intestinal epithelial cells adapt the expression of these genes to the amount of extracellular Zn available in order to maintain Zn homeostasis. Cell line-dependent differences in the regulation of Zn homeostasis were detected.
Keywords: Differentiation status, Intestinal epithelial cell, Intracellular Zn concentration, Metallothionein, Metal-regulatory transcription factors, Zn transporter
Introduction
Zinc (Zn) has manifold functions and is essential for multiple processes in mammals. In commercial piglet rearing, Zn is often used as a feed supplement to promote weight gain and performance [1] and to reduce the incidence of diarrhea [2, 3]. Pharmacological doses of Zn fed to piglets for approximately 2 weeks after weaning have been shown to reduce damage to intestinal cells caused by pathogenic bacteria [4], albeit with the disadvantage of promoting a more diverse and antibiotic-resistant population of coliforms in the gut [5]. In addition to promoting antibiotic-resistant Escherichia coli, high Zn can also be toxic to the host [6]. Therefore, effective mechanisms are required to maintain Zn homeostasis in cells (especially intestinal epithelial cells) and to protect the organism from excessive Zn accumulation.
Several Zn transporters regulate the cellular influx and efflux of Zn. Zinc transporter 1 (ZnT1, SLC30A1) is located at the basolateral membrane of mainly intestinal epithelial cells [7, 8] and lowers intracellular Zn concentrations by mediating Zn efflux [9]. In previous studies, high Zn levels have been shown to upregulate the ZnT1 mRNA level in several tissues, including rat small intestine [10]. Zn transporter 4 (ZIP4, SLC39A4) is localized at the apical membrane, most notably in the small intestine [11, 12]. Its expression is regulated at both the transcriptional and post-transcriptional levels, and its activation leads to an increase in intracellular Zn concentration [13]. In Zn-deficient states, the expression of ZIP4 has been shown to be upregulated in the small intestine of mice [11, 14], whereas Zn supplementation leads to the downregulation of ZIP4 in the small intestine of rats [15]. Metallothionein (MT), a protein that regulates the intracellular Zn level by strongly binding free Zn, is also increased during Zn supplementation [16, 17]. The basal and metal-induced transcription of MT and of ZnT1 is regulated by the metal-response element-binding transcription factor-1 (MTF1) [18, 19], which directly senses increases in intracellular Zn concentration [20].
Based on the assumption that the tight regulation of intracellular Zn homeostasis in enterocytes of weaned piglets is a crucial necessity, the aim of this study was to elucidate the mechanism(s) which regulate intracellular Zn concentrations and maintain Zn homoeostasis in porcine intestinal epithelial cells challenged with increasing extracellular concentrations of Zn. Porcine intestinal epithelial cell line IPEC-J2 cells were used as a model system in our experiments, and the results obtained were compared with those from the well-established human intestinal cell line Caco-2.
As the response of human intestinal cells has previously been shown to depend on differentiation status [21], we also addressed the question of whether preconfluent (‘undifferentiated’) or postconfluent (‘differentiated’) cells are differently affected by increasing Zn concentrations or by the duration of Zn exposure (6 or 24 h). Readout variables of this complex approach included the Zn concentration within the cells, mRNA and protein expression of ZnT1, ZIP4, and MT, and MTF1 mRNA levels.
Methods and materials
Cells and culture conditions
Piglet intestinal epithelial cells (cell line IPEC-J2), established from the jejunum of a newborn pig [22], were kindly provided by Prof. A. Blikslager (North Carolina State University, Raleigh, NC) and maintained in in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium (1:1) supplemented with 5 % fetal bovine serum (FBS; Biochrom, Berlin, Germany), 2.5 mmol/l l-glutamine (Biochrom), insulin (5 µg/ml), transferrin (5 µg/ml), sodium selenite (5 ng/ml) (ITS; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), epidermal growth factor (5 ng/ml, Biochrom), and penicillin–streptomycin (10 000 U penicillin and 10 mg streptomycin/100 ml medium; Sigma Aldrich Chemie GmbH). The IPEC-J2 cells were subcultured following trypsinization (0.15 g/l porcine trypsin, 0.06 g/l EDTA).
Human epithelial intestinal cells from the colorectal adenocarcinoma line Caco-2 [ATCC Catalog No. HTB-37; American Type Culture Collection (ATCC), Manassas, VA] were cultured in Eagle’s Minimum Essential Medium with Earle’s buffered saline solution and 2 mmol/l l-glutamine (ATCC) containing 1.0 mmol/l sodium pyruvate, 0.1 mmol/l nonessential amino acids, and 1.5 g/l sodium bicarbonate. To this medium, 20 % FBS and penicillin–streptomycin were added.
Incubation with ZnSO4
A 4 mM stock solution of ZnSO4 was added to the respective cell culture media, which contained 10 % FBS on the day of the experiments. At the beginning of the experiments, the media of the cells were replaced by media containing the respective Zn concentration.
Intracellular Zn concentration
For the measurement of intracellular Zn concentration, cells were grown in 25-cm2 cell culture flasks for 1 day (IPEC-J2, preconfluent), 7 days (IPEC-J2, postconfluent), 2–3 days (Caco-2, preconfluent), or 19–21 days (Caco-2, postconfluent). On the day of the experiment, the medium in each flask was replaced with new medium containing 10 % FBS and the concentration of ZnSO4 to be tested (0, 50, 100, and 200 µM). After an incubation of 6 or 24 h, the cell culture flasks were rinsed twice with phosphate-buffered saline (PBS) solution without Ca2+ and Mg2+ (Biochrom), following which 1 ml PBS was added to the flask and the cell harvested with a cell scraper. The harvested cells were first centrifuged at 200 g for 5 min, then the PBS supernatant was removed, and the cell pellet suspended in 200 µl PBS, of which 180 µl was stored at −20 °C for atomic absorption spectrometry (AAS) and 20 µl was stored at −80 °C for protein analysis.
After solubilization of the samples with 0.061 mol/l hydrochloric acid, cellular Zn uptake was measured by using AAS in a Jena AAS VARIO 6 spectrometer (Analytik Jena, Jena, Germany). To normalize the intracellular Zn concentration, total protein content was measured using the Bradford protein assay (Bio-rad Laboratories GmbH, München, Germany) in a Microplate Manager 6 (Bio-Rad) and the amount of Zn (in mg Zn/g protein) then calculated.
Real-time quantitative PCR
Cells were seeded at a density of 105 on 24-well TPP tissue culture plates (Biochrom), allowed to differentiate for 1–2 days (IPEC-J2, preconfluent), 7–10 days (IPEC-J2, postconfluent), 3–4 days (Caco-2, preconfluent), or 21 days (Caco-2, postconfluent), and then treated with increasing concentrations of ZnSO4 (0–200 µM) for 6 or 24 h.
The sampling procedure, RNA isolation, quality control, and cDNA synthesis methods, and the protocol for real-time quantitative PCR were as described in Lodemann et al. [23]. Briefly, the samples were stored in RNAlater RNA Stabilization Reagent (Qiagen GmbH, Hilden, Germany) at −20 °C after sampling. The Nucleospin RNA II kit (Macherey–Nagel GmbH & Co. KG, Düren, Germany) was used to isolate total RNA. Only samples with an RNA integrity number of >7 (2100 Bioanalyzer; Agilent Technologies, Santa Clara, CA) were reverse-transcribed to cDNA with the iScript™ cDNA Synthesis kit (Bio-Rad). The primers were obtained from Eurofins MWG Synthesis GmbH (Ebersberg, Germany) and are shown in Table 1.
Table 1.
Oligonucleotide primers and amplicon length of PCR products used in the experiments
| Gene | Primer sequence | Amplicon length (bp) | Accession number | Reference |
|---|---|---|---|---|
| ACTB (h) | (S) 5′-TTG CCG ACA GGA TGC AGA AGG A-3′ | 129 | NM_001101 | Baudry et al. [25] |
| (AS) 5′-AGG TGG ACA GCG AGG CCA GGA T-3′ | ||||
| TBP (h) | (S) 5′-TGC ACA GGA GCC AAG AGT GAA-3′ | 132 | NM_003194 | Dallol et al. [26] |
| (AS) 5′-CAC ATC ACA GCT CCC CAC CA-3′ | ||||
| UBC (h) | (S) 5′-ATT TGG GTC GCG GTT CTT G-3′ | 133 | M26880 | Vandesompele et al. [27] |
| (AS) 5′-TGC CTT GAC ATT CTC GAT GGT-3′ | ||||
| ZNT1 (SLC30A1) (h) | (S) 5′-GAC CAG GAG GAG ACC AAC AC -3′ | 95 | BC121015.1 | |
| (AS) 5′-TCA CCA CTT CTG GGG TTT TC-3′ | ||||
| ZIP4 (h) | (S) 5′-CCA GTG TGT GGG ACA CGG TAT-3′ | 64 | NM_130849.3 | Jou et al. [39] |
| (AS) 5′-TGT TCC GAC AGT CCA TAT GCA-3′ | ||||
| MT1A (h) | (S) 5′-CTT GGG ATC TCC AAC CTC AC-3′ | 137 | NM_005946.2 | |
| (AS) 5′-AGG AGC AGC AGC TCT TCT TG-3′ | ||||
| MTF1 (h) | (S) 5′-AAG GTG CAA CCC TCA CTC TG-3′ | 144 | BC014454.1 | |
| (AS) 5′- CTC CTC GGT GAG TCT TCT GG-3′ | ||||
| GAPDH (p) | (S) 5′-ACT CAC TCT TCT ACC TTT GAT GCT-3′ | 100 | DQ178124 | Erkens et al. [28] |
| (AS) 5′-TGT TGC TGT AGC CAA ATT CA-3′ | ||||
| TBP (p) | (S) 5′-GAT GGA CGT TCG GTT TAG G-3′ | 124 | DQ 178129 | Erkens et al. [28] |
| (AS) 5′-AGC AGC ACA GTA CGA GCA A-3′ | ||||
| YWHAZ (p) | (S) 5′-ATG CAA CCA ACA CAT CCT ATC-3′ | 178 | DQ178130 | Erkens et al. [28] |
| (AS) 5′-GCA TTA TTA GCG TGC TGT CTT-3′ | ||||
| ZNT1 (SLC30A1) (p) | (S) 5′-AGG AGG AGA CCA ACA TCC TG-3′ | 138 | NM_001139470.1 | |
| (AS) 5′-CCT GGT CCG GTT CTC TGA TA-3′ | ||||
| ZIP4 (p) | (S) 5′-TGC TGA CCT TGC TGT CCT C-3′ | 166 | XM_001925360.2 | |
| (AS) 5′-GGG AGT CCT GGC TTC TCA G-3′- | ||||
| MT1A (p) | (S) 5′ TGC TCT CTG CTT GGT CTC AC-3′ | 138 | M29515.1 | |
| (AS) 5′ AGG AGC AGC AGC TCT TCT TG-3′ | ||||
| MTF1 (p) | (S) 5′- AGT CGG AAT GTC CAG AAA CG-3′ | 155 | NM_001243544.1 | |
| (AS) 5′- GCA GCC CTC CTG ATT ACA GA-3′ |
ACTB (h) = Actin, beta, Homo sapiens; TBP (h) = TATA box binding protein, Homo sapiens; UBC (h) = ubiquitin C, Homo sapiens; ZNT1 (h) = SLC30A1 (Solute carrier family 30) member 1, Homo sapiens; ZIP4 (h2) = SLC39A4 (Solute carrier family 39) member 4, Homo sapiens); MT1A (h) = metallothionein 1A, Homo sapiens; MTF1 (h) = metal-regulatory transcription factor 1, Homo sapiens; GAPDH (p) = glyceraldehyde-3-phosphate dehydrogenase, Sus scrofa; TBP (p) = TATA box binding protein, Sus scrofa; YWHAZ (p) = tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide, Sus scrofa; ZnT1 (p) = SLC30A1 (Solute carrier family 30, member 1 (SLC30A1), Sus scrofa; ZIP4 (p) = SLC39A4 (Solute carrier family 39, member 4, Sus scrofa); MT1A (p) = metallothionein 1A, Sus scrofa ; MTF1 (p) = metal-regulatory transcription factor 1, Sus scrofa
The reactions for real-time quantitative PCR were performed in triplicate; the final reaction volume (15 µl) contained iQ SYBR Green Supermix (Bio-Rad), primers (0.3 µl of 20 pmol/µl each), and 5 µl cDNA, and the samples were run in a thermal cycler (iCyclerIQ–Real-Time PCR Detection System; Bio-Rad) with the following cycling parameters: an initial denaturation for 3 min at 95 °C; 35 amplification cycles at 95 °C for 30 s and 58 °C for 2 min. The relative amount of the target genes [ZnT1, ZIP4, metallothionein 1A (MT1A), MTF1] in each experimental group compared with that of the control group (0 µM for the respective group) was calculated using iQ5 software, which uses a modification of the ΔΔCt equation, initially introduced by Livak and Schmittgen [24]. Three reference genes were used for normalization [IPEC-J2: GAPDH, YWHAZ, TBP; Caco-2: ACTB, TBP, UBC; see footnote to Table 1).
Western blot analysis
Cells were cultured in cell culture flasks (25 cm2) and allowed to differentiate as described in the preceding section. Only cells which had been exposed to Zn for 24 h were used in subsequent analyses. The cell lysates were prepared as described previously [29]. The protein concentration was measured using a 2-D Quant Kit (GE Healthcare Life Sciences, Pittsburgh, PA). Proteins and a pre-stained protein molecular-weight marker (Fermentas, St. Leon Rot, Germany) were resolved in 15 % polyacrylamide gels by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Using the Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad), we then transferred samples for MT analysis onto nitrocellulose membranes with a pore size of 0.2 µm (GE Healthcare Life Sciences) for 1 h at 6.8 mA/cm2; samples for ZnT1 and ZIP4 analysis were transferred onto nitrocellulose membranes with a pore size of 0.45 µm. The uniform transfer of the proteins was confirmed by Ponceau staining. MT western blotting was then performed following the protocol of Mizzen et al. [30].
The membranes used for the detection of ZnT1 and ZIP4 were blocked with 5 % (w/v) non-fat milk powder (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) prepared in Tris-buffered saline containing 0.1 % Tween20 at room temperature for 1 h. The membranes were then incubated with either a 1:1000 dilution of a rabbit polyclonal antibody against SLC39A4 (ZIP4) (Antibodies-online GmBH, Aachen, Germany) or a 1:2000 dilution of rabbit polyclonal antibody against SLC30A1 (ZnT1) (Antibodies-online) at 4 °C overnight. After several washes, the membranes were incubated in a 1:25,000 dilution of an anti-mouse horseradish-peroxidase-conjugated secondary antibody (GE Healthcare Life Sciences). The signals were detected by chemiluminescence with the ECL ADVANCE Western Blotting Detection kit (GE Healthcare Life Sciences) according to the manufacturer’s instructions.
Statistical analysis
Statistical evaluations were carried out with the IBM SPSS Statistics program for Windows, version 21 (IBM Deutschland GmbH, Ehningen, Germany). Unless otherwise stated, results are given as the mean ± standard error of the mean. The number of independent experiments included in the statistical evaluation is indicated by ‘N’ in the relevant legends. Results were considered to be significant at P ≤ 0.05.
For the determinations of intracellular Zn concentrations and ZnT1, ZIP4, MT1A, and MTF1 mRNA expression, we first subjected the data to an overall three-way variance analysis with the fixed factors ‘concentration’ (Zn concentrations: 0, 50, 100, 200 µM), ‘sampling time’ (6 or 24 h), and ‘differentiation status’ (preconfluent or postconfluent cells). For the graphic presentation of the results the data have separated according to the factors differentiation status (preconfluent or postconfluent cells) and sampling time (6 or 24 h), and a one-way variance analysis with the factor concentration and posthoc Scheffé or Dunnett test were performed.
Results
Intracellular Zn concentrations
The Zn concentration was measured by AAS in preconfluent and postconfluent cells incubated with increasing ZnSO4 concentrations (0, 50, 100, 200 µmol/l) for 6 or 24 h. In the overall analysis, extracellular Zn concentration affected the intracellular Zn concentration in both cell lines (P < 0.001). However, the intracellular Zn concentration of both cell lines increased only numerically in the concentration range between 0–100 µM extracellular Zn; a sharp and significant increase occurred at 200 µM extracellular Zn (Fig. 1a, c, d). In Caco-2 cells, the intracellular Zn concentration was higher in postconfluent versus preconfluent cells (P < 0.001) and higher after 24 versus 6 h of Zn exposure (P < 0.001).
Fig. 1.
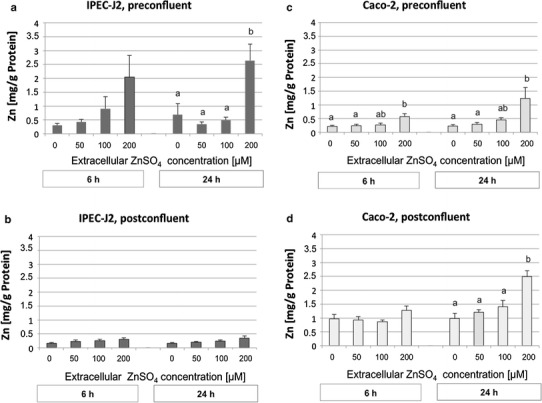
Intracellular zinc (Zn) accumulation (mg Zn/g protein) after treatment with 0, 50, 100, or 200 µM of ZnSO4 for 6 or 24 h, respectively, measured by atomic absorption spectrometry. a Preconfluent IPEC-J2 cells, b postconfluent IPEC-J2 cells, c preconfluent Caco-2 cells, d postconfluent Caco-2 cells. N = 5 (IPEC-J2 pre-and postconfluent; Caco-2 preconfluent), N = 4 (Caco-2, postconfluent) independent experiments. Data are presented as the mean ± standard error of the mean (SEM). Different lowercase letters indicate significant differences (P ≤ 0.05) between the Zn concentrations for each condition. Cell lines are defined in “Cells and culture conditions”
In IPEC-J2 cells, the intracellular Zn level was also dependent on differentiation status, although, in contrast to Caco-2 cells, preconfluent cells contained more Zn than postconfluent cells (P < 0.001).
Zn transporters (ZnT1, ZIP4)
Upregulation of ZnT1 mRNA occurred in a dose-dependent manner (P < 0.001) in both IPEC-J2 and Caco-2 cells, with a significant increase in ZnT1 mRNA abundance occurring at both 100 or 200 µM extracellular Zn (Fig. 2a–c). The changes in ZnT1 mRNA abundance were highest after 6 h of Zn exposure, subsequently decreasing with increasing duration of exposure to Zn until the 24-h time point (P < 0.001). Interestingly, an inverse relationship seemed to exist between intracellular Zn concentration and ZnT1 mRNA abundance. Lower intracellular Zn concentrations in preconfluent versus postconfluent Caco-2 cells (Fig. 1c, d) coincided with higher changes in ZnT1 mRNA abundance in preconfluent versus postconfluent cells (P < 0.01) (Fig. 2c, d). Similarly, lower intracellular Zn concentrations in postconfluent versus preconfluent IPEC-J2 cells coincided with, at least numerically, higher changes in ZnT1 mRNA abundance, especially in postconfluent IPEC-J2 cells exposed to 200 µM extracellular Zn.
Fig. 2.
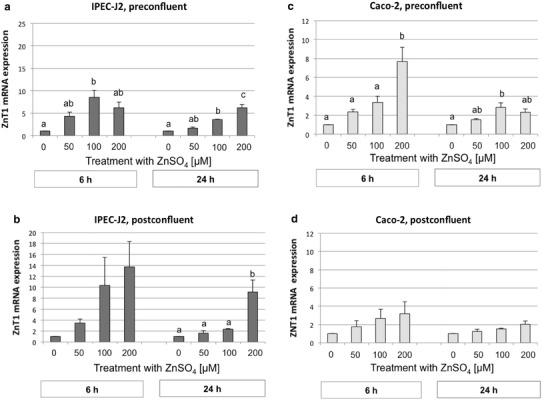
Zinc transporter 1 (ZnT1) mRNA expression in preconfluent IPEC-J2 (a) and Caco-2 (c) cells and postconfluent IPEC-J2 (b) and Caco-2 (d) cells (normalized fold expression; mean ± SEM) after treatment with 0, 50, 100, or 200 µM ZnSO4 for 6 and 24 h, respectively. The relative amount of the target genes in the experimental groups compared to the control group was calculated using the ΔΔCt method; N = 3 independent experiments. Different lowercase letters indicate significant differences (P ≤ 0.05) between ZnSO4 concentrations at 6 or 24 h
The same results were obtained for ZnT1 protein expression as for ZnT1 mRNA expression (Fig. 3). In postconfluent IPEC-J2 cells, protein expression was higher at 200 µM after the 24-h incubation; in contrast, postconfluent Caco-2 cells did not show any distinct effect at the protein level from exposure to 200 µM Zn for 24 h. Similar results were obtained in preconfluent cells (data not shown).
Fig. 3.
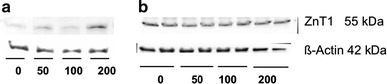
ZnT1 protein expression in postconfluent IPEC-J2 (a) and Caco-2 (b) cells (60 µg protein per lane) after treatment with 0, 50, 100, or 200 µM ZnSO4, respectively, for 24 h. N = 3 independent experiments
With regard to ZIP4 mRNA expression, significant effects of exposure to external Zn were only observed for the factor ‘concentration’ in the overall analysis (P < 0.001). In Caco-2 cells, this change was only numerical when the data were subdivided according to differentiation status and sampling time (Fig. 4c, d). In IPEC-J2 cells, ZIP4 mRNA abundance was downregulated by increasing Zn concentrations, with the lowest values at 200 µM ZnSO4 (Fig. 4a, b). Similar results were obtained for ZIP4 protein expression in preconfluent (data not shown) and postconfluent IPEC-J2 and Caco-2 cells (Fig. 5).
Fig. 4.
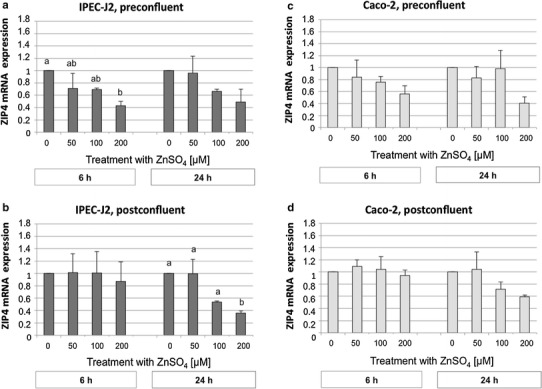
Zinc transporter 4 (ZIP4) mRNA expression in preconfluent IPEC-J2 (a) and Caco-2 (c) cells and postconfluent IPEC-J2 (b) and Caco-2 (d) cells (normalized fold expression; mean ± SEM) after treatment with 0, 50, 100, or 200 µM ZnSO4 for 6 or 24 h, respectively. The relative amount of the target genes in the experimental groups compared to the control group was calculated using the ΔΔCt method. N = 3 independent experiments. Different lowercase letters indicate significant differences (P ≤ 0.05) between ZnSO4 concentrations at 6 or 24 h
Fig. 5.
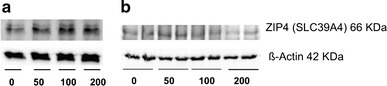
ZIP4 protein expression in postconfluent IPEC-J2 (a) and Caco-2 (b) cells (30 µg protein per lane for Caco-2 cells; 40 µg protein per lane for IPEC-J2 cells) after treatment with 0, 50, 100, or 200 µM ZnSO4 for 24 h. N = 3 independent experiments
Metallothionein
The mRNA of metallothionein 1A (MT1A), a Zn-binding protein, was upregulated with increasing Zn concentrations in both the IPEC-J2 and Caco-2 cell lines (P < 0.001). In both cell lines, the relative changes in MT1A mRNA abundance were not significantly influenced by the differentiation status of the cells nor the duration of Zn exposure. MT1A mRNA expression was significantly upregulated at 200 µM Zn and 24 h of incubation in postconfluent cells (Fig. 6b, d). Although a numerical rise was also observed in preconfluent cells, this increase was significant only in Caco-2 cells at 6 h (Fig. 6c).
Fig. 6.
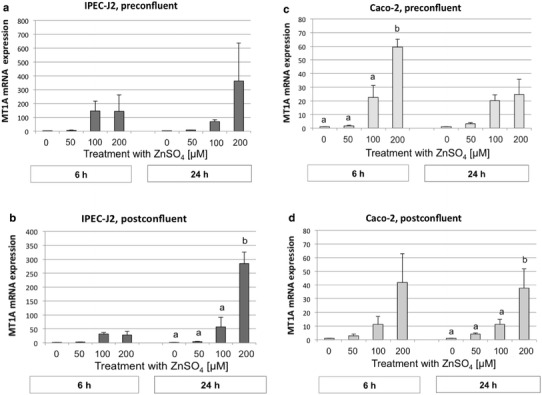
Metallothionein 1A (MT1A) mRNA expression in preconfluent IPEC-J2 (a) and Caco-2 (c) cells and postconfluent IPEC-J2 (b) and Caco-2 (d) cells (normalized fold expression; mean ± SEM) after treatment with 0, 50, 100, or 200 µM ZnSO4 for 6 or 24 h, respectively. The relative amount of the target genes in the experimental groups compared to the control group was calculated using the ΔΔCt method. N = 3 independent experiments. Different lowercase letters indicate significant differences (P ≤ 0.05) between ZnSO4 concentrations at 6 or 24 h
The abundance of MT protein was also upregulated with increasing Zn concentrations in preconfluent (data not shown) and postconfluent Caco-2 cells and IPEC-J2 cells (Fig. 7).
Fig. 7.
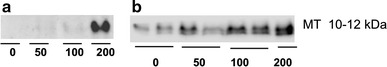
Metallothionein (MT) protein expression in post-confluent IPEC-J2 (a) and Caco-2 (b) cells (60 µg protein per lane) after treatment with 0, 50, 100, or 200 µM ZnSO4 for 24 h. N = 3 independent experiments
Metal-regulatory transcription factor-1
The mRNA of MTF1 was detected in both cell lines, but showed rather small changes in response to increasing Zn concentration (Fig. 8). Statistical significance for the factor Zn concentration was observed only in IPEC-J2 cells (P < 0.001) together with a significant effect of sampling time (P < 0.01) and differentiation status (P < 0.001). The higher MTF1 abundance appeared to occur selectively after a 24-h incubation at 200 µM extracellular ZnSO4 in preconfluent IPEC-J2 cells (Fig. 8a).
Fig. 8.
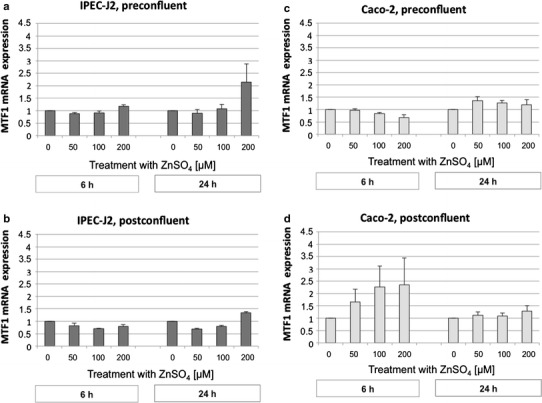
Metal-response element-binding transcription factor-1 (MTF1) mRNA expression of preconfluent IPEC-J2 (a) and Caco-2 (c) cells and postconfluent IPEC-J2 (b) and Caco-2 (d) cells (normalized fold expression; mean ± SEM) after treatment with 0, 50, 100, or 200 µM ZnSO4 for 6 or 24 h, respectively. The relative amount of the target genes in the experimental groups compared to the control group was calculated using the ΔΔCt method. N = 3 independent experiments
In Caco-2 cells, only the differentiation status significantly affected MTF1 mRNA abundance (P < 0.01). Postconfluent cells showed the numerically highest changes in MTF1 mRNA abundance at 6 h of Zn supplementation (Fig. 8d). Notably, the higher expression of MTF1 in postconfluent Caco-2 cells (Fig. 8d) corresponded with the higher intracellular Zn concentrations in these cells (Fig. 1d).
Discussion
The feed of weaned piglets is often supplemented with high Zn concentrations to improve growth performance after weaning and reduce the incidence of postweaning diarrhea. We have examined the regulation of cellular Zn concentrations and homeostasis in a porcine intestinal epithelial cell line (IPEC-J2). For comparison, parallel studies were carried out using the well-established human intestinal cell line Caco-2.
The cells were exposed to increasing Zn concentrations in vitro to simulate luminal Zn concentrations after high Zn supply in vivo. Dintzis et al. reported luminal intestinal Zn concentrations of 50–80 µM Zn (164 ppm Zn) in pigs 24 h after consumption of a Zn-supplemented feed [31]. Pieper et al. fed high dietary doses of Zn (2425 ppm) to weaned piglets and determined that the jejunal concentration of Zn was 152 µM after 6 days of exposure (32-day-old piglets) and 232 µM after 28 days of exposure (54-day-old piglets) [32]. In the present study, the intracellular Zn content increased in a dose-dependent manner, with a significant increase at 200 µM of extracellular Zn in IPEC-J2 cells; this increase was also observed in Caco-2 cells. We therefore are in agreement with Beyersmann and Haase [33] and suggest that, up to a certain concentration (100 µM), cells seem to tolerate increasing Zn exposure, as the intracellular Zn concentration is controlled by Zn transporters [34–36] and binding proteins (e.g., MTs) [37]. The assumption of Zn binding to MT is supported by the concomitant increase in MT levels and, in terms of viability of the cells, by previous observations of Lodemann et al. [23] in Caco-2 cells.
Preconfluent porcine IPEC-J2 cells showed the most intense changes in intracellular Zn levels upon exposure to extracellular Zn supplementation. Correspondingly, MTF1 mRNA abundance, which regulates the induction of ZnT1 and MT1A transcription, was significantly upregulated in undifferentiated IPEC-J2 cells compared with differentiated ones, followed by at least a numerical increase in the expression of MT1A. Since MT can strongly bind free intracellular Zn, this finding can be interpreted as an attempt of the cell to trap the high amounts of Zn intracellularly. This result is in accordance with observations by Beyersmann and Haase [38] who showed that MT is overexpressed in proliferating tissues. However, this regulation appears to have a limited capacity and does not seem to prevent toxic effects in preconfluent IPEC-J2 cells because the viability of preconfluent IPEC-J2 cells has been shown to be compromised at 100–200 µM [23], with Caco-2 cells being affected to a smaller extent.
In contrast to the preconfluent state, postconfluent IPEC-J2 cells predominantly seemed to utilize a transport-dominated regulation of Zn homeostasis via ZnT1 induction. The binding of Zn via the induction of MT1A may play the dominant role in preconfluent IPEC-J2 cells as the efficient induction of ZnT1 might be limited in this early differentiation state. We base this assumption on changes in the baseline expression pattern of Zn transporters during the maturation process of the small intestine [39]; based on their results, the authors of this latter study proposed that the cellular regulation of Zn absorption in the pig is developmentally regulated.
In contrast to IPEC-J2 cells, Caco-2 cells showed a higher intracellular Zn accumulation with advancing maturation than did IPEC-J2 cells, with the increase in the mRNA and protein expression of ZnT1 being twofold higher in preconfluent Caco-2 cells than in postconfluent cells. We therefore propose that undifferentiated Caco-2 cells initially try to dispose of the high intracellular amount of Zn via an induction of ZnT1. In postconfluent Caco-2 cells, however, a store-operated regulation by MT might be induced. The latter notion is supported by the high basal MT protein levels and a correspondingly high increase in intracellular Zn concentration in postconfluent Caco-2 cells.
Our results also suggest that not only the concentration of externally applied Zn, but also the time of exposure has an impact on the transcription of homeostatic transport proteins. Linking the present findings with previous studies [40, 41], it would appear that increases in ZnT1 mRNA level due to high Zn exposure occur within 6–12 h after exposure to high Zn levels; after 24 h, there is generally a lower or no increase in the mRNA level of ZnT1 [40].
In our study, MTF1 mRNA levels increased only moderately with increasing Zn concentrations. A significant effect of Zn concentration on MTF1 mRNA could only be seen in IPEC-J2 cells at 200 µM ZnSO4. Such small changes are in agreement with previous studies in human prostate, breast cancer, and cervical carcinoma cells [42–44], whereas, to our knowledge, no comparable studies have as yet been conducted in porcine small intestinal cells. Although the changes in MTF1 mRNA abundance at various levels of extracellular Zn were rather small, they nevertheless mirrored the changes in ZnT1 expression, suggesting that, especially in IPEC-J2 cells, MTF1 controls the regulation of ZnT1. However, MTF1 regulates the expression of target genes not primarily via its transcription but mainly via its nuclear translocation and binding to the promoter region of the respective target genes [45].
We observed the downregulation of ZIP4 mRNA in both cell lines at 200 µM ZnSO4. In previous studies with rat and porcine intestinal tissue, ZIP4 mRNA abundance was also downregulated when the animals were supplemented with high dietary Zn concentrations [15, 16]. To our knowledge, no previous studies have examined the changes in ZIP4 protein expression in porcine tissue or a porcine cell line in response to Zn supplementation. Whereas ZIP4 protein expression followed the downregulation of ZIP4 mRNA in Caco-2 cells with increasing Zn concentrations, this was not evident in IPEC-J2 cells. The reasons for this differential response are not clear at present.
Conclusion
The results of our experiments show that intestinal epithelial cells of both porcine and human origin were able to adapt the expression of MT, MTF1 and Zn transporters ZnT1 and ZIP4 to the amount of extracellular Zn. In the presence of high Zn levels, the cells responded by either upregulating or downregulating mRNA and protein levels of genes related to Zn homeostasis. However, other factors, such as application time and differentiation status, had a modulating influence on this regulation. We also noted that an increase in intracellular Zn concentration occurred with high levels of extracellular Zn supplementation despite the induction of compensatory mechanisms for reduced Zn uptake (ZIP4) and increased Zn export (ZnT1). Our data support previous conclusions drawn by Martin et al. [16] who propose that supplementation of feed with high Zn levels to piglets in vivo for longer than 2 weeks can induce imbalances in the Zn homeostasis of these piglets and result in distinct cell damage with potential toxic effects for the animal. The potential toxic effects might be mediated not only by the concentration of the externally applied Zn, but also by the time of exposure. Further, we observed significant differences between the porcine and human intestinal epithelial cells, indicating that species-dependent diversities do exist that must be taken into account. In our study, the IPEC-J2 cells, which are nontransformed porcine intestinal epithelial cells, were more sensitive to increasing concentrations of ZnSO4 than the Caco-2 cells, and this observation has also been made in previous studies based on such parameters as transepithelial electrical resistance and cell viability. Our results extend these findings in showing that maturity plays a predominant role in the regulation of intracellular Zn homeostasis in this porcine cell model as postconfluent cells could regulate intracellular Zn concentration more efficiently than preconfluent IPEC-J2 cells, likely due to a more distinct increase in ZnT1 in conjunction with MTF1. Given that IPEC-J2 cells have previously been used as a porcine-specific infection model for, as an example, transmissible gastroenteritis of pigs, we propose that this model may also be suitable to study Zn effects in such challenge models in vitro.
Acknowledgments
We thank M. Grunau and U. Scholz for technical support. This work was supported by the Forschungskommission der Freien Universität Berlin, the DFG (Grant No. SFB 852/1), and the H. -W. Schaumann-Stiftung.
Conflict of Interest
None.
References
- 1.Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102(5):687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- 2.Holm A, Poulsen HD. Zinc oxide in treating E. coli diarrhea in pigs after weaning. Compend Contin Educ Pract Vet. 1996;18:26–29. [Google Scholar]
- 3.Owusu-Asiedu A, Nyachoti CM, Marquardt RR. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody, zinc oxide, fumaric acid, or antibiotic. J Anim Sci. 2003;81(7):1790–1798. doi: 10.2527/2003.8171790x. [DOI] [PubMed] [Google Scholar]
- 4.Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli . J Nutr. 2003;133(12):4077–4082. doi: 10.1093/jn/133.12.4077. [DOI] [PubMed] [Google Scholar]
- 5.Bednorz C, Oelgeschlager K, Kinnemann B, Hartmann S, Neumann K, Pieper R, Bethe A, Semmler T, Tedin K, Schierack P, Wieler LH, Guenther S. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. J Med Microbiol. 2013;303(6–7):396–403. doi: 10.1016/j.ijmm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Zodl B, Zeiner M, Sargazi M, Roberts NB, Marktl W, Steffan I, Ekmekcioglu C. Toxic and biochemical effects of zinc in Caco-2 cells. J Inorg Biochem. 2003;97(4):324–330. doi: 10.1016/S0162-0134(03)00312-X. [DOI] [PubMed] [Google Scholar]
- 7.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci USA. 1998;95(9):4841–4846. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu YY, Kirschke CP, Huang L. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J Histochem Cytochem. 2007;55(3):223–234. doi: 10.1369/jhc.6A7032.2006. [DOI] [PubMed] [Google Scholar]
- 9.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 10.Liuzzi JP, Blanchard RK, Cousins RJ. Differential regulation of zinc transporter 1, 2, and 4 mRNA expression by dietary zinc in rats. J Nutr. 2001;131(1):46–52. doi: 10.1093/jn/131.1.46. [DOI] [PubMed] [Google Scholar]
- 11.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278(35):33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. 2002;71(1):66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 14.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101(40):14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimura T, Matsui T, Funaba M. Regulatory responses to excess zinc ingestion in growing rats. Br J Nutr. 2012;107(11):1655–1663. doi: 10.1017/S0007114511004867. [DOI] [PubMed] [Google Scholar]
- 16.Martin L, Lodemann U, Bondzio A, Gefeller EM, Vahjen W, Aschenbach JR, Zentek J, Pieper R. A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets. J Nutr. 2013;143(8):1205–1210. doi: 10.3945/jn.113.177881. [DOI] [PubMed] [Google Scholar]
- 17.Martinez MM, Hill GM, Link JE, Raney NE, Tempelman RJ, Ernst CW. Pharmacological zinc and phytase supplementation enhance metallothionein mRNA abundance and protein concentration in newly weaned pigs. J Nutr. 2004;134(3):538–544. doi: 10.1093/jn/134.3.538. [DOI] [PubMed] [Google Scholar]
- 18.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275(44):34803–34809. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 19.Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 1993;12(4):1355–1362. doi: 10.1002/j.1460-2075.1993.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Kimura T, Laity JH, Andrews GK. The zinc-sensing mechanism of mouse MTF-1 involves linker peptides between the zinc fingers. Mol Cell Biol. 2006;26(15):5580–5587. doi: 10.1128/MCB.00471-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zemann N, Zemann A, Klein P, Elmadfa I, Huettinger M. Differentiation- and polarization-dependent zinc tolerance in Caco-2 cells. Eur J Nutr. 2011;50(5):379–386. doi: 10.1007/s00394-010-0146-3. [DOI] [PubMed] [Google Scholar]
- 22.Berschneider HM (1989) Development of normal cultured small intestinal epithelial cell line which transports Na and Cl (abstract). Gastroenterology 96:A41
- 23.Lodemann U, Einspanier R, Scharfen F, Martens H, Bondzio A. Effects of zinc on epithelial barrier properties and viability in a human and a porcine intestinal cell culture model. Tox In Vitro. 2013;27(2):834–843. doi: 10.1016/j.tiv.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Baudry D, Faussillon M, Cabanis MO, Rigolet M, Zucker JM, Patte C, Sarnacki S, Boccon-Gibod L, Junien C, Jeanpierre C. Changes in WT1 splicing are associated with a specific gene expression profile in Wilms’ tumour. Oncogene. 2002;21(36):5566–5573. doi: 10.1038/sj.onc.1205752. [DOI] [PubMed] [Google Scholar]
- 26.Dallol A, Da Silva NF, Viacava P, Minna JD, Bieche I, Maher ER, Latif F. SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res. 2002;62(20):5874–5880. [PubMed] [Google Scholar]
- 27.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erkens T, Van Poucke M, Vandesompele J, Goossens K, Van Zeveren A, Peelman LJ. Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BMC Biotechnol. 2006;6:41. doi: 10.1186/1472-6750-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bondzio A, Gabler C, Badewien-Rentzsch B, Schulze P, Martens H, Einspanier R. Identification of differentially expressed proteins in ruminal epithelium in response to a concentrate-supplemented diet. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G260–G268. doi: 10.1152/ajpgi.00304.2010. [DOI] [PubMed] [Google Scholar]
- 30.Mizzen CA, Cartel NJ, Yu WH, Fraser PE, McLachlan DR. Sensitive detection of metallothioneins-1, -2 and -3 in tissue homogenates by immunoblotting: a method for enhanced membrane transfer and retention. J Biochem Biophys Methods. 1996;32(2):77–83. doi: 10.1016/0165-022X(95)00044-R. [DOI] [PubMed] [Google Scholar]
- 31.Dintzis FR, Laszlo JA, Nelsen TC, Baker FL, Calvert CC. Free and total ion concentrations in pig digesta. J Anim Sci. 1995;73(4):1138–1146. doi: 10.2527/1995.7341138x. [DOI] [PubMed] [Google Scholar]
- 32.Pieper R, Starke I, Vahjen W, Zentek J. Influence of high levels of dietary zinc oxide on intestinal zinc fractions and their correlation with microbial ecophysiology in pigs. Proc Nutr Physiol Soc. 2013;22:54. [Google Scholar]
- 33.Haase H, Beyersmann D. Uptake and intracellular distribution of labile and total Zn(II) in C6 rat glioma cells investigated with fluorescent probes and atomic absorption. Biometals. 1999;12(3):247–254. doi: 10.1023/A:1009232311677. [DOI] [PubMed] [Google Scholar]
- 34.Eide DJ. The SLC39 family of metal ion transporters. Pflug Arch. 2004;447(5):796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 35.Haase H. Zinkhomöostase in säugerzellen: untersuchungen zur aufnahme, intrazellulären verteilung und toxizität. Herdecke: GCA; 2001. [Google Scholar]
- 36.Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflug Arch. 2004;447(5):744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- 37.Vallee BL. The function of metallothionein. Neurochem Int. 1995;27(1):23–33. doi: 10.1016/0197-0186(94)00165-Q. [DOI] [PubMed] [Google Scholar]
- 38.Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14(3–4):331–341. doi: 10.1023/A:1012905406548. [DOI] [PubMed] [Google Scholar]
- 39.Jou MY, Philipps AF, Kelleher SL, Lonnerdal B. Effects of zinc exposure on zinc transporter expression in human intestinal cells of varying maturity. J Pediatr Gastroenterol Nutr. 2010;50(6):587–595. doi: 10.1097/MPG.0b013e3181d98e85. [DOI] [PubMed] [Google Scholar]
- 40.Bobilya DJ, Gauthier NA, Karki S, Olley BJ, Thomas WK. Longitudinal changes in zinc transport kinetics, metallothionein and zinc transporter expression in a blood–brain barrier model in response to a moderately excessive zinc environment. J Nutr Biochem. 2008;19(2):129–137. doi: 10.1016/j.jnutbio.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen H, Qin H, Guo J. Cooperation of metallothionein and zinc transporters for regulating zinc homeostasis in human intestinal Caco-2 cells. Nutr Res. 2008;28(6):406–413. doi: 10.1016/j.nutres.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200(2):187–195. doi: 10.1016/S0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 43.Ostrakhovitch EA, Olsson PE, von Hofsten J, Cherian MG. P53 mediated regulation of metallothionein transcription in breast cancer cells. J Cell Biochem. 2007;102(6):1571–1583. doi: 10.1002/jcb.21381. [DOI] [PubMed] [Google Scholar]
- 44.Otsuka F, Okugaito I, Ohsawa M, Iwamatsu A, Suzuki K, Koizumi S. Novel responses of ZRF, a variant of human MTF-1, to in vivo treatment with heavy metals. Biochim Biophys Acta. 2000;1492(2–3):330–340. doi: 10.1016/S0167-4781(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 45.Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem. 2000;275(13):9377–9384. doi: 10.1074/jbc.275.13.9377. [DOI] [PubMed] [Google Scholar]


