Abstract
To test the hypothesis that topical menthol-induced reactivity of cold sensation and cutaneous vasoconstriction to local cooling is augmented in individuals with a cold constitution, we examined thermal sensation and cutaneous vasoconstrictor responses at menthol-treated and untreated sites in the legs during local skin cooling in young women complaining of chilliness (C group) and young women with no complaint as a normal control group (N group). During local skin cooling, the sensitivity to cold sensation was greater in the C group than in the N group. The application of menthol enhanced the cold sensation at a low temperature in the N group, but not in the C group. Cutaneous vasoconstrictor responses to local skin cooling were not altered by menthol treatment in either of the two groups. These findings suggest the desensitization of menthol-activated cold receptors in the legs of C group subjects, and a minor role of cold receptor activity in cutaneous vasoconstrictor response to local cooling.
Keywords: Cold sensation, Cold receptor, TRPM8, Skin blood flow, Local temperature
Introduction
In humans, skin exposure to a cool environment produces a cold sensation. Cold-induced somatosensory responses usually exhibit inter-individual differences. In Japan, even at temperate thermal conditions when most people do not feel cold, women often suffer from severe chilliness of the body, especially in the lower extremities [1–5]. This unusual feeling of coldness in individuals is called “hi-e-sho” in Japanese, and has been identified more frequently in females than in males [1, 2, 5]. Cold-sensitive females were also characterized by higher adrenergic vasoconstrictor sensitivity in the distal skin sites of the lower extremities [6]. However, the underlying mechanisms resulting in higher sensitivity to cold sensation are currently unknown. The higher thermal sensitivity to cold would be at least due to: (1) a greater activity in somatosensory neurons in the brain at a given peripheral thermosensitive input, and/or (2) greater thermoreceptor excitation in the cutaneous endings of somatosensory neurons at a similar temperature [6]. Therefore, a possible mechanism may be an up-regulation of cold receptor function in the skin. Pharmacological blockade of the cold receptor TRPM8 (transient receptor potential melastatin-8) channels attenuates cutaneous vasoconstriction and behavioral cold defense [7], whereas application of menthol, a specific agonist of TRPM8 channels, to the skin of whole trunk in mice induces heat-gain responses, including a rise in oxygen consumption and tail skin vasoconstriction [8]. Based upon these findings, we speculate that topical application of menthol may increase cold sensation to local skin cooling in individuals suffering from unusual coldness.
Direct skin cooling induces cutaneous vasoconstriction, as well as cold sensation. Blockade of sensory nerves using topical anesthesia at the cooled skin site revealed the initial vasodilator response to direct local cooling [9]. These findings suggest that sensory nerve endings at site of the cooled skin were involved in the inhibition of vasodilation or activation of vasoconstriction. Relative to normal control females, cold-sensitive young females showed increased cutaneous vasoconstriction in the distal portion of the extremities during whole-body skin cooling [6, 10]. If cold receptor sensitization occurs in cold-sensitive individuals, then topical application of menthol will further increase cutaneous vasoconstrictor response to local cooling of the skin.
This study was conducted to obtain a better understanding of the specific mechanisms controlling thermal sensation during local skin cooling in young females complaining of unusual chilliness. We hypothesized that the sensitivity to cold sensation and cutaneous vasoconstriction in response to cold receptor activation by the topical application of menthol in the lower extremities are greater in young females complaining of unusual chilliness than in normal young females.
Methods
Subjects
The ethics committees of the medical care and research institutions approved all experiments. All subjects were fully informed of the methods and risks before written informed consent was obtained. A total of 16 Japanese female subjects participated in the experiments. Subjects were chosen based upon a 10-question interview, as previously reported [10], which contains questions concerning the typical complaints of individuals suffering from unusual chilliness. Subjects were entered into the study if they answered yes more than six times [n = 8, cold-sensitive group (C)], or fewer than three times [n = 8, normal group (N)] in the interview. Those that answered yes between three and six times were excluded from this study. The average age was 20 ± 1 and 21 ± 1 years, average weight was 50.2 ± 1.6 and 48.2 ± 2.2 kg, and average height was 159.0 ± 1.3 and 158.1 ± 2.9 cm in the C and N groups, respectively. All subjects were healthy nonsmokers, not obese (body mass index of 17–23 kg/m2), and not taking any medications. Selected subjects were confirmed to be healthy by a medical examination and had regular menstrual cycles. The menstrual cycle was not considered because previous reports showed that the menstrual cycle had no effect on thermal perception and on autonomic cold-defense response in young female subjects [11], and that the vasoconstrictor response to local cooling was unaffected by reproductive hormone status [12]. Differences in response between follicular and luteal phases were not seen in the present studies and therefore their data were combined for analysis.
Topical application of menthol
Menthol acts on the highly sensitive cold receptor TRPM8, which is located on the cell membrane of sensory neurons [13, 14]. Low concentrations (<2 %) of menthol elicit a cool sensation [15–17], whereas high concentrations (2–5 %) cause irritation and local anesthesia [18]. However, little is known about the relationship between the dose of topical menthol and the responses of skin blood flow (SkBF) in humans. In a pilot study, we examined the effects of various concentrations [0 % (control), 0.2, 0.5, 1, and 3 %] of menthol solution in 25 % ethanol on the SkBF in the lateral aspect of the calves of 7 healthy female subjects. Topical application of 3 % menthol increased baseline SkBF and caused irritation in some subjects, while the other concentrations did not change baseline SkBF and thermal sensation at a local temperature of 35 °C. Additionally, application of 1 or 3 % menthol decreased vasoconstrictor responses to local cooling. Based upon these findings, we chose the 0.5 % menthol solution in 25 % ethanol for local application to an area (~8 cm2) on the calf and dorsal foot of one leg. Application of 25 % ethanol to skin sites on the opposite leg was used as a placebo control.
Measurements
Sublingual temperature (T sl) was measured with a polyethylene-sealed thermocouple. Skin temperatures (T sk) at four sites (chest, upper forearm, thigh, and calf) were measured with copper–constantan thermocouples. The mean T sk for each of the four skin sites was calculated based upon the Ramanathan weighting formula [19], with an accuracy of ±0.1 °C. Heart rate (HR) was determined using electrocardiogram (ECG) with a telemetric device (BSM-2401; Nihon Kohden, Tokyo, Japan). SkBF at five sites (the lateral aspects of the middle part of left and right calf, the dorsal aspects of the middle part of left and right dorsal foot, and the middle part of the left forearm) was measured using laser Doppler flowmetry (ALF21; Advance, Tokyo, Japan). The local temperature of the 6.3-cm2 area surrounding the site of SkBF measurement on the calf and dorsal foot was controlled using local temperature controllers comprising a custom-built metal sleeve for the flow probe. A thermocouple between the skin surface and the sleeve allowed for measurement and feedback control. The local temperature controllers were put on the skin area where 0.5 % menthol or 25 % ethanol vehicle was applied. The local temperature of the area surrounding the site of SkBF measurement on the forearm was not controlled. Temperature and SkBF were recorded every 1 s using a data acquisition system (UAS-108S; Unique Medical, Tokyo, Japan). Mean arterial pressure (MAP) was measured every 5 min using a Dynamap automated oscillometric blood pressure device (model 8100; Criticon, Tampa, FL, USA).
During local cooling, thermal sensation was evaluated every 5 min using a visual analogue scale. Subjects were asked to report thermal sensation separately for each of the four local cooling sites by marking their current cold sensation on a 15-cm line rating scale, which was labeled “cold” 2.5 cm from the left end and “not at all” 2.5 cm from the right end. Additionally, subjects could mark their degree of cold sensation beyond the “cold” or “not-at-all” points, if necessary. Cold sensation scores were calculated using the distance from the reported cold sensation to the “not at all” point, producing a negative value.
Experimental protocol
The subjects arrived in the laboratory at 0900 hours after having abstained from caffeine and alcohol for at least 1 day and from food for at least 2 h. Dressed in sleeveless shirts and short pants, they entered an experimental room maintained at an ambient temperature (T a) of 29.5 °C with a relative humidity of 60 %, and rested in a supine position on a bed. It has previously been verified that a T a of 29.5 °C is within the neutral range of thermal comfort for Japanese women, wearing similar clothes, who do or do not suffer from unusual chilliness [6, 10].
The subjects rested in a supine position for ~1 h before data collection. During this period, 0.5 % menthol and 25 % ethanol vehicle, and then measuring devices, were applied to the skin. Data collection began with a 5-min baseline control period at a local temperature of 35 °C at the four SkBF measurement sites. The sites were then cooled in −2.5 °C steps every 5 min to 25 °C over 20 min. In each temperature step, local temperature at the measurement sites was slowly decreased by 2.5 °C for 1 min and kept a constant level for 4 min during the assessments of skin thermal sensation. The 35–25 °C T sk was used to simulate the local cold stress induced in the lower extremities during mild whole-body cold exposure [6, 10].
Data analysis
Measurements were averaged over 5-min periods for data analysis. Cutaneous vascular conductance (CVC) was calculated as SkBF/MAP. Changes in CVC were expressed as the percent change over 5 min from the 5-min baseline control period. Differences in physical characteristics between the two groups were compared by Student’s t tests. Differences in the measurement values between the C and N groups or the cooling and control periods were assessed by repeated measures ANOVA. Any significant differences between the means of the two groups or periods at a specific time point were subsequently identified by post hoc analysis using the Newman–Keuls procedure. Regression analysis was conducted using the standard least-square method. The differences in the slopes of the regression lines between the C and N groups, the menthol-treated and control sites, or the calf and dorsal foot were assessed using three-way ANOVA with the Bonferroni post hoc test. All data are expressed as the mean ± SE, and P values of <0.05 were considered significant.
Results
There was no difference between the two groups in T sl (C 36.8 ± 0.1 °C, N 36.9 ± 0.1 °C, P = 0.56), mean T sk (C 34.6 ± 0.1 °C, N 34.6 ± 0.1 °C, P = 0.94), MAP (C 68.8 ± 2.1 mmHg, N 68.5 ± 1.5 mmHg, P = 0.91), and HR (C 61.7 ± 1.1 beats/min, N 65.7 ± 1.2 beats/min, P = 0.06) during the pre-cooling baseline period. Direct cooling of a small area of the lower extremities induced a cold sensation and decreased the SkBF at the cooled sites without any changes in T sl (P = 0.85), mean T sk (P = 0.99), MAP (P = 0.87), HR (P = 0.98), and SkBF in the forearm (P = 0.41).
As depicted in Fig. 1, the rating scores of cold sensation decreased linearly with the decrease in local temperature during cooling. There were significant correlations (r = 0.887–0.998, P < 0.05) between local T sk and the rating scores of cold sensation in each site for all subjects. The slopes of the regression lines are shown in Table 1. With respect to the slopes of the relationships between local T sk and the rating scores of cold sensation, a three-way ANOVA revealed significant main effects between the C and N groups (P < 0.001), the menthol-treated and control sites (P < 0.05), and the calf and dorsal foot (P < 0.01), with a non-significant interaction among the three factors (P = 0.88). Regardless of whether or not the menthol was applied, the slopes of the regression lines for local T sk and the rating scores for the calf and dorsal foot were greater in the C group (P < 0.001) than in the N group, and the slopes for local T sk and the rating score were greater in the dorsal foot (P < 0.01) than in the calf (Table 1). The application of menthol increased cold sensation in the cooled area of the legs in the N group (P < 0.05), but not the C group (P = 0.78).
Fig. 1.
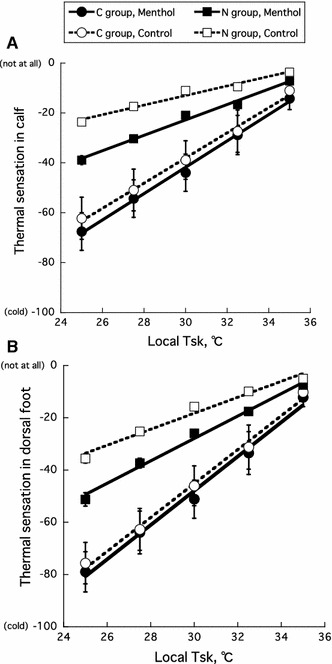
Relationships between local skin temperature (T sk) and thermal sensation in the calf (a) and dorsal foot (b) of cold-sensitive (C) and normal (N) groups. Closed and open circles represent data for menthol and control conditions, respectively. A score of −100 indicates cold and a score of 0 indicates not cold at all
Table 1.
The sensitivities for cold sensation and vasoconstrictor responses during local cooling
| C group | N group | |||
|---|---|---|---|---|
| Menthol | Control | Menthol | Control | |
| Cold sensation (unit/°C) | ||||
| Calf | 5.28 ± 0.60*# | 5.04 ± 0.67*# | 3.09 ± 0.55†# | 1.92 ± 0.32# |
| Dorsal foot | 6.56 ± 0.54 * | 6.49 ± 0.60 * | 4.29 ± 0.50† | 3.06 ± 0.50 |
| CVC responses (%/°C) | ||||
| Calf | 5.41 ± 0.54 | 6.03 ± 0.44 | 5.71 ± 0.28 | 6.54 ± 0.33 |
| Dorsal foot | 5.25 ± 0.45 | 6.13 ± 0.61 | 5.47 ± 0.18 | 5.00 ± 0.39 |
Values represent the mean ± SE
* Significant difference between the C and N groups (P < 0.05)
†Significant difference between the menthol and control sites within a group (P < 0.05)
#Significant difference between the calf and dorsal foot within a group (P < 0.05)
During the pre-cooling baseline period, there was no difference between the two groups in CVC at the control sites (C 5.3 ± 0.6 units, N 6.1 ± 0.5 units, P = 0.57) and menthol-treated sites (C 6.4 ± 1.0 units, N 6.0 ± 0.5 units, P = 0.64) in the calf. In addition, there was no difference between the two groups in CVC at the control sites (C 6.7 ± 1.5 units, N 4.5 ± 0.6 units, P = 0.20) and menthol treated sites (C 6.2 ± 0.8 units, N 6.0 ± 0.5 units, P = 0.85) in the dorsal foot during the pre-cooling baseline period. As shown in Fig. 2, local skin cooling decreased CVC in the calf and dorsal foot with decreasing local temperature. The application of menthol did not alter the responses of CVC during local cooling in the C and N groups. There were significant correlations (r = 0.880–0.997, P < 0.05) between local T sk and CVC in each site for all subjects. With respect to the slopes of the relationships between the local T sk and CVC, there was no significant main effects between the C and N groups (P = 0.94), the menthol-treated and control sites (P = 0.13), and the calf and dorsal foot (P = 0.13), with a non-significant interaction among the three factors (P = 0.39) (Table 1).
Fig. 2.
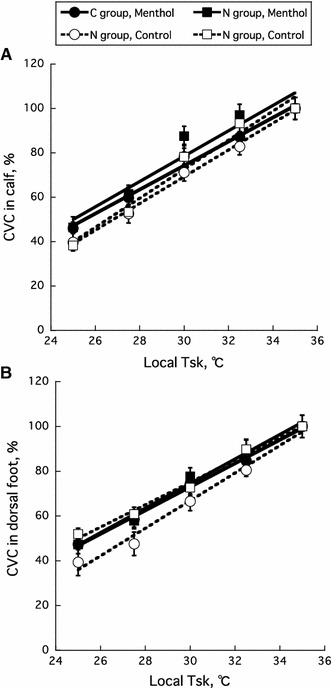
Relationships between local T sk and cutaneous vascular conductance (CVC) in the calf (a) and dorsal foot (b) of cold-sensitive (C) and normal (N) groups. Closed and open circles represent data for menthol and control conditions, respectively
Discussion
There were two major findings of the current study. First, the topical application of 0.5 % menthol increased the sensitivity of cold sensation in the calf and dorsal foot to decreasing local T sk in the N group, but did not alter sensitivities in the C group. Second, the application of menthol did not change the local cooling-induced vasoconstrictor response of leg skin in the two groups. These findings suggest: (1) the specific control of cold sensation by cold receptor TRPM8 channels in the C group; and (2) a minor role of cold receptor activity in cutaneous vasoconstrictor response to direct skin cooling.
In the present study, subjects were required to report thermal sensation at locally cooled skin sites using a visual analogue scale. The cooling-evoked cold sensation was not so intense as to induce significant activation of autonomic nerves, because blood pressure, HR, and SkBF in the forearm did not change during local cooling of the calf and dorsal foot. The cold sensation at a lower T sk was greater in the C group than in the N group during local cooling (Fig. 1), suggesting a higher thermal sensitivity to cold in the lower extremities of young women complaining of chilliness. It has been reported that cold discomfort of the body or lower extremities at a given T sk was greater in the C group than in the N group during the whole-body mild cold exposure by reducing the room temperature from 29.5 to 23.5 °C [6, 10]. The present findings of higher cold sensation sensitivity to local cooling in the C group was obtained at a room temperature of 29.5 °C, which falls within the neutral temperature range of thermal comfort. Sato and Tamura [20] have also reported that cold sensation thresholds in the hand, foot, and loin at room temperature (28 °C) were lower in young females with cold constitutions (C group) than in normal control females, suggesting a higher sensitivity to cold in the C group. It is speculated that the higher thermal sensitivity to cold in the C group is due to a greater thermoreceptor excitation in the cutaneous endings of somatosensory neurons at a similar temperature, and/or greater activity in somatosensory neurons in the brain at a given peripheral thermosensitive input.
We hypothesized that the cause of the inter-individual differences in cold sensation may partly be due to menthol-sensitive skin cold receptor function. The thermal sensation at 35 °C was not altered by the application of 0.5 % menthol, suggesting that menthol-sensitive cold receptors were not activated under neutral temperature conditions in human skin. The application of menthol increased cold sensation by 40–60 % in the cooled area of the legs in the N group, but not in the C group (Table 1). In the C group, the ineffectiveness of topical menthol on cold sensation at a lower T sk was not due to a maximal intensity of cold sensation being reached, because more intense cooling stimuli was able to increase the cold sensation (Fig. 1). Since the application of 0.5 % menthol was determined from a pilot study using healthy female subjects, the concentration of menthol might be not high enough to induce activation of TRPM8 channels for the subjects in the C group. Nevertheless, the different sensorial responses between the two groups at a given concentration would suggest a desensitization of menthol-activated TRPM8 channels in cold receptors of the cooled skin areas in the C group. It is also possible that the concentration of menthol was too high to elicit an expected cold sensation for the subjects in the C group, although the dose did not cause irritation or skin vasodilation in the subjects. Therefore, a greater excitation in temperature-sensitive TRP channels, excluding TRPM8 channels in skin themoreceptors at a similar temperature, might be responsible for the greater thermal sensitivity to cold in the C group. In the C groups, the putative deactivation of menthol-activated TRPM8 channels would act to weaken the excessive thermal discomfort resulting from cold extremities in a cool environment.
Another explanation for the greater thermal sensitivity to cold in the C group is the role of upstream mechanisms of thermoreceptors, including greater activity in somatosensory neurons in the brain. Human studies using functional magnetic resonance imaging (fMRI) have suggested that the insular cortex rather than parietal cortex is implicated in the genesis of temperature sensation during innocuous cooling stimulation [21]. It is unclear whether brain activity in specific regions during cold stress is enhanced in cold-sensitive humans. Further studies are warranted to elucidate brain mechanisms creating inter-individual differences in thermal sensation.
Enhanced alpha 2 receptor sensitivity to catecholamine and reduced nitric oxide have been proposed as vasomotor mechanisms of local cooling [22–25]. While transient receptor potential vanilloid 1 (TRPV1) receptors play a substantial role in the hyperemic response to local skin heating [26, 27], the role of TRP channels in the vasoconstrictor response to local skin cooling is currently not well understood. Johnson et al. [28] have reported that topical application of 3 % menthol to human skin caused increased SkBF at the treated sites, and that vasodilation was markedly reduced by pretreatment of atropine or N G-nitro-l-arginine methyl ester (l-NAME). In preliminary studies, we also observed that application of 3 % menthol increased baseline SkBF and caused irritation in normal healthy subjects. Thus, TRPM8 channels may act as a vasodilator component in the local control of SkBF. Cold sensation was enhanced by 0.5 % menthol during local cooling in the N group, whereas the application of menthol did not change baseline SkBF or enhance the vasoconstrictor response to local cooling in either group. These findings suggest that TRPM8 channels do not play a significant role in local control of cutaneous vasoconstriction during direct skin cooling in humans.
In conclusion, the findings of the current study suggest a desensitization of menthol-activated cold receptors in the lower extremities during local cooling in young females complaining of unusual chilliness, and a minor role of cold receptor activity in cutaneous vasoconstrictor response to direct skin cooling. Higher cold sensation sensitivity during cold exposure in the tested skin areas may be due to upstream mechanisms from skin cold receptors and/or activities in thermosensitive TRP channels, excluding TRPM8 channels in skin themoreceptors.
Acknowledgments
The authors thank the subjects who participated in this experiment. This study was supported by Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (24500688).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Miura T, Katano Y, Sumimoto K, Kanayama N. Study on chilliness and lifestyle in young women. Jpn J Matern Health. 2001;42:784–789. [Google Scholar]
- 2.Sadakata M, Satoh E, Sayama M. The skin surface temperature in the women with excessive sensitivity to cold (HIESHO) in the neutral-temperature environment. The study of the measurement-part helping to make the judging guideline and characteristic of the skin surface temperature . Biomed Thermol. 2007;27:1–7. [Google Scholar]
- 3.Tanaka H, Shikimi T. Thermal adjustment to mild-cold or mild-hot water immersion test in young women with cold constitution. Jpn Red Cross Med J. 2005;56:507–511. [Google Scholar]
- 4.Yamada N, Bekku N, Yoshimura H. Determinants for discriminating young woman with and without chilliness. Jpn J Neuropsychopharmacol. 2007;27:191–199. [PubMed] [Google Scholar]
- 5.Yamada N, Yoshimura H. Determinants of chilliness among young women and their application to psychopharmacological trials. Jpn J Neuropsychopharmacol. 2009;29:171–179. [PubMed] [Google Scholar]
- 6.Yamazaki F. The cutaneous vasoconstrictor response in lower extremities during whole-body and local skin cooling in young women with a cold constitution. J Physiol Sci. 2015;65:397–405. doi: 10.1007/s12576-015-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tajino K, Matsumura K, Kosada K, Shibakusa T, Inoue K, Fushiki T, Hosokawa H, Kobayashi S. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2128–R2135. doi: 10.1152/ajpregu.00377.2007. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and non-neuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol. 2005;288:H1573–H1579. doi: 10.1152/ajpheart.00849.2004. [DOI] [PubMed] [Google Scholar]
- 10.Nagashima K, Yoda T, Yagishita T, Taniguchi A, Hosono T, Kanosue K. Thermal regulation and comfort during a mild-cold exposure in young Japanese women complaining of unusual coldness. J Appl Physiol. 2002;92:1029–1035. doi: 10.1152/japplphysiol.00399.2001. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda-Nakamura M, Yasuhara S, Nagashima K. Effect of menstrual cycle on thermal perception and autonomic thermoregulatory responses during mild cold exposure. J Physiol Sci. 2015;65:339–347. doi: 10.1007/s12576-015-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol. 1999;87:1719–1723. doi: 10.1152/jappl.1999.87.5.1719. [DOI] [PubMed] [Google Scholar]
- 13.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 14.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 15.Gillis DJ, House JR, Tipton MJ. The influence of menthol on thermoregulation and perception during exercise in warm, humid conditions. Eur J Appl Physiol. 2010;110:609–618. doi: 10.1007/s00421-010-1533-4. [DOI] [PubMed] [Google Scholar]
- 16.Green BG. Menthol inhibits the perception of warmth. Physiol Behav. 1986;38:833–838. doi: 10.1016/0031-9384(86)90050-8. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer K, Braun HA, Isenberg C. Effect of menthol on cold receptor activity. Analysis of receptor processes. J Gen Physiol. 1986;88:757–776. doi: 10.1085/jgp.88.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Tamura T (2006) Characteristics of the skin temperature and the cold and warm thresholds in young women with cold constitutions known as “Hie-sho”. In: Proceedings of the 30th symposium on human-environment system, pp 101–104
- 21.le Hua H, Strigo IA, Baxter LC, Johnson SC, Craig AD. Anteroposterior somatotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol. 2005;289:R319–R325. doi: 10.1152/ajpregu.00123.2005. [DOI] [PubMed] [Google Scholar]
- 22.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. α-Adreneroceptors and cold-induced vasoconstriction in human finger skin. Am J Physiol. 1988;255:H1000–H1003. doi: 10.1152/ajpheart.1988.255.5.H1000. [DOI] [PubMed] [Google Scholar]
- 23.Flavahan NA, Lindblad L-E, Verbeuren TJ, Shepherd JT, Vanhoutte PM. Cooling and α1 and α2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol. 1985;249:H950–H955. doi: 10.1152/ajpheart.1985.249.5.H950. [DOI] [PubMed] [Google Scholar]
- 24.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol. 2006;574:849–857. doi: 10.1113/jphysiol.2006.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in the human skin. J Appl Physiol. 2006;100:42–50. doi: 10.1152/japplphysiol.00139.2005. [DOI] [PubMed] [Google Scholar]
- 26.Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R894–R901. doi: 10.1152/ajpregu.2001.281.3.R894. [DOI] [PubMed] [Google Scholar]
- 27.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol. 2010;588:4317–4326. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;296:H1868–H1877. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


