Abstract
Chronic low blood pressure is typically accompanied by symptoms such as fatigue, reduced drive, dizziness, headaches and cold limbs. Reduced cognitive performance, diminished cerebral blood flow and autonomic dysregulation have been furthermore documented in this condition. The present contribution reports two studies exploring systemic hemodynamics in chronic hypotension and their modification through vasopressor application. In study I, effects of the alpha-sympathomimetic midodrine were examined in 54 hypotensive individuals using a placebo-controlled double-blind design. Hemodynamic parameters were assessed at rest and during mental stress. They were derived from continuous blood pressure recordings using Modelflow analysis. The drug led to marked increases in blood pressure, total peripheral resistance and stroke volume. However, due to strong heart rate deceleration, cardiac output remained virtually unchanged. In study II, 40 hypotensive and 40 normotensive control persons were compared with respect to hemodynamics. While groups did not differ in total peripheral resistance, hypotensives exhibited markedly diminished stroke volume and heart rate, resulting in a reduction in cardiac output of 25% at rest and of 33% during mental stress. The data provide relevant knowledge about the hemodynamic mediation of chronic hypotension. In contrast to elevated blood pressure, which is mainly determined by increased peripheral resistance, reduced cardiac output may be the cardinal hemodynamic aberration in chronic hypotension. Midodrine proved to be effective in elevating blood pressure. However, given the cardiac origin of chronic hypotension and the lack of drug effect on cardiac output, alpha-sympathomimetic treatment may be suboptimal.
Keywords: Hypotension, Blood pressure, Cardiac output, Baroreflex, Mental stress
Introduction
Hypotension has been defined by the World Health Organization (WHO) [1] as low blood pressure with a systolic reading below 110 mmHg in males and below 100 mmHg in females. The concept of chronic (“essential”) hypotension refers to a condition of inappropriately reduced blood pressure independent of the presence of further pathological conditions. It has to be distinguished from secondary hypotension (e.g., due to blood loss or medication) as well as from the orthostatic form consisting of circulatory problems when assuming an upright position [2, 3].
Chronic low blood pressure is typically accompanied by symptoms such as fatigue, reduced drive, dizziness, headaches and cold limbs [2]. Even though it is usually not regarded as a severe medical condition, a considerable impact of chronic hypotension on subjective well-being and quality of life has been documented in a number of large population-based studies [4–6]. Furthermore, reduced cognitive performance, diminished cerebral blood flow and cortical activation have been found in chronic hypotension [2]. As etiological factors, low body weight, reduced liquid intake and autonomic dysregulations in terms of habitually reduced sympathetic and increased parasympathetic outflow, as well as aberrations in baroreflex function, were considered [2, 7].
The present contribution deals with alterations in systemic hemodynamics in chronic hypotension and their modification through pharmacological blood pressure elevation. The major hemodynamic factors determining systemic blood pressure are cardiac output and peripheral resistance [8]. Increased total peripheral resistance is the most relevant hemodynamic aberrance in chronically elevated blood pressure [9, 10]. Augmented cardiac output may furthermore contribute to this condition, at least during its early phase [10–12]. In contrast, knowledge about hemodynamic peculiarities in chronic hypotension remains sparse. Hypotheses may be derived from the findings on autonomic origins of this condition. Increased parasympathetic tone and reduced sympathetic outflow are thought to be accompanied by diminished heart rate and cardiac contractility resulting in reduced cardiac output [13]. In addition, diminished vascular tone due to reduced alpha-adrenergic activity cannot be ruled out [8].
Sympathomimetic drugs are commonly used for antihypotensive treatment. The majority of clinical trials evaluating the therapeutic effects of these substances were conducted with patients suffering from, mostly neurogenic, orthostatic hypotension [14–16]. In mixed samples of persons with chronic and orthostatic hypotension, reductions of subjective symptoms under the sympathomimetic etilefrine were reported [17, 18]. Studies aiming specifically at pharmacological treatment of chronic hypotension have focused exclusively on reduction of cognitive deficits [19, 20]. Hemodynamic effects of sympathomimetic agents have not been investigated so far in this population. The alpha-adrenergic agonist midodrine, which was applied in the present study, is highly effective in inducing arterial and venous vasoconstriction [21]. The substance has been shown to significantly increase blood pressure in healthy subjects, as well as patients with neurogenic orthostatic hypotension [14, 16, 22, 23].
The present report includes two studies. Study I investigated hemodynamic effects of the sympathomimetic midodrine in a placebo-controlled double-blind design. In study II, individuals with chronic hypotension were compared to a normotensive control sample with respect to hemodynamics. In order to get a more comprehensive picture, in both studies cardiovascular parameters were obtained during resting conditions as well as under mental stress. For the latter purpose, a mental arithmetic task was applied.
Methods
Study I
Participants
The study sample was comprised of 54 chronic hypotensive individuals according to the WHO definition [1]. None of them suffered from a relevant physical disease or mental disorder. Health status was assessed by means of an anamnestic interview and a questionnaire covering diseases of the cardiovascular, respiratory, gastro-intestinal and uro-genital systems, the thyroid, the liver, as well as metabolic diseases and psychiatric disorders. None of the participants used any kind of medication affecting the cardiovascular or central/peripheral nervous system. In addition, individuals using medication with known interactions with midodrine were excluded from participation.
Subjects were randomly assigned to one of two study groups administered with either midodrine or placebo. The midodrine group included 22 women and 5 men; the placebo group 20 women and 7 men. Information regarding blood pressure and heart rate at the beginning of the experimental session, as well as age, body height and BMI are given in Table 1. Nine women of the midodrine and seven of the placebo group were using oral contraceptives.
Table 1.
Sample characteristics: systolic blood pressure, diastolic blood pressure, heart rate, age, body height and body mass index
| Midodrine | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | M | SD | Min | Max | |
| Systolic blood pressure (mmHg) | 94.5 | 6.1 | 81.0 | 108.0 | 96.7 | 7.4 | 84.0 | 108.0 |
| Diastolic blood pressure (mmHg) | 60.4 | 4.5 | 50.7 | 69.0 | 60.7 | 6.3 | 44.7 | 73.0 |
| Heart rate (beats/min) | 69.7 | 7.4 | 55.3 | 97.0 | 68.7 | 10.6 | 44.0 | 88.0 |
| Age (years) | 26.1 | 4.8 | 19 | 39 | 26.9 | 5.5 | 20 | 41 |
| Body height (cm) | 167.5 | 7.4 | 157 | 186 | 168.1 | 8.5 | 155 | 186 |
| Body mass index (kg/m2) | 20.9 | 2.1 | 17.9 | 26.6 | 20.7 | 2.0 | 17.0 | 25.3 |
M, means; SD, standard deviations; min, minimal; max, maximal values
Recording of hemodynamic data
Blood pressure was monitored continuously (Finometer Model-2, Finapres Medical Systems, The Netherlands). The cuff of the device was applied to the mid-phalanx of the second finger of the right hand. In order to control for the influence of hydrostatic level errors, the height correction unit integrated in the device was used. For periodic recalibration, the device’s Physiocal feature [24] was in operation. The signal was digitized at a sample rate of 200 Hz.
Substance administration
Dosage of midodrine (trade name in Germany: Gutron®) was 0.4 mg per kg body weight. The placebo consisted of 4.8 mg of alcohol per kg body weight, equivalent to the alcohol content of Gutron®. Both the drug and the placebo were dispensed in a glass of water. The group assignment and substance administration were carried out by an assistant who was not otherwise involved in the experiment. Both the subjects and the experimenter were blind to the respective conditions.
Procedure
The study was approved by a local ethics commission, and subjects gave informed consent prior to the experiment. In order to determine hypotension, blood pressure was taken during screening sessions, which were conducted at least 1 week prior to the main experiment, and once more at the beginning of the experimental session. For this purpose, after a resting period of 10 min, three blood pressure measurements were taken with 5-min rest intervals in between. Sphygmomanometrical measurements were performed in a seated position using an automatic inflation monitor (MIT, TYP M CR15; Omron, USA). Women with a mean systolic blood pressure below 100 mmHg and men with a mean value below 110 mmHg were included. The criteria had to be fulfilled at both the screening and experimental sessions.
Hemodynamic indices were obtained at rest and during mental stress induced by a serial subtraction task. During the 5-min resting phase, participants were asked to sit still, not to speak and to relax with their eyes open. The subtraction task consisted of a 3 min interval during which subjects had to count down from 700, subtracting 17 each time and saying the numbers out loud. They were asked to perform the task as quickly and as accurately as possible.
Due to the fact that a maximal effect of orally applied midodrine develops after approximately 1 h, the administration was followed by a 60-min break. After this break, three further measurements of blood pressure and heart rate were taken in the described form. Thereafter, hemodynamic assessment was repeated. Subjects were requested not to smoke or drink either alcohol or beverages containing caffeine for 3 h prior to the screening and experimental sessions.
Data analysis
Hemodynamic parameters were determined based on the Modelflow method [25] using the software Beatscope 1.1a. (Finapres Medical Systems, The Netherlands). The Modelflow technique makes it possible to model blood flow from arterial pressure by computing a flow wave that is integrated to obtain beat-to-beat stroke volume (in ml). Heart rate (in beats/min), cardiac output (in l/min) and total peripheral resistance [in mmHg/(l*min−1)] were also included in the analysis.
All indices were averaged over the time intervals of the resting and mental stress conditions. Possible drug effects on sphygmomanometrically assessed blood pressure were evaluated using ANOVA procedures with experimental group (midodrine versus placebo) as a between subjects factor and point of measurement (before versus after substance administration) as a within subjects factor. In the ANOVA models for continuously assessed stroke volume, heart rate, cardiac output and total peripheral resistance, experimental condition (rest versus mental stress) was applied as a further within subjects factor. In order to control for differences between both experimental groups at the pretest stage, t-tests were computed for each of the dependent variables.
Study II
Participants
Forty subjects with chronic hypotension according to the WHO [1] were compared to 40 normotensive control persons (32 women and eight men in each group). Criteria for exclusion, as well as the methods for their determination, were identical with those used in study I. Fifteen of the women in the hypotensive group and 20 in the control group were using oral contraceptives. Both study groups were matched according to age and gender. Table 2 provides information about blood pressure as recorded just before the experimental procedure, age, body height and body mass index (BMI).
Table 2.
Means (M) and standard deviations (SD) of systolic blood pressure, diastolic blood pressure, age, body height and body mass index in both samples
| Chronic hypotension | Control group | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Systolic blood pressure (mmHg) | 96.5 | 4.8 | 121.8 | 5.3 |
| Diastolic blood pressure (mmHg) | 57.7 | 4.5 | 77.2 | 5.9 |
| Age (years) | 27.7 | 5.7 | 27.8 | 5.4 |
| Body height (cm) | 168.3 | 9.1 | 171.0 | 8.2 |
| Body mass index (kg/m2) | 20.2 | 2.0 | 22.5 | 3.4 |
Procedure
Participants gave informed consent prior to the experiment. In order to assign subjects to the two study groups, blood pressure was taken during screening sessions, which were conducted at least 1 week prior to the main experiment, and once more at the beginning of the experimental session. Just as in study I, after a resting period of 10 min, three blood pressure measurements were taken with 5-min rest intervals in between. Females with a mean systolic blood pressure of <100 mmHg and males with a mean value below 110 mmHg were assigned to the hypotensive group. The inclusion criterion for the control group was systolic blood pressure between 115 and 140 mmHg. The criteria had to be fulfilled at both the screening and experimental sessions.
Continuous hemodynamic recordings were made at rest (5 min) and during mental stress induced by the arithmetic task (3 min) described in study I. The same apparatus as in study I was applied for this purpose. Subjects were requested not to smoke, drink alcohol or beverages containing caffeine for 3 h prior to both sessions.
Data analysis
The same hemodynamic parameters as in study I were obtained by means of Modelflow [25] using the software Beatscope 1.1a. (Finapres Medical Systems, The Netherlands). All indices were averaged over the time intervals of the resting and mental stress conditions. The statistical analyses included analysis of variance (ANOVA) procedures for repeated measures. Blood pressure group (hypotensive versus control group) served as a between-subjects factor and experimental condition (rest versus mental stress) as a within-subjects factor.
Results
Study I
The group receiving midodrine and the placebo group did not differ with respect to any of the dependent variables at the first point of measurement (systolic blood pressure: P = 0.25, diastolic blood pressure: P = 0.86, heart rate: P = 0.67, stroke volume at rest: P = 0.56, cardiac output at rest: P = 0.84, total peripheral resistance at rest: P = 0.94, stroke volume during stress: P = 0.80, cardiac output during stress: P = 0.80 and total peripheral resistance during stress: P = 0.53), nor did the two groups differ in terms of age (P = 0.56), body height (P = 0.80) and BMI (P = 0.78).
The changes in sphygmomanometrically assessed blood pressure between both points of measurement are displayed in Fig. 1. Systolic and diastolic values increased in the midodrine group and slightly decreased under placebo. ANOVA revealed a significant interaction between point of measurement and experimental group for both parameters (both P < 0.01).
Fig. 1.
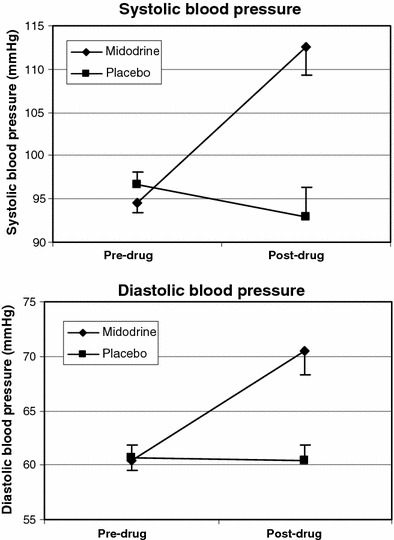
Blood pressure before and after substance application (bars denote standard errors of the means)
Figure 2 displays the continuously assessed hemodynamic parameters. Stroke volume, measured both at rest and during mental stress, increased under midodrine and remained almost constant under placebo. The drug effect is confirmed by a significant interaction between point of measurement and experimental group (P < 0.01). Overall, stroke volume was somewhat higher during stress; the factor condition, however, reached only the 10% significance level (P = 0.06). Heart rate decreased markedly in the midodrine group and remained unchanged in the placebo group under both conditions (interaction point of measurement by group: P < 0.01). In both experimental groups heart rate was higher during stress, which is corroborated by a significant effect of condition (P < 0.01). Regarding cardiac output, only a slight increase at rest was observed under midodrine, the interaction between point of measurement and group not being significant (P = 0.55). In both groups cardiac output was significantly higher during stress than at rest (condition: P < 0.01). A significant drug-induced increase in total peripheral resistance was furthermore obtained (interaction point of measurement by group: P = 0.02). Total peripheral resistance was overall somewhat higher under stress than at rest (condition: P = 0.06).
Fig. 2.
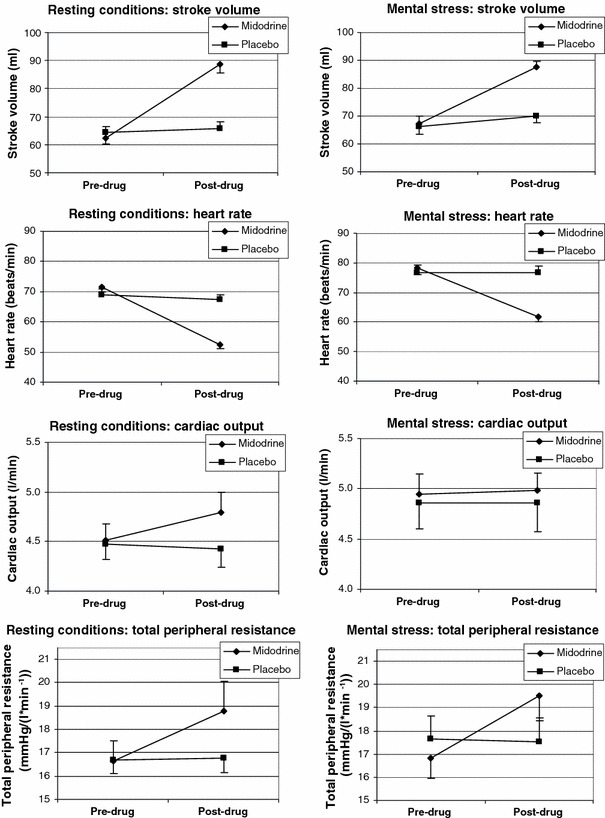
Stroke volume, heart rate, cardiac output and total peripheral resistance before and after substance application (bars denote standard errors of the means)
Study II
Stroke volume, heart rate and cardiac output were markedly lower in chronic hypotension than in the normotensive control group, whereas total peripheral resistance was slightly higher in hypotensives (see Fig. 3). The values of all parameters were higher during mental stress than at rest. The ANOVA revealed a significant main effect of group for stroke volume (P < 0.01), heart rate (P < 0.01) and cardiac output (P < 0.01), as well as a main effect of condition on each of the hemodynamic variables (stroke volume: P = 0.04, heart rate: P < 0.01, cardiac output: P < 0.01, total peripheral resistance: P < 0.01). In the case of cardiac output, a significant interaction between group and condition was also found (P < 0.01), indicating a stronger increase under stress in the control group.
Fig. 3.
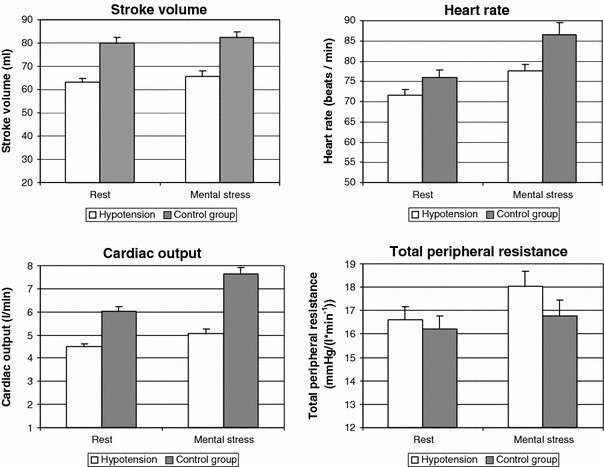
Stroke volume, heart rate, cardiac output and total peripheral resistance in hypotensive and control subjects (bars denote standard errors of the means)
Discussion
The present report includes two studies on systemic hemodynamics in chronic low blood pressure. Study I investigated hemodynamic effects of the vasopressor agent midodrine. The drug caused increases in blood pressure, stroke volume and total peripheral resistance, as well as a reduction in heart rate. Cardiac output was not significantly affected. In study II, individuals with chronic hypotension were compared to a normotensive control sample. Hypotensives showed markedly reduced stroke volume, heart rate and cardiac output. In both studies, comparable effects were found for resting as well as mental stress conditions.
Information concerning the treatment of chronic hypotension remains sparse [2]. In study I, sympathomimetic treatment led to a rise in both systolic and diastolic blood pressure into the normotensive range. This is in line with previously reported blood pressure enhancement through this substance in healthy volunteers and patients with neurogenic orthostatic hypotension [16, 22, 23]. As a selective alpha-receptor agonist, midodrine induces arterial and venous vasoconstriction, leading to increased peripheral resistance and arterial pressure [21]. In contrast, midodrine does not directly affect cardiac contractility. Nonetheless, under the drug, stroke volume increased by 41% at rest and by 30% during mental load. This may be regarded as an indirect effect resulting from elevated vascular resistance. It can be ascribed to the Frank-Starling mechanism, according to which the force of myocardial contraction rises as a consequence of increased diastolic filling pressure [8]. Only a small non-significant drug-induced increase in cardiac output was observed. The enhancement of stroke volume appeared not to be sufficient to compensate for the marked heart rate reduction. The heart deceleration is the result of a compensatory response to blood pressure increase mediated by the arterial baroreflex [8]. Its relatively large extent may be attributed to peculiarities in baroreflex function related to chronic hypotension. Baroreflex sensitivity, expressed as change in heart rate per unit of blood pressure change, is habitually increased in hypotension [2, 7]. Recent research has suggested that the baroreflex is involved not only in the buffering of transient changes in arterial pressure, but also in the long-term setting of blood pressure, and thus may contribute to the origin of chronic hypotension [2, 26, 27].
The group comparison in study II revealed a reduction in cardiac output in chronic hypotension by 25% at rest and by 33% during mental stress. This remarkable reduction was due to lowered heart rate and stroke volume. However, hypotensive and normotensive individuals did not differ in terms of total peripheral resistance. This pattern of results may provide relevant information about the hemodynamic origin of chronic hypotension. There is broad evidence of increased peripheral resistance in chronically elevated blood pressure, indicating that this condition is mainly determined by vascular factors [9, 10]. In contrast, the present data suggest that in the causation of chronic hypotension, cardiac factors such as reduced heart rate, stroke volume and cardiac output play a dominant role. One may hypothesize that lowered cardiac output results in reduced organ perfusion in chronic hypotension. This is in accordance, for instance, with the assumption of diminished perfusion of the skin vessels that causes lowered skin temperatures and the frequent experience of cold limbs [2]. Cerebral blood flow was also shown to be affected by chronic low blood pressure. Even though the brain is generally well protected against hypo-perfusion [28], substantially reduced cerebral resting perfusion and deficient blood flow adjustment to situational requirements occur in hypotension [29]. Alterations in cerebral blood flow regulation have been shown to be of crucial importance to the cognitive deficits that accompany chronic hypotension [2, 19, 29]. A permanent reduction in brain perfusion may furthermore be relevant to the connection between low blood pressure and the occurrence of geriatric cognitive disorders that has been documented in a number of longitudinal studies [30–32].
Both of the present studies revealed a slight increase in stroke volume and more pronounced increases in heart rate, cardiac output and total peripheral resistance during mental stress as compared to resting conditions. This can be attributed to stress-related elevation of general cardiac arousal, mediated by autonomic nervous system and hormonal pathways [33]. The reduced increase of cardiac output in hypotension observed in study II is consistent with a number of previous findings on reduced cardiovascular reactivity to mental and physical stress in chronic low blood pressure [34–36].
One limitation of a part of the present results pertains to the estimation of stroke volume using the Modelflow technique [25]. While this method allows assessing intraindividual hemodynamic changes with high precision, its accuracy in estimating absolute values of stroke volume and thus in quantifying inter-individual differences is under debate [37]. However, some data support the utility of Modelflow also for inter-individual comparisons at least at group level. For instance, Wesseling et al. [25] showed reasonable consistence between absolute measures of cardiac output derived from Modelflow and thermodilution (7% mean difference between estimates obtained with both methods). The validity of the method in quantifying changes in stroke volume under defined conditions has been well established [37, 38]. Nonetheless, possible distortion of the observed effects of midodrine on stroke volume due to drug-induced changes in arterial vascular properties cannot be completely ruled out.
To sum up, the present studies extend our knowledge about the mediation of chronically reduced blood pressure by hemodynamic factors. In contrast to essential hypertension, which is predominantly determined by vascular factors, chronic hypotension would appear to be caused by cardiac factors, such as reduced heart rate, stroke volume and cardiac output. The sympathomimetic midodrine led to marked increases in blood pressure, stroke volume and total peripheral resistance, but, because of pronounced heart deceleration, not in cardiac output. Therefore, the drug’s efficiency in improving organ perfusion is possibly suboptimal. Future research may focus on drugs that also display beta-sympathetic properties and thus have a direct impact on heart activity, i.e., positive inotrope and chronotrope effects. This seems of particular importance considering the assumed cardiac origin of chronic hypotension.
Acknowledgements
This study was funded by the German Research Foundation (project no. DU 447/1-1). The cooperation between the Universities of Munich and Jaén was supported by the German Academic Exchange Service and the Spanish Ministry of Education and Science.
References
- 1.WHO . Arterial hypertension: technical report series no. 628. Genova: World Health Organisation; 1978. [PubMed] [Google Scholar]
- 2.Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to essential hypotension. Clin Autonom Res. 2007;17:69–76. doi: 10.1007/s10286-006-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7:451–458. doi: 10.1016/S1474-4422(08)70088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilgrim JA, Stansfield S, Marmot M. Low blood pressure, low mood? BMJ. 1992;304:75–78. doi: 10.1136/bmj.304.6819.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosengren A, Tibblin G, Wilhelmsen L. Low systolic blood pressure and self perceived wellbeing in middle aged men. BMJ. 1993;306:243–246. doi: 10.1136/bmj.306.6872.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessely S, Nickson J, Cox B. Symptoms of low blood pressure: a population study. BMJ. 1990;301:362–365. doi: 10.1136/bmj.301.6748.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duschek S, Dietel A, Schandry R, Reyes del Paso GA. Increased baroreflex sensitivity and reduced cardiovascular reactivity in chronic low blood pressure. Hypertens Res. 2008;31:1873–1878. doi: 10.1291/hypres.31.1873. [DOI] [PubMed] [Google Scholar]
- 8.Levy MN, Pappano AJ. Cardiovascular physiology. Philadelphia: Mosby Elsevier; 2007. [Google Scholar]
- 9.Cain AE, Khalil RA. Pathophysiology of essential hypertension: role of the pump, the vessel, and the kidney. Semin Nephrol. 2002;22:3–16. [PubMed] [Google Scholar]
- 10.Lund-Johansen P. Newer thinking on the hemodynamics of hypertension. Curr Opin Cardiol. 1994;9:505–511. doi: 10.1097/00001573-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lovallo WR, al’Absi M. Hemodynamics during rest and behavioral stress in normotensive men at high risk for hypertension. Psychophysiology. 1998;35:47–53. doi: 10.1017/S004857729896108X. [DOI] [PubMed] [Google Scholar]
- 12.Lund-Johansen P. Twenty-year follow-up of hemodynamics in essential hypertension during rest and exercise. Hypertension. 1991;18(suppl 5):SIII54–SIII61. doi: 10.1161/01.hyp.18.5_suppl.iii54. [DOI] [PubMed] [Google Scholar]
- 13.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann H, Brannan T, Krakoff L, Yahr MD, Mandeli J. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine) Neurology. 1988;38:951–956. doi: 10.1212/wnl.38.6.951. [DOI] [PubMed] [Google Scholar]
- 15.Oldenburg O, Mitchell A, Nurnberger J, et al. Ambulatory norepinephrine treatment of severe autonomic orthostatic hypotension. J Am Coll Cardiol. 2001;37:219–223. doi: 10.1016/S0735-1097(00)01062-7. [DOI] [PubMed] [Google Scholar]
- 16.Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1988;51:120–124. doi: 10.1212/wnl.51.1.120. [DOI] [PubMed] [Google Scholar]
- 17.Bismarck M, Rust G. Erste Erfahrungen mit dem Antihypotonikum Thomasin (=Etilefrin) unter ambulanten Bedingungen (eine Doppelblindstudie) Z Ärztl Fortb. 1982;76:153–156. [PubMed] [Google Scholar]
- 18.Schandry R. Die Verbesserung der subjektiven Befindlichkeit bei orthostatischer Hypotonie unter dem Einfluss blutdrucksteigernder Therapie [Improvement of subjective wellbeing in orthostatic hypotension due to blood pressure elevating therapy] Med Welt. 1999;50:160–165. [Google Scholar]
- 19.Duschek S, Hadjamu M, Schandry R. Enhancement of cerebral blood flow and cognitive performance due to pharmacological blood pressure elevation in chronic hypotension. Psychophysiology. 2007;44:145–153. doi: 10.1111/j.1469-8986.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- 20.Duschek S, Hadjamu M, Schandry R. Dissociation between cortical activation and cognitive performance in the pharmacological treatment of chronic hypotension. Biol Psychol. 2007;75:277–285. doi: 10.1016/j.biopsycho.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.McTavish D, Goa KL. Midodrine. A review of its pharmacological properties and therapeutic use in orthostatic hypotension and secondary hypotensive disorders. Drugs. 1989;38:757–777. doi: 10.2165/00003495-198938050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Grobecker HF, Kees F. Pharmacokinetic parameters and haemodynamic actions of midodrine in young volunteers. Int Angiol. 1993;12:119–124. [PubMed] [Google Scholar]
- 23.Schrage WG, Eisenach JH, Dinenno FA. Effects of midodrine on exercise-induced hypotension and blood pressure recovery in autonomic failure. J Appl Physiol. 2004;97:1978–1984. doi: 10.1152/japplphysiol.00547.2004. [DOI] [PubMed] [Google Scholar]
- 24.Wesseling KH, De Wit B, Van der Hoeven GMA, Van Goudoever J, Settels JJ. Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis. 1995;36:67–82. [Google Scholar]
- 25.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 26.Hesse C, Charkoudian N, Liu Z, Joyner MJ, Eisenach JH. Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension. 2007;50:41–46. doi: 10.1161/HYPERTENSIONAHA.107.090308. [DOI] [PubMed] [Google Scholar]
- 27.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 28.Paulson OB. Blood-brain barrier, brain metabolism and cerebral blood flow. Eur Neuropsychopharmacol. 2002;12:495–501. doi: 10.1016/S0924-977X(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 29.Duschek S, Schandry R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology. 2004;41:905–913. doi: 10.1111/j.1469-8986.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruitenberg A, Skoog I, Ott A, et al. Blood pressure and risk of dementia. Results from the Rotterdam study and the Gothenburg H-70 study. Dement Geriatr Cogn Disord. 2001;12:33–39. doi: 10.1159/000051233. [DOI] [PubMed] [Google Scholar]
- 31.Heijer T, Skoog I, Oudkerkm M, et al. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003;24:307–313. doi: 10.1016/S0197-4580(02)00088-X. [DOI] [PubMed] [Google Scholar]
- 32.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 33.Hugdahl K. Psychophysiology. Cambridge: Harvard University Press; 2001. [Google Scholar]
- 34.Cadalbert B (1997) Die Psychophysiologie des niedrigen Blutdrucks: Kreislaufregulation, Lebensgewohnheiten und Beschwerden [The psychophysiology of low blood pressure: Cardiovascular regulation, life style, and complaints]. Frankfurt a.M.: Peter Lang
- 35.Duschek S, Matthias E, Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behav Med. 2005;30:149–158. doi: 10.3200/BMED.30.4.149-160. [DOI] [PubMed] [Google Scholar]
- 36.Duschek S, Schandry R. Deficient adjustment of cerebral blood flow to cognitive activity due to chronically low blood pressure. Biol Psychol. 2006;72:311–317. doi: 10.1016/j.biopsycho.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Bogert LWJ, Lieshout JJ. Non-invasive arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 38.Jellema W, Wesseling KH, Groeneveld ABJ, Stoutenbeek CP, Thijs LG. Continuous cardiac output in septic shock by simulating a model of the aortic input in pomparison with bolus injection thermodilution. Anesthesiology. 1999;90:1317–1328. doi: 10.1097/00000542-199905000-00016. [DOI] [PubMed] [Google Scholar]


