Abstract
Clarification of the functiogenesis of the embryonic central nervous system (CNS) has long been problematic, because conventional electrophysiological techniques have several limitations. First, early embryonic neurons are small and fragile, and the application of microelectrodes is challenging. Second, the simultaneous monitoring of electrical activity from multiple sites is limited, and as a consequence, spatiotemporal response patterns of neural networks cannot be assessed. We have applied multiple-site optical recording with a voltage-sensitive dye to the embryonic CNS and paved a new way to analyze the functiogenesis of the CNS. In this review, we discuss key points of optical recording in the embryonic CNS and introduce recent progress in optical investigations on the embryonic CNS with special emphasis on the development of the chick olfactory system. The studies clearly demonstrate the usefulness of voltage-sensitive dye recording as a powerful tool for elucidating the functional organization of the vertebrate embryonic CNS.
Keywords: Optical recording, Voltage-sensitive dye, Chick embryo, Development, Olfactory system, Oscillation
Introduction
Highly organized neural circuits in the central nervous system (CNS) give rise to the complex and sophisticated functions of the nervous system. During ontogenesis, when do the elements of the CNS, such as neurons and synapses, develop their structure and function? How are the neural circuits precisely formed within the CNS? Although these questions are fundamental issues in neuroscience, little is known about the “functiogenesis”, which is a coined word to contrast with “morphogenesis” and means the emergence and development of excitability, action potentials, synaptic transmission, and network property [1, 2], despite large amounts of anatomical and molecular genetic information. This situation has partially resulted from methodological limitations of conventional electrophysiological means, with which it is difficult to record neural activities from small and fragile embryonic neurons.
Voltage-sensitive dye (VSD) recording enables us to monitor transmembrane voltage changes from excitable cells that are inaccessible by conventional electrophysiological techniques. Furthermore, the introduction of multi-channel photodiode arrays has provided powerful tools for monitoring the spatiotemporal dynamics of neural activity [for reviews see 3–6]. We have applied optical recording with VSDs to the embryonic nervous system and established its feasibility to analyze the dynamics of neural activity in the CNS [for reviews see 7–9].
In this review, we first discuss technical issues such as optical recording systems and VSDs that are suitable for monitoring small optical changes in the embryonic nervous system. Next, we summarize the application to date of VSDs to the embryonic nervous system. Finally, as an example of optical recording in the embryonic CNS, we introduce recent advances in optical studies on the embryonic chick olfactory system [10, 11]. The functiogenesis of the embryonic brainstem and a widely spreading depolarization wave observed in the embryonic CNS are reviewed elsewhere [7, 12–15], and we will not describe these issues in this review.
Technical considerations for VSD recording in the embryonic nervous system
For VSD recording in the embryonic nervous system, we need to take the special characteristics of embryos into consideration. First, the immaturity of the embryonic nervous system has some advantages in VSD recording. (1) The embryonic preparation has a loose cellular-interstitial structure, which allows the VSD to diffuse sufficiently from the surface to deeper tissues and stain neurons relatively well. (2) Incident light passes easily through the preparation because of the high translucency of the embryonic tissues. This enables us to detect optical signals from deeper regions in the preparation. (3) In absorption measurements, the high translucency also generates a large signal-to-noise ratio (S/N), since the S/N is proportional to the square root of the transmitted background light intensity, if the dominant noise source is shot noise [5, 6]. (4) The thinness and loose structure of the embryonic preparation reduce light scattering, which deteriorates the signals and decreases the spatial resolution of the optical measurements. (5) Although the optical signal detected by one detector is the sum of many signals from different cell populations, the shape of the optical signal is relatively simple in the embryonic nervous system because of its simpler structure and lower complexity of neural populations.
On the other hand, the fragility and immaturity of embryonic neurons also have some disadvantages. (1) It is difficult or impossible to directly compare the optical signal with the electrophysiological signal because conventional electrophysiological methods are hard to apply. (2) Postsynaptic potentials in the embryonic nervous system fatigue easily, and we need to detect the optical signals without signal averaging. (3) Embryonic postsynaptic potentials are usually long-lasting (duration > 1 s), and the waveforms of the optical signals are easily deteriorated by various noise sources.
In these situations, it is necessary to improve VSD recording to clearly monitor neural activity in the embryonic nervous system. Informative reviews concerning optical recording systems have been published [5, 6, 9, 16–19]. Here, we mention two important points: the optical recording system, especially the detector, and the VSD.
Optical recording system
The recording systems are composed of optics, a detector, and a recording system with a computer (Fig. 1). In VSD recording, high sensitivity and high temporal resolution together with an adequate spatial resolution are needed to record small and millisecond-domain optical signals (10−4–10−2 as a fractional change: the change in light intensity divided by DC-background intensity). For monitoring embryonic neural activity, the following points should be considered: (1) the temporal resolution should be sufficient to detect the action potential. (2) Slow changes in the membrane potential, such as embryonic postsynaptic potentials (duration >1 s), should be detectable. (3) The S/N should be as large as possible to record neural activity without averaging because of rapid fatigue of the embryonic postsynaptic potential. (4) The dynamic range should match the fractional changes of the signals to be recorded.
Fig. 1.
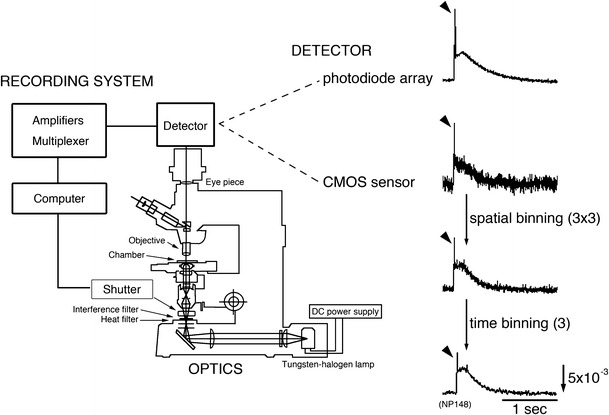
Schematic drawing of the optical recording system and optical signals induced by olfactory nerve (N.I) stimulation in a 12-day embryonic chick olfactory bulb. These signals were detected at the same region in one preparation using a 464-element photodiode array (NeuroPDA, RedShirtImaging, Fairfield, CT, USA), or a 128 × 128-element CMOS sensor (NeuroCMOS, RedShirtImaging, Fairfield, CT, USA) with a single sweep. One element of the photodiode array collects light from a round area (diameter = 46 µm), whereas that of the CMOS sensor collects light from a 12.5-µm2 square region. The frame rate of the photodiode array is almost the same as that of the CMOS sensor (≈1 kHz). To increase the S/N, the optical signal detected using the CMOS sensor was processed with spatial binning (average of 3 × 3 elements) and time binning (average of three points). The direction of the arrow in the lower right indicates an increase in transmitted light intensity (a decrease in dye absorption), and the length of the arrow represents the stated value of the fractional change. Arrowheads indicate spike-like signals corresponding to action potentials
In absorption measurements in in vitro embryonic preparations, the signals arise from a baseline of high-intensity transmitted light. As a detector, a photodiode array or a complementary metal oxide semiconductor (CMOS) sensor is desirable because other detectors such as a charge-coupled device (CCD) sensors would saturate at high light intensities. Compared with the CMOS sensor, the photodiode array enables us to record optical signals with a larger S/N, although the spatial resolution is lower. To detect optical signals with a similar S/N, the CMOS sensor needs signal averaging or spatial/temporal binning (Fig. 1). There is a possibility that the spatial/temporal binning deteriorates the shapes of optical signals by combining differing signals. In Fig. 1, a spike-like signal corresponding to an action potential may become smaller after the spatial/temporal binning. In our studies, we usually employ a home-made optical recording system with a 34 × 34 photodiode array [7, 20] and a 464-element photodiode system (NeuroPDA, RedShirtImaging, Fairfield, CT, USA).
Voltage-sensitive dyes
Extensive research has led to marked progress in the development of VSDs for measuring rapid transmembrane voltage changes [for reviews see 21–23]. The VSDs are classified into absorption and fluorescence dyes. The high translucency of embryonic tissue gives a large S/N in absorption measurements, and thus, absorption, rather than fluorescence. VSDs have usually been employed in embryonic preparations [7, 9]. Figure 2 shows examples of optical recording of the depolarization wave [for a review see 14] observed in the embryonic chick CNS stained with a merocyanine-rhodanine absorption dye, NK2761, (Fig. 2a) and a styryl fluorescence dye, di-2-ANEPEQ, (Fig. 2b) [24]. Even with di-2-ANEPEQ, which performed the best among the fluorescence dyes screened, its S/N was inferior (approximately 20 %) to that of NK2761.
Fig. 2.
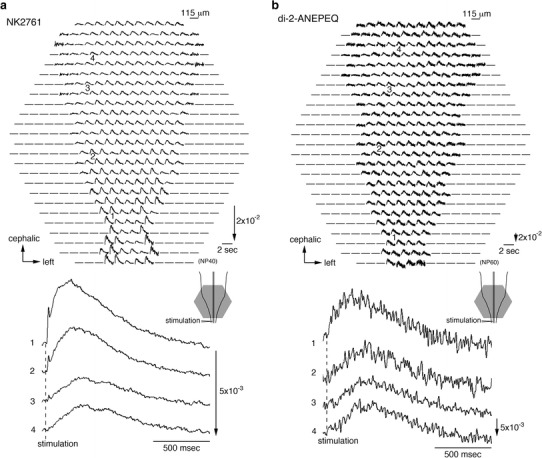
Multiple-site optical recording of neural responses to upper spinal cord stimulation in 7-day embryonic chick brainstem-spinal cord preparations. The preparation was stained with a merocyanine-rhodanine absorption dye, NK2761 (a), or a styryl fluorescence dye, di-2-ANEPEQ (b). The optical signals evoked by electrical stimulation (200 µA/1 ms) were recorded simultaneously from 464 contiguous regions of the preparation with a magnification of ×4 (an objective) ×1.67 (an eyepiece). Electrical stimulation elicited a propagating depolarization wave, widely propagating correlated activity, in the spinal cord and brainstem. Illustrations of the preparations are shown in the lower right, in which the detected areas are marked with gray hexagons. The recording was made in a single sweep. For each recording, enlarged traces of the optical signals detected from four regions indicated by 1–4 are shown in the lower panels. Modified from Ref. [24]
Absorption VSDs have also been favored because they are less phototoxic than fluorescence dyes and can be used in experiments requiring prolonged imaging [25–27]. In absorption VSDs, NK2761 (produced by Hayashibara Biochemical Laboratories Inc./Kankoh-Shikiso Kenkyusho, Okayama, Japan) [28] is the most commonly used dye in embryonic hearts [for reviews see 29, 30] and nervous systems [31, 32, for reviews see 7, 9]. Large optical signals have also been obtained using NK2776, NK3224, and NK3225, with no serious pharmacological or phototoxic effects on the evoked action potential [33].
On the other hand, fluorescence VSDs may be useful for detecting optical signals in preparations of later developmental stages or postnatal/posthatched animals because of their lower translucency. Among the fluorescence VSDs, di-2-ANEPEQ had the largest S/N in the embryonic chick brain, while (1) its photobleaching and detachment of dye bound to the cell membrane were faster (Fig. 3) and (2) the recovery of neural responses after staining was slower than those of di-4-ANEPPS and di-3-ANEPPDHQ (Fig. 4) [24]. Di-4-ANEPPS and di-3-ANEPPDHQ showed large S/Ns (Fig. 4a), together with less photobleaching (Fig. 3). These styryl dyes also exhibited large S/N in cardiac myocyte preparations [34]. However, these dyes also required a relatively long time for the recovery of neural activity after staining (Fig. 4). Furthermore, the background fluorescence intensity changed unstably with time (Fig. 3), the mechanism of which is unknown. Using fluorescence VSDs in the embryonic nervous system, we need to pay attention to the adequacy of the recovery time.
Fig. 3.
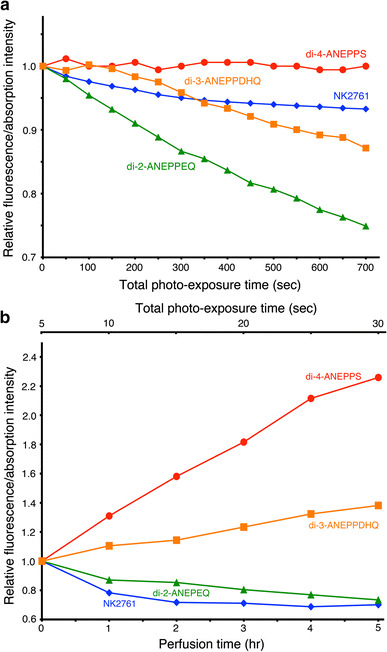
a Effects of illumination on the fluorescence/absorption background intensity of the dye. The ordinate represents the relative fluorescence/absorption intensity (DC-background light intensity at each time divided by that at time 0), and the abscissa is the time of continuous illumination. b Effects of perfusion on the fluorescence/absorption background intensity of the dye. The incident light was turned off except during the measuring period. The ordinate represents the relative fluorescence/absorption intensity (DC-background light intensity at each time divided by that at time 0). The lower abscissa is the time after staining (perfusion rate: 1 ml/min), and the upper abscissa is the total illumination time. In each figure, the results of an absorption dye (NK2761) and three fluorescence dyes (di-4-ANEPPS, di-3-ANEPPDHQ, and di-2-ANEPEQ) are shown. Modified from Ref. [24]
Fig. 4.
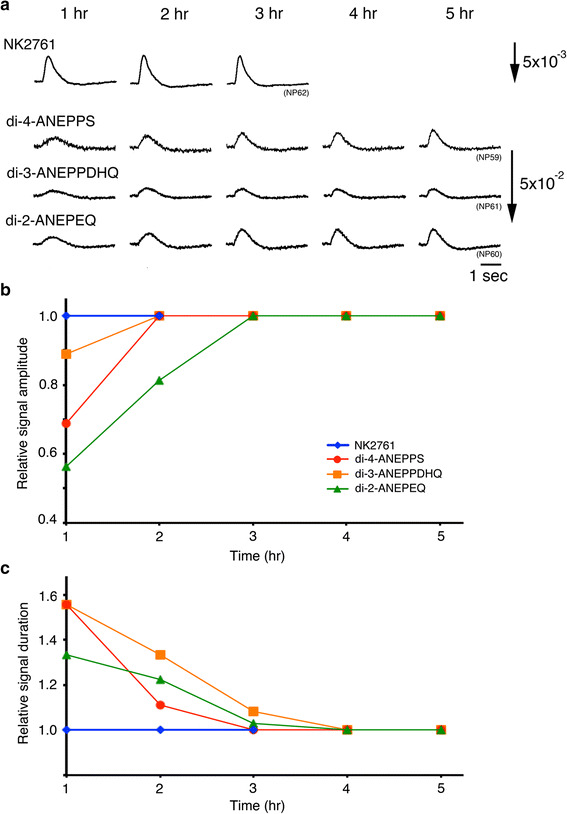
Time-dependent changes in optical signals after staining. The preparations were stained with an absorption dye and three fluorescence dyes. a Optical signals related to the depolarization wave detected with a magnification of ×10 ×1.67 in single sweeps at 1, 2, 3, 4, and 5 h after staining. b, c Amplitudes (b) and durations (c) of the optical signal normalized to the values at 1 h after staining are plotted against time. The signal duration was measured at 50 % of the maximum signal amplitude. Modified from Ref. [24]
Application of VSDs to the embryonic nervous system
Since the first application of VSDs to the embryonic nervous system [31, 32], extensive investigations have been performed in the brainstem, forebrain, spinal cord, and peripheral nervous system of chick embryos as well as rat and mouse fetuses. Table 1 summarizes VSD analysis of the functiogenesis of the embryonic nervous system. Some of these investigations were discussed previously in detail [7, 8, 9, 12, 13, 25, 35].
Table 1.
Application of voltage-sensitive dyes to the embryonic nervous system
| Chick olfactory system (I) | Sato et al. [10, 11] |
| Chick visual system (II) | Miyakawa et al. [36] |
| Chick oculomotor, trochlear, and abducens nuclei (III, IV, VI) | Glover et al. [37]* |
| Chick trigeminal nucleus (V) | Momose-Sato and Sato [38] |
| Sakai et al. [31] | |
| Sato et al. [39] | |
| Chick vestibulo-cochlear nucleus (VIII) | Asako et al. [40] |
| Glover et al. [37]* | |
| Momose-Sato et al. [41] | |
| Sato and Momose-Sato [42] | |
| Chick glossopharyngeal nucleus (IX) | Momose-Sato et al. [43] |
| Sato and Momose-Sato [44, 45] | |
| Sato et al. [46–48] | |
| Chick vagal nucleus (X) (including pharmacological studies on NTS neurons) | Kamino et al. [32, 49] |
| Komuro et al. [50] | |
| Momose-Sato and Sato [51] | |
| Momose-Sato et al. [43, 52–57] | |
| Sato and Momose-Sato [44] | |
| Sato et al. [48, 58–62] | |
| Chick spinal cord | Arai et al. [63, 64] |
| Mochida et al. [65] | |
| Rat trigeminal nucleus (V) | Momose-Sato et al. [66, 67] |
| Rat facial nucleus (VII) | Momose-Sato et al. [68] |
| Rat glossopharyngeal nucleus (IX) | Momose-Sato et al. [69] |
| Rat vagal nucleus (X) | Momose-Sato et al. [69–71] |
| Sato et al. [72, 73] | |
| Rat spinal cord | Demir et al. [74] |
| Rat respiratory center | Onimaru and Homma [75] |
| Mouse vagal nucleus (X) | Momose-Sato and Sato [76] |
| Mouse respiratory center | Ikeda et al. [77] |
| Onimaru et al. [78] | |
| Peripheral nervous system | Momose-Sato et al. [79, 80] |
| Sakai et al. [31, 81] | |
| Correlated wave activity | |
| Chick | Arai et al. [64] |
| Komuro et al. [82] | |
| Mochida et al. [83, 84] | |
| Momose-Sato and Sato [85] | |
| Momose-Sato et al. [86–89] | |
| Rat | Momose-Sato et al. [67, 68] |
| Ren et al. [90] | |
| Mouse | Momose-Sato et al. [91, 92] |
| Dye screening and improvements in optical recording systems | Hirota et al. [20] |
| Momose-Sato et al. [33] | |
| Mullah et al. [24] | |
| Tsau et al. [93] | |
| Wenner et al. [94] | |
Roman numerals in parentheses indicate the number of cranial nerves, which were stimulated to induce the response, except for the oculomotor, trochlear, and abducens nuclei (III, IV, and VI), which were identified in the vestibulo-ocular reflex pathway by stimulation of the vestibular nerve
The application of voltage-sensitive dyes in postnatal/posthatched animals is a major branch of optical studies in developmental neuroscience. However, this issue is beyond the scope of this review and not mentioned here. Modified from Ref. [9]
“*” shows that the results are presented in abstract form
Functiogenesis of the chick olfactory system
As an example of the application of VSD recording to the embryonic nervous system, we show investigations of the functiogenesis of the chick olfactory pathway. The chick embryo is easy to handle for ontogenetic analysis. In addition, the chick olfactory nerve (N.I) is long, and we can selectively stimulate the N.I with a suction electrode. Furthermore, re-evaluation of the basic structure of the avian brain has been facilitated in comparison with the mammalian brain by virtue of elucidation of the chick genome [95, 96]. This allows us to gain a deeper understanding of vertebrate brain function through the study of birds.
Optical detection of neural responses in the olfactory bulb (OB) and forebrain
In a 9-day embryonic N.I-OB-forebrain preparation, N.I stimulation elicited neural responses in the OB and forebrain (Fig. 5a). In the OB, the optical response was composed of a fast spike-like signal, corresponding to a sodium-dependent action potential, and a long-lasting slow signal, corresponding to an excitatory postsynaptic potential (EPSP) [10] (Fig. 5b). Concerning the EPSP-related slow signal, the following features were commonly observed in the OB [10]. (1) Synaptic transmission was mediated by glutamate, and N-methyl-d-aspartate (NMDA) and non-NMDA receptors were functionally expressed. (2) The initial phase of the EPSP was attributed to non-NMDA receptors, while the later phase was mediated by NMDA receptors. (3) The EPSP has a duration in the order of seconds. (4) The EPSP readily fatigued. These characteristics were also seen in the embryonic brainstem [for reviews see 7, 8, 9], and may be the basic principles of synaptic transmission in the embryonic CNS.
Fig. 5.
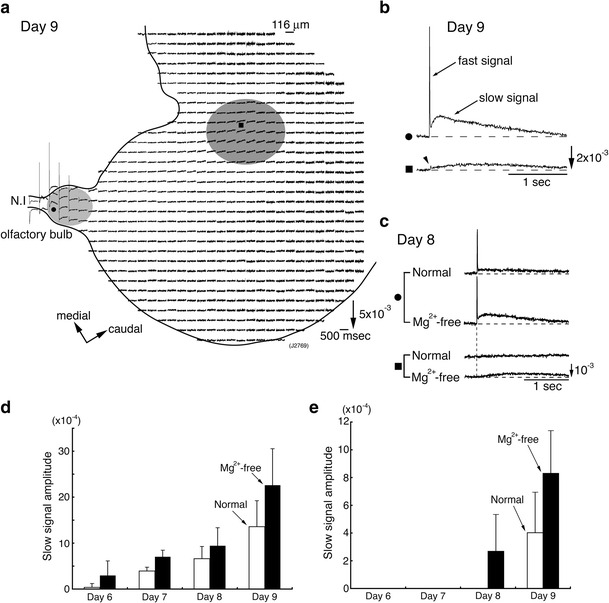
a Multiple-site optical recording of neural responses to N.I stimulation in a 9-day embryonic N.I-OB-forebrain preparation. In this recording, two response areas were identified: in the OB (shadowed in light gray), and in the forebrain (shadowed in dark gray). b Enlarged traces of the optical signals detected from the OB (upper trace marked by a black circle in a) and the forebrain (lower trace marked by a black square in a). In the lower trace, a small spike-like signal corresponding to the presynaptic action potential was identified (marked by an arrowhead). c Appearance of the slow signal in the forebrain of an 8-day chick embryo upon the removal of Mg2+ from the bathing solution. In the forebrain (marked by a black square), no slow signal was observed in normal solution, whereas a small slow signal was evoked in Mg2+-free solution. d, e Comparison of the slow signal amplitude in the OBs (d) and the forebrains (e) at 6- to 9-day embryonic stages. The slow signal amplitudes in normal (white bars) and Mg2+-free saline (black bars) are presented as the mean + SD in the fractional change. Modified from Ref. [10]
In the forebrain of a 9-day embryo, the slow signal was observed in a restricted area, which corresponds to the higher-ordered nucleus receiving inputs from the N.I (Fig. 5a) [10]. In pigeons [97–99], efferents of the OB project (1) to the medial septal nucleus and to more dorsal parts of the medial telencephalon through a medial olfactory tract (MOT), (2) to the piriform cortex through a lateral olfactory tract (LOT), and (3) to the olfactory tubercle and rostral part of the lobus parolfactorius (medial striatum: [100]) through an intermediate olfactory tract (IOT). In our recording, we could not determine the anatomical region for the response area in the forebrain because there are some differences between chicks and pigeons and also between embryos and adults.
Development of functional synaptic connections in the OB and forebrain
At an 8-day embryonic stage, N.I stimulation elicited neural responses in the OB but not in the forebrain in normal physiological solution (Fig. 5c). The removal of Mg2+ from the bathing solution, which activated the NMDA receptor function, enhanced the slow signal in the OB. In the forebrain, the removal of Mg2+ elicited a small slow signal, indicating that the synaptic function latently emerged in the forebrain at this stage. The ontogenetic investigation (Fig. 5d, e) revealed that: (1) functional synaptic transmission in normal physiological solution appears at around the 6 ~ 7-day embryonic stage in the OB and at around the 9-day embryonic stage in the forebrain, (2) NMDA receptor function is latently expressed earlier than the non-NMDA receptor function at the 6-day embryonic stage in the OB and the 8-day embryonic stage in the forebrain, and that (3) functional synaptic transmission is gradually strengthened during development.
Oscillations in the embryonic OB
Since its discovery by Adrian in the hedgehog [101], it has been known that odorant molecules induce oscillations, i.e., stereotyped sinusoidal neural activity, in the OB of various species [102]. In the embryonic chick OB, oscillation was also optically detected in addition to fast and slow signals (Fig. 6).
Fig. 6.
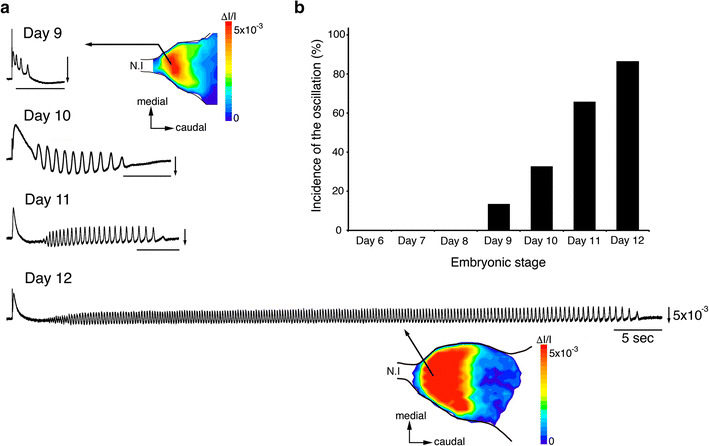
a Oscillations evoked by N.I stimulation in 9 ~ 12-day embryonic chick OBs. Color-coded maps of the oscillation in 9- and 12-day preparations are also shown. b Comparison of the incidence of the oscillation in the OB at 6- to 12-day embryonic stages. Modified from Ref. [11]
The oscillation had some unique characteristics [11]. (1) The oscillation was distributed in the distal half of the OB near the N.I. (2) The onset was around the 9-day embryonic stage. (3) The incidence increased with development. (4) The duration and mean frequency showed preparation-to-preparation variations even at the same developmental stage. (5) The duration became longer as development proceeded, while the relative area did not change during the embryonic 9- to 12-day stages. (6) The mean frequency became higher with development, while it remained in the range of the theta oscillation at all stages. These results indicate that the oscillation in the chick OB dynamically changes during embryogenesis. Such changes may continue beyond the 12-day embryonic stage, suggesting that the neural network responsible for the oscillation is still not fully differentiated for at least 12 days.
Developmental sequence of neural function in the chick olfactory system
Figure 7 summarizes the functional development of the chick olfactory system. In the OB, functional synaptic transmission appears at around the 6 ~ 7-day embryonic stage. Previous morphological investigations demonstrated the developmental sequence of olfactory structure formation in chicken embryos [95, 103–108]: (1) the nasal placode forms at the 2-day embryonic stage; (2) the differentiation of nasal pits, the formation of the OB, and growth of the N.I towards the brain occur by the 4-day embryonic stage; (3) the olfactory tuberculum and piriform cortex appear at the 5-day embryonic stage; (4) the olfactory epithelium is observed within the nasal capsule at the 6-day embryonic stage; and (5) the axons of olfactory neurons reach the OB by the 7-day embryonic stage.
Fig. 7.
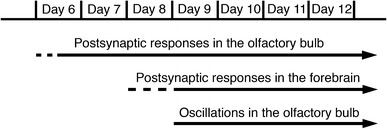
Functiogenesis of neural activity in the chick olfactory system
Compared with morphogenesis, functional synaptic connections in the OB are considered to be formed at almost the same time as the N.I fibers project into the OB. This result raises the possibility that the responses detected as slow signals are not mediated by mature synapses. Using VSD recording combined with electron microscopic observations, we have shown that synaptic transmission occurs before mature synaptic structures have been formed in the embryonic rat trigeminal system [66]. Similar phenomena were observed in embryonic chick ciliary ganglia [109] and developing neuromuscular junctions in culture obtained from Xenopus embryos [110]. The precise communication mechanisms in these immature synapses are not yet known. Functional communication at immature synapses can be mediated by transmitter release from arriving growth cones [111], chemical transmission between motile axonal and dendritic filopodia, and transmission at morphologically unspecialized pre- and postsynaptic contacts [112].
Optical responses to N.I stimulation in the forebrain were detected from around the 9-day embryonic stage. This means that functional neural circuits from the first- to higher-ordered nucleus are generated by the 9-day embryonic stage. In the chick brainstem sensory nuclei related to the N.VIII [41] and the N.X [62], synaptic transmission between the first- and higher-order nuclei is established by the 7-day embryonic stage. These profiles suggest that neural circuits from the first- to higher-ordered nuclei related to the cranial nerves have the ability to transfer sensory information from the early stages of development, irrespective of being in the brainstem or forebrain.
The appearance of the oscillation was ontogenetically later than the emergence of functional synaptic transmission in the OB. It has been reported that the theta oscillation is supported by low-frequency burst firing of external tufted cells in the glomerular layer [113]. If this is the case in the chick embryo, our results suggest that intra-bulbar neural circuits responsible for the oscillation mature later than synapses between the N.I and mitral/tufted cells.
The role of the oscillation during functiogenesis is unclear. The oscillation in the embryonic chick OB showed a gradual increase in duration with development, and the prolonged oscillation possibly causes a sustained intracellular Ca2+ concentration. This might be beneficial for the development of the olfactory system, because the elevated intracellular Ca2+ concentration plays an essential role in the development of the CNS, such as neural growth, survival, and differentiation [114–116].
Acknowledgments
We are grateful to Emeritus Professor Kohtaro Kamino for his instruction to optical analysis of functiogenesis of the embryonic nervous system. We also wish to acknowledge all of our collaborators at Tokyo Medical and Dental University, Kanto Gakuin University and Komazawa Women’s University. This work was supported by JSPS KAKENHI Grant Number JP15K06720 (K.S), JP26569968 (Y. M-S) and The Japan Epilepsy Research Foundation (Y. M-S).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
The 92nd annual meeting of the Physiological Society of Japan.
References
- 1.Kamino K. Personal recollections: regarding the pioneer days of optical recording of membrane potential using voltage-sensitive dyes. Neurophoton. 2015;2:021002. doi: 10.1117/1.NPh.2.2.021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai T, Kamino K. Functiongenesis of cardiac pacemaker activity. J Physiol Sci. 2016;66:293–301. doi: 10.1007/s12576-015-0431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen LB. Changes in neuron structure during action potential propagation and synaptic transmission. Physiol Rev. 1973;53:373–418. doi: 10.1152/physrev.1973.53.2.373. [DOI] [PubMed] [Google Scholar]
- 4.Cohen LB, Salzberg BM. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- 5.Salzberg BM. Optical recording of electrical activity in neurons using molecular probes. In: Barker JL, McKelvy JF, editors. Current methods in cellular neurobiology. New York: Wiley; 1983. pp. 139–187. [Google Scholar]
- 6.Grinvald A, Frostig RD, Lieke E, Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988;68:1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- 7.Momose-Sato Y, Sato K, Kamino K. Optical approaches to embryonic development of neural function in the brainstem. Prog Neurobiol. 2001;63:151–197. doi: 10.1016/S0301-0082(00)00023-X. [DOI] [PubMed] [Google Scholar]
- 8.Glover JC, Sato K, Momose-Sato Y. Using voltage-sensitive dye recording to image the functional development of neuronal circuits in vertebrate embryos. Dev Neurobiol. 2008;68:804–816. doi: 10.1002/dneu.20629. [DOI] [PubMed] [Google Scholar]
- 9.Momose-Sato Y, Sato K, Kamino K. Monitoring population membrane potential signals during development of the vertebrate nervous system. In: Canepari M, Zecevic D, Bernus O, editors. Membrane potential imaging in the nervous system and heart. New York: Springer; 2015. pp. 213–242. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Kinoshita M, Momose-Sato Y. Optical mapping of spatiotemporal emergence of functional synaptic connections in the embryonic chick olfactory pathway. Neurosci. 2007;144:1334–1346. doi: 10.1016/j.neuroscience.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Hayashi S, Inaji M, Momose-Sato Y. Oscillations in the embryonic chick olfactory bulb: initial expression and development revealed by optical imaging with a voltage-sensitive dye. Eur J Neurosci. 2016;43:1111–1121. doi: 10.1111/ejn.13189. [DOI] [PubMed] [Google Scholar]
- 12.Momose-Sato Y, Sato K. Optical recording of vagal pathway formation in the embryonic brainstem. Auto Neurosci. 2006;126–127:39–49. doi: 10.1016/j.autneu.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Momose-Sato Y, Sato K. The embryonic brain and development of vagal pathways. Resp Physiol Neurobiol. 2011;178:163–173. doi: 10.1016/j.resp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Momose-Sato Y, Sato K. Large-scale synchronized activity in the embryonic brainstem and spinal cord. Front Cell Neurosci. 2013;7:36. doi: 10.3389/fncel.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momose-Sato Y, Sato K. Development of spontaneous activity in the avian hindbrain. Front Neural Circuits. 2016;10:63. doi: 10.3389/fncir.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinvald A. Real-time optical mapping of neuronal activity: from single growth cones to the intact mammalian brain. Annu Rev Neurosci. 1985;8:263–305. doi: 10.1146/annurev.ne.08.030185.001403. [DOI] [PubMed] [Google Scholar]
- 17.Cohen LB, Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. In: De Weer P, Salzberg BM, editors. Optical methods in cell physiology. New York: Wiley; 1986. pp. 71–99. [PubMed] [Google Scholar]
- 18.Wu J-Y, Cohen LB. Fast multisite optical measurement of membrane potential. In: Mason WT, editor. Fluorescent and luminescent probes for biological activity. Boston: Academic Press; 1993. pp. 389–404. [Google Scholar]
- 19.Wu J-Y, Lam Y-W, Falk CX, Cohen LB, Fang J, Loew L, Prechtl JC, Kleinfeld D, Tsau Y. Voltage-sensitive dyes for monitoring multineuronal activity in the intact central nervous system. Histochem J. 1998;30:169–187. doi: 10.1023/A:1003295319615. [DOI] [PubMed] [Google Scholar]
- 20.Hirota A, Sato K, Momose-Sato Y, Sakai T, Kamino K. A new simultaneous 1020-site optical recording system for monitoring neural activity using voltage-sensitive dyes. J Neurosci Meth. 1995;56:187–194. doi: 10.1016/0165-0270(94)00123-X. [DOI] [PubMed] [Google Scholar]
- 21.Dasheiff RM. Fluorescent voltage-sensitive dyes: applications for neurophysiology. J Clinical Neurophysiol. 1988;5:211–235. doi: 10.1097/00004691-198807000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Loew LM. How to choose a potentiometric membrane probe. In: Loew LM, editor. Spectroscopic membrane probes. Boca Raton, Florida: CRC Press; 1988. pp. 139–151. [Google Scholar]
- 23.Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol. 1995;46:463–506. doi: 10.1016/0301-0082(95)00010-S. [DOI] [PubMed] [Google Scholar]
- 24.Mullah SH-E-R, Komuro R, Yan P, Hayashi S, Inaji M, Momose-Sato Y, Loew LM, Sato K. Evaluation of voltage-sensitive fluorescence dyes for monitoring neuronal activity in the embryonic central nervous system. J Memb Biol. 2013;246:679–688. doi: 10.1007/s00232-013-9584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donovan MJ, Bonnot A, Mentis GZ, Arai Y, Chub N, Shneider NA, Wenner P. Imaging the spatiotemporal organization of neural activity in the developing spinal cord. Dev Neurobiol. 2008;68:788–803. doi: 10.1002/dneu.20620. [DOI] [PubMed] [Google Scholar]
- 26.Parsons TD, Kleinfeld D, Raccuia F, Salzberg BM. Optical recording of the electrical activity of synaptically interacting neurons in culture using potentiometric probes. Biophys J. 1989;56:213–221. doi: 10.1016/S0006-3495(89)82666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons TD, Salzberg BM, Obaid AL, Raccuia-Behling F, Kleinfeld D. Long-term optical recording of patterns of electrical activity in ensembles of cultured Aplysia neurons. J Neurophysiol. 1991;66:316–333. doi: 10.1152/jn.1991.66.1.316. [DOI] [PubMed] [Google Scholar]
- 28.Fujii S, Hirota A, Kamino K. Action potential synchrony in embryonic precontractile chick heart: optical monitoring with potentiometric dyes. J Physiol (Lond) 1981;319:529–541. doi: 10.1113/jphysiol.1981.sp013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamino K, Hirota A, Komuro H. Optical indications of electrical activity and excitation-contraction coupling in the early embryonic heart. Adv Biophys. 1989;25:45–93. doi: 10.1016/0065-227X(89)90004-X. [DOI] [PubMed] [Google Scholar]
- 30.Kamino K. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol Rev. 1991;71:53–91. doi: 10.1152/physrev.1991.71.1.53. [DOI] [PubMed] [Google Scholar]
- 31.Sakai T, Hirota A, Komuro H, Fujii S, Kamino K. Optical recording of membrane potential responses from early embryonic chick ganglia using voltage-sensitive dyes. Dev Brain Res. 1985;17:39–51. doi: 10.1016/0165-3806(85)90130-0. [DOI] [PubMed] [Google Scholar]
- 32.Kamino K, Katoh Y, Komuro H, Sato K. Multiple-site optical monitoring of neural activity evoked by vagus nerve stimulation in the embryonic chick brain stem. J Physiol (Lond) 1989;409:263–283. doi: 10.1113/jphysiol.1989.sp017496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Momose-Sato Y, Sato K, Sakai T, Hirota A, Matsutani K, Kamino K. Evaluation of optimal voltage-sensitive dyes for optical monitoring of embryonic neural activity. J Memb Biol. 1995;144:167–176. doi: 10.1007/BF00232802. [DOI] [PubMed] [Google Scholar]
- 34.Rohr S, Salzberg BM. Multiple site optical recording of transmembrane voltage in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J. 1994;67:1301–1315. doi: 10.1016/S0006-3495(94)80602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Momose-Sato Y, Sato K, Kamino K. Application of voltage-sensitive dyes to the embryonic central nervous system. In: Fagan J, Davidson JN, Shimizu N, editors. Recent research developments in membrane biology. Kerara: Research Signpost; 2002. pp. 159–181. [Google Scholar]
- 36.Miyakawa N, Sato K, Momose-Sato Y. Optical detection of neural function in the chick visual pathway in the early stages of embryogenesis. Eur J Neurosci. 2004;20:1133–1144. doi: 10.1111/j.1460-9568.2004.03572.x. [DOI] [PubMed] [Google Scholar]
- 37.Glover JC, Momose-Sato Y, Sato K (2003) Functional visualization of emerging neural circuits in the brain stem of the chicken embryo using optical recording. In: 33rd Annu Meeting Society for Neuroscience Abstract vol 148.7
- 38.Momose-Sato Y, Sato K. Optical survey of initial expression of synaptic function in the embryonic chick trigeminal sensory nucleus. Neurosci Lett. 2014;570:92–96. doi: 10.1016/j.neulet.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Momose-Sato Y, Mochida H, Arai Y, Yazawa I, Kamino K. Optical mapping reveals the functional organization of the trigeminal nuclei in the chick embryo. Neurosci. 1999;93:687–702. doi: 10.1016/S0306-4522(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 40.Asako M. Doi T, Matsumoto A, Yang SM, Yamashita T Spatial and temporal patterns of evoked neural activity from auditory nuclei in chick brainstem detected by optical recording. Acta Otolaryngol. 1999;119:900–904. doi: 10.1080/00016489950180252. [DOI] [PubMed] [Google Scholar]
- 41.Momose-Sato Y, Glover JC, Sato K. Development of functional synaptic connections in the auditory system visualized with optical recording: afferent-evoked activity is present from early stages. J Neurophysiol. 2006;96:1949–1962. doi: 10.1152/jn.00319.2006. [DOI] [PubMed] [Google Scholar]
- 42.Sato K, Momose-Sato Y. Optical detection of developmental origin of synaptic function in the embryonic chick vestibulo-cochlear nuclei. J Neurophysiol. 2003;89:3215–3224. doi: 10.1152/jn.01169.2002. [DOI] [PubMed] [Google Scholar]
- 43.Momose-Sato Y, Kinoshita M, Sato K. Embryogenetic expression of glossopharyngeal and vagal excitability in the chick brainstem as revealed by voltage-sensitive dye recording. Neurosci Lett. 2007;423:138–142. doi: 10.1016/j.neulet.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Sato K, Momose-Sato Y. Optical detection of convergent projections in the embryonic chick NTS. Neurosci Lett. 2004;371:97–101. doi: 10.1016/j.neulet.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 45.Sato K, Momose-Sato Y. Optical mapping reveals developmental dynamics of Mg2+-/APV-sensitive components of glossopharyngeal glutamatergic EPSPs in the embryonic chick NTS. J Neurophysiol. 2004;92:2538–2547. doi: 10.1152/jn.00372.2004. [DOI] [PubMed] [Google Scholar]
- 46.Sato K, Momose-Sato Y, Sakai T, Hirota A, Kamino K. Responses to glossopharyngeal stimulus in the early embryonic chick brainstem: spatiotemporal patterns in three dimensions from repeated multiple-site optical recording of electrical activity. J Neurosci. 1995;15:2123–2140. doi: 10.1523/JNEUROSCI.15-03-02123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato K, Mochida H, Sasaki S, Momose-Sato Y. Developmental organization of the glossopharyngeal nucleus in the embryonic chick brainstem slice as revealed by optical sectioning recording. Neurosci Lett. 2002;327:157–160. doi: 10.1016/S0304-3940(02)00414-7. [DOI] [PubMed] [Google Scholar]
- 48.Sato K, Mochida H, Yazawa I, Sasaki S, Momose-Sato Y. Optical approaches to functional organization of glossopharyngeal and vagal motor nuclei in the embryonic chick hindbrain. J Neurophysiol. 2002;88:383–393. doi: 10.1152/jn.2002.88.1.383. [DOI] [PubMed] [Google Scholar]
- 49.Kamino K, Komuro H, Sakai T, Sato K. Optical assessment of spatially ordered patterns of neural response to vagal stimulation in the embryonic chick brainstem. Neurosci Res. 1990;8:255–271. doi: 10.1016/0168-0102(90)90032-A. [DOI] [PubMed] [Google Scholar]
- 50.Komuro H, Sakai T, Momose-Sato Y, Hirota A, Kamino K. Optical detection of postsynaptic potentials evoked by vagal stimulation in the early embryonic chick brain stem slice. J Physiol (Lond) 1991;442:631–648. doi: 10.1113/jphysiol.1991.sp018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Momose-Sato Y, Sato K. Primary vagal projection to the contralateral non-NTS region in the embryonic chick brainstem revealed by optical recording. J Memb Biol. 2005;208:183–191. doi: 10.1007/s00232-005-0829-5. [DOI] [PubMed] [Google Scholar]
- 52.Momose-Sato Y, Sakai T, Komuro H, Hirota A, Kamino K. Optical mapping of the early development of the response pattern to vagal stimulation in embryonic chick brain stem. J Physiol (Lond) 1991;442:649–668. doi: 10.1113/jphysiol.1991.sp018813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Momose-Sato Y, Sakai T, Hirota A, Sato K, Kamino K. Optical mapping of early embryonic expressions of Mg2+-/APV-sensitive components of vagal glutaminergic EPSPs in the chick brainstem. J Neurosci. 1994;14:7572–7584. doi: 10.1523/JNEUROSCI.14-12-07572.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Momose-Sato Y, Sato K, Sakai T, Hirota A, Kamino K. A novel γ-aminobutyric acid response in the embryonic brainstem as revealed by voltage-sensitive dye recording. Neurosci Lett. 1995;191:193–196. doi: 10.1016/0304-3940(95)11590-S. [DOI] [PubMed] [Google Scholar]
- 55.Momose-Sato Y, Sato K, Hirota A, Sakai T, Kamino K. Optical characterization of a novel GABA response in early embryonic chick brainstem. Neurosci. 1997;80:203–219. doi: 10.1016/S0306-4522(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 56.Momose-Sato Y, Sato K, Hirota A, Kamino K. GABA-induced intrinsic light-scattering changes associated with voltage-sensitive dye signals in embryonic brain stem slices: coupling of depolarization and cell shrinkage. J Neurophysiol. 1998;79:2208–2217. doi: 10.1152/jn.1998.79.4.2208. [DOI] [PubMed] [Google Scholar]
- 57.Momose-Sato Y, Kinoshita M, Sato K. Development of vagal afferent projections circumflex to the obex in the embryonic chick brainstem visualized with voltage-sensitive dye recording. Neurosci. 2007;148:140–150. doi: 10.1016/j.neuroscience.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 58.Sato K, Momose-Sato Y, Sakai T, Hirota A, Komuro H, Kamino K. Optical assessment of spatial patterning of strength-duration relationship for vagal responses in the early embryonic chick brainstem. Jpn J Physiol. 1993;43:521–539. doi: 10.2170/jjphysiol.43.521. [DOI] [PubMed] [Google Scholar]
- 59.Sato K, Momose-Sato Y, Hirota A, Sakai T, Kamino K. Optical studies of the biphasic modulatory effects of glycine on excitatory postsynaptic potentials in the chick brainstem and their embryogenesis. Neurosci. 1996;72:833–846. doi: 10.1016/0306-4522(95)00581-1. [DOI] [PubMed] [Google Scholar]
- 60.Sato K, Momose-Sato Y, Arai Y, Hirota A, Kamino K. Optical illustration of glutamate-induced cell swelling coupled with membrane depolarization in embryonic brain stem slices. Neuroreport. 1997;8:3559–3563. doi: 10.1097/00001756-199711100-00028. [DOI] [PubMed] [Google Scholar]
- 61.Sato K, Mochida H, Sasaki S, Yazawa I, Kamino K, Momose-Sato Y. Optical responses to micro-application of GABA agonists in the embryonic chick brain stem. Neuroreport. 2001;12:95–98. doi: 10.1097/00001756-200101220-00026. [DOI] [PubMed] [Google Scholar]
- 62.Sato K, Miyakawa N, Momose-Sato Y. Optical survey of neural circuit formation in the embryonic chick vagal pathway. Eur J Neurosci. 2004;19:1217–1225. doi: 10.1111/j.1460-9568.2004.03218.x. [DOI] [PubMed] [Google Scholar]
- 63.Arai Y, Momose-Sato Y, Sato K, Kamino K. Optical mapping of neural network activity in chick spinal cord at an intermediate stage of embryonic development. J Neurophysiol. 1999;81:1889–1902. doi: 10.1152/jn.1999.81.4.1889. [DOI] [PubMed] [Google Scholar]
- 64.Arai Y, Mentis GZ, Wu J-Y, O’Donovan MJ. Ventrolateral origin of each cycle of rhythmic activity generated by the spinal cord of the chick embryo. PLoS One. 2007;2(5):e417. doi: 10.1371/journal.pone.0000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mochida H, Sato K, Arai Y, Sasaki S, Yazawa I, Kamino K, Momose-Sato Y. Multiple-site optical recording reveals embryonic organization of synaptic networks in the chick spinal cord. Eur J Neurosci. 2001;13:1547–1558. doi: 10.1046/j.0953-816x.2001.01528.x. [DOI] [PubMed] [Google Scholar]
- 66.Momose-Sato Y, Honda Y, Sasaki H, Sato K. Optical mapping of the functional organization of the rat trigeminal nucleus: initial expression and spatiotemporal dynamics of sensory information transfer during embryogenesis. J Neurosci. 2004;24:1366–1376. doi: 10.1523/JNEUROSCI.4457-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Momose-Sato Y, Honda Y, Sasaki H, Sato K. Optical imaging of large-scale correlated wave activity in the developing rat CNS. J Neurophysiol. 2005;94:1606–1622. doi: 10.1152/jn.00044.2005. [DOI] [PubMed] [Google Scholar]
- 68.Momose-Sato Y, Sato K, Kinoshita M. Spontaneous depolarization waves of multiple origins in the embryonic rat CNS. Eur J Neurosci. 2007;25:929–944. doi: 10.1111/j.1460-9568.2007.05352.x. [DOI] [PubMed] [Google Scholar]
- 69.Momose-Sato Y, Nakamori T, Sato K. Functional development of the vagal and glossopharyngeal nerve-related nuclei in the embryonic rat brainstem: optical mapping with a voltage-sensitive dye. Neurosci. 2011;192:781–792. doi: 10.1016/j.neuroscience.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Momose-Sato Y, Sato K, Kamino K. Optical identification of calcium-dependent action potentials transiently expressed in the embryonic rat brainstem. Neurosci. 1999;90:1293–1310. doi: 10.1016/S0306-4522(98)00517-X. [DOI] [PubMed] [Google Scholar]
- 71.Momose-Sato Y, Nakamori T, Mullah SH-E-R, Sato K. Optical survey of vagus nerve-related neuronal circuits in the embryonic rat brainstem. Neurosci Lett. 2013;535:140–145. doi: 10.1016/j.neulet.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 72.Sato K, Momose-Sato Y, Hirota A, Sakai T, Kamino K. Optical mapping of neural responses in the embryonic rat brainstem with reference to the early functional organization of vagal nuclei. J Neurosci. 1998;18:1345–1362. doi: 10.1523/JNEUROSCI.18-04-01345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato K, Yazawa I, Mochida H, Sasaki S, Kamino K, Momose-Sato Y. Optical detection of embryogenetic expression of vagal excitability in the rat brain stem. Neuroreport. 2000;11:3759–3763. doi: 10.1097/00001756-200011270-00033. [DOI] [PubMed] [Google Scholar]
- 74.Demir R, Gao B-X, Jackson MB, Ziskind-Conhaim L. Interactions between multiple rhythm generators produce complex patterns of oscillation in the developing rat spinal cord. J Neurophysiol. 2002;87:1094–1105. doi: 10.1152/jn.00276.2001. [DOI] [PubMed] [Google Scholar]
- 75.Onimaru H, Homma I. Developmental changes in the spatio-temporal pattern of respiratory neuron activity in the medulla of late fetal rat. Neurosci. 2005;131:969–977. doi: 10.1016/j.neuroscience.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 76.Momose-Sato Y, Sato K. Development of synaptic networks in the mouse vagal pathway revealed by optical mapping with a voltage-sensitive dye. Eur J Neurosci. 2016;44:1906–1918. doi: 10.1111/ejn.13283. [DOI] [PubMed] [Google Scholar]
- 77.Ikeda K, Onimaru H, Yamada J, Inoue K, Ueno S, Onaka T, Toyoda H, Arata A, Ishikawa TO, Taketo MM, Fukuda A, Kawakami K. Malfunction of respiratory-related neuronal activity in Na+, K+ -ATPase α2subunit-deficient mice is attributable to abnormal Cl- homeostasis in brainstem neurons. J Neurosci. 2004;24:10693–10701. doi: 10.1523/JNEUROSCI.2909-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Onimaru H, Ikeda K, Kawakami K. Defective interaction between dual oscillators for respiratory rhythm generation in Na+, K+-ATPase α2 subunit-deficient mice. J Physiol (Lond) 2007;584:271–284. doi: 10.1113/jphysiol.2007.136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Momose-Sato Y, Komuro H, Sakai T, Hirota A, Kamino K. Optical monitoring of cholinergic postsynaptic potential in the embryonic chick ciliary ganglion using a voltage-sensitive dye. Biomed Res. 1991;12(Suppl 2):139–140. [Google Scholar]
- 80.Momose-Sato Y, Komuro H, Hirota A, Sakai T, Sato K, Kamino K. Optical imaging of the spatiotemporal patterning of neural responses in the embryonic chick superior cervical ganglion. Neurosci. 1999;90:1069–1083. doi: 10.1016/S0306-4522(98)00500-4. [DOI] [PubMed] [Google Scholar]
- 81.Sakai T, Komuro H, Katoh Y, Sasaki H, Momose-Sato Y, Kamino K. Optical determination of impulse conduction velocity during development of embryonic chick cervical vagus nerve bundles. J Physiol (Lond) 1991;439:361–381. doi: 10.1113/jphysiol.1991.sp018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komuro H, Momose-Sato Y, Sakai T, Hirota A, Kamino K. Optical monitoring of early appearance of spontaneous membrane potential changes in the embryonic chick medulla oblongata using a voltage-sensitive dye. Neurosci. 1993;52:55–62. doi: 10.1016/0306-4522(93)90181-E. [DOI] [PubMed] [Google Scholar]
- 83.Mochida H, Sato K, Arai Y, Sasaki S, Kamino K, Momose-Sato Y. Optical imaging of spreading depolarization waves triggered by spinal nerve stimulation in the chick embryo: Possible mechanisms for large-scale coactivation of the CNS. Eur J Neurosci. 2001;14:809–820. doi: 10.1046/j.0953-816x.2001.01692.x. [DOI] [PubMed] [Google Scholar]
- 84.Mochida H, Sato K, Momose-Sato Y. Switching of the transmitters that mediate hindbrain correlated activity in the chick embryo. Eur J Neurosci. 2009;29:14–30. doi: 10.1111/j.1460-9568.2008.06569.x. [DOI] [PubMed] [Google Scholar]
- 85.Momose-Sato Y, Sato K. Maintenance of the large-scale depolarization wave in the embryonic chick brain against deprivation of the rhythm generator. Neurosci. 2014;266:186–196. doi: 10.1016/j.neuroscience.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 86.Momose-Sato Y, Sato K, Mochida H, Yazawa I, Sasaki S, Kamino K. Spreading depolarization waves triggered by vagal stimulation in the embryonic chick brain: optical evidence for intercellular communication in the developing central nervous system. Neurosci. 2001;102:245–262. doi: 10.1016/S0306-4522(00)00477-2. [DOI] [PubMed] [Google Scholar]
- 87.Momose-Sato Y, Miyakawa N, Mochida H, Sasaki S, Sato K. Optical analysis of depolarization waves in the embryonic brain: a dual network of gap junctions and chemical synapses. J Neurophysiol. 2003;89:600–661. doi: 10.1152/jn.00337.2002. [DOI] [PubMed] [Google Scholar]
- 88.Momose-Sato Y, Mochida H, Sasaki S, Sato K. Depolarization waves in the embryonic CNS triggered by multiple sensory inputs and spontaneous activity: optical imaging with a voltage-sensitive dye. Neurosci. 2003;116:407–423. doi: 10.1016/S0306-4522(02)00585-7. [DOI] [PubMed] [Google Scholar]
- 89.Momose-Sato Y, Mochida H, Kinoshita M. Origin of the earliest correlated neuronal activity in the chick embryo revealed by optical imaging with voltage-sensitive dyes. Eur J Neurosci. 2009;29:1–13. doi: 10.1111/j.1460-9568.2008.06568.x. [DOI] [PubMed] [Google Scholar]
- 90.Ren J, Momose-Sato Y, Sato K, Greer JJ. Rhythmic neuronal discharge in the medulla and spinal cord of fetal rats in the absence of synaptic transmission. J Neurophysiol. 2006;95:527–534. doi: 10.1152/jn.00735.2005. [DOI] [PubMed] [Google Scholar]
- 91.Momose-Sato Y, Nakamori T, Sato K. Spontaneous depolarization wave in the mouse embryo: origin and large-scale propagation over the CNS identified with voltage-sensitive dye imaging. Eur J Neurosci. 2012;35:1230–1241. doi: 10.1111/j.1460-9568.2012.07997.x. [DOI] [PubMed] [Google Scholar]
- 92.Momose-Sato Y, Nakamori T, Sato K. Pharmacological mechanisms underlying the switching from the large-scale depolarization wave to segregated activity in the mouse CNS. Eur J Neurosci. 2012;35:1242–1252. doi: 10.1111/j.1460-9568.2012.08040.x. [DOI] [PubMed] [Google Scholar]
- 93.Tsau Y, Wenner P, O’Donovan MJ, Cohen LB, Loew LM, Wuskell JP. Dye screening and signal-to-noise ratio for retrogradely transported voltage-sensitive dyes. J Neurosci Meth. 1996;70:121–129. doi: 10.1016/S0165-0270(96)00109-4. [DOI] [PubMed] [Google Scholar]
- 94.Wenner P, Tsau Y, Cohen LB, O’Donovan MJ, Dan Y. Voltage-sensitive dye recording using retrogradely transported dye in the chicken spinal cord: staining and signal characteristics. J Neurosci Meth. 1996;70:111–120. doi: 10.1016/S0165-0270(96)00108-2. [DOI] [PubMed] [Google Scholar]
- 95.Jones RB, Roper TJ. Olfaction in the domestic fowl: a critical review. Physiol Behav. 1997;62:1009–1018. doi: 10.1016/S0031-9384(97)00207-2. [DOI] [PubMed] [Google Scholar]
- 96.The Avian Brain Nomenclature Consortium Avian brains and a new understanding of vertebrate brain evolution. Nature Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rieke GK, Wenzel BM. Forebrain Projections of the pigeon olfactory bulb. J Morphol. 1978;158:41–56. doi: 10.1002/jmor.1051580105. [DOI] [PubMed] [Google Scholar]
- 98.Reiner A, Karten HJ. Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol. 1985;27:11–27. doi: 10.1159/000118717. [DOI] [PubMed] [Google Scholar]
- 99.Dubbeldam JL. Birds. In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, editors. The central nervous system of vertebrate. Berlin: Springer; 1998. pp. 1525–1636. [Google Scholar]
- 100.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gütürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adrian ED. Olfactory reactions in the brain of the hedgehog. J Physiol (Lond) 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendoza AS, Breipohl W, Miragall F. Cell migration from the chick olfactory placode: a light and electron microscopic study. J Embryol Exp Morph. 1982;69:47–59. [PubMed] [Google Scholar]
- 104.Croucher SJ, Tickle C. Characterization of epithelial domains in the nasal passages of chick embryos: spatial and temporal mapping of a range of extracellular matrix and cell surface molecule during development of the nasal placode. Development. 1989;106:493–509. doi: 10.1242/dev.106.3.493. [DOI] [PubMed] [Google Scholar]
- 105.Rogers LJ. The development of brain and behaviour in the chicken. Wallingford: CAB International; 1995. [Google Scholar]
- 106.Drapkin PT, Silverman A-J. Development of the chick olfactory nerve. Dev Dyn. 1999;214:349–360. doi: 10.1002/(SICI)1097-0177(199904)214:4<349::AID-AJA7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 107.Fornaro M, Geuna S, Fasolo A, Giacobin-Robecchi MG. Evidence of very early neuronal migration from the olfactory placode of the chick embryo. Neurosci. 2001;107:191–197. doi: 10.1016/S0306-4522(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 108.Lalloué FL, Ayer-Le Lièvre CS. Experimental study of early olfactory neuron differentiation and nerve formation using quail-chick chimeras. Int J Dev Biol. 2005;49:193–200. doi: 10.1387/ijdb.041933fl. [DOI] [PubMed] [Google Scholar]
- 109.Landmesser L, Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol (Lond) 1972;222:691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takahashi T, Nakajima Y, Hirosawa K, Nakajima S, Onodera K. Structure and physiology of developing neuromuscular synapses in culture. J Neurosci. 1987;7:473–481. doi: 10.1523/JNEUROSCI.07-02-00473.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hume RI, Role LW, Fischbach GD. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305:632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- 112.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 113.Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kater SB, Mattson MP, Cohan C, Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988;11:315–321. doi: 10.1016/0166-2236(88)90094-X. [DOI] [PubMed] [Google Scholar]
- 115.Gu X, Spitzer NC. Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev Neurosci. 1997;19:33–41. doi: 10.1159/000111183. [DOI] [PubMed] [Google Scholar]
- 116.Wong ROL, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nature Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]


