Abstract
Japanese women can experience a sensation of cold feet in daily life. It is possible that this sensation of coldness in feet may be associated with female hormones, but to date the effect of menstrual cycle phase on the skin temperature (Tsk) of the foot during local cooling is unknown. We therefore examined Tsk and partial cutaneous blood flow in the foot during the follicular (F) and luteal (L) phases of the menstrual cycle in women experiencing local cooling. Tsk was measured in the toes and the dorsum of the left foot using infrared thermography, while cutaneous blood flow was evaluated in the big toe of the left foot using laser Doppler flowmetry (LDF), both at 28 °C. Mild local cooling (24.7 °C) was then applied for 30 min to the right foot. During cooling of the right foot, no significant differences in Tsk were observed between the F and L phases in either the toes of the left foot or the dorsum of the left foot of all subjects. However, cutaneous blood flow determined by LDF in the big toe of the left foot was greater in the F phase than in the L phase. These results suggest that the menstrual cycle phase did not affect Tsk in the foot, but it did affect cutaneous blood flow in the big toe during mild local cooling.
Keywords: Menstrual cycle, Skin temperature, Skin blood flow, Toes, Dorsum of foot
Introduction
Japanese women can experience a sensation of cold feet in daily life. In a questionnaire survey, women who had a subjective feeling of coldness during daily living activities (n = 66) reported a sensation of coldness in feet [1]. Plasma estradiol (E2) concentration decreases over time with the onset of menopause, and it also fluctuates during the menstrual cycle in young women. In one study which compared young women and menopausal women, the menopausal women were found to experience a peripheral thermal sensation that was loosely associated with smaller changes in the skin temperature (Tsk) of the toe and finger during stepwise changes in ambient temperature from 35 to 18 °C, 35 to 22 °C, 35 to 26 °C, and 35 to 30 °C [2]. In normal female rats, body temperature during exposure to cold (5 °C for 2 h) was found to be lower in the diestrus phase (characterized by a lower E2 level) than in the proestrus phase (characterized by a higher E2 level) [3]. Additionally, body temperature during cold exposure (5 °C for 2 h) decreased to a greater extent in ovariectomized rats than in ovariectomized rats that were administered systemic E2 [3]. Systemic E2 administration to ovariectomized rats facilitates thermoregulatory behavior, as assessed by tail-hiding behavior during cold exposure (16 °C for 2 h) [4]. Taken together, these results indicate that E2 fluctuation in the menstrual cycle may affect the sensation of cold feet in women in daily living activities.
In a study conducted in young Japanese women, Matsuda-Nakamura et al. reported that the Tsk in the big toe during a change in ambient temperature from 29 to 23.5 °C was lower in the luteal phase (L phase) than in the follicular phase (F phase) [5], suggesting that menstrual cycle phase might influence the control of Tsk in the big toe under cold ambient temperatures. However, the effect of menstrual cycle phase on Tsk in the toes and in each area of the dorsum of the foot during mild local cooling is unknown. In the present study, to elucidate the effect of menstrual cycle phase on foot Tsk during mild local cooling in young women, we examined Tsk and partial cutaneous blood flow in the toes and in three areas of the dorsum of the foot during the F and L phases while applying of mild local cooling.
Methods
Subjects
Six young Japanese women (age 20 ± 2 years; body weight 51.3 ± 3.3 kg; body height 158.1 ± 1.6 cm; body mass index 20.1 ± 0.9) participated in the study. All subjects completed a ten-question hie-sho interview [6] on coldness of body, and those subjects who scored more than seven were defined as experiencing hie-sho. Women experience a coldness sensation even in an air-conditioned room where most people feel thermally comfortable called “hie-sho”. All of the subjects completing the questions were not hie-sho (score of the interview 3.5 ± 0.9). All subjects provided informed consent to participate in the experiment, which was approved by the Human Investigation Committee of Nara Women’s University.
Experimental protocols
The experiments were conducted both when the women were in the F (pre-ovulatory) phase of their menstrual cycle, on the day that an ovulation prediction kit showed a positive result for the first time (New dotest® LH; ROHTO Pharmaceutical Co., Ltd., Osaka, Japan), and in the L phase, at 3 days before the estimated date of the next menses. The ovulation prediction kit detected increased levels of urinary lutenizing hormone prior to ovulation day. Our previous study had shown that the E2 concentration is at a relatively higher level and the progesterone concentration is at a relatively lower level on the first day that the ovulation prediction kit had a positive result. In our previous study (unpublished), the late luteal phase (L) is defined as 1–5 days before the start of menstruation, and during this phase the E2 concentration is lower and the progresterone concentration is higher than their counterparts in the F phase. In the current study, we adhered fundamentally these previously described definitions of the F and L menstrual phases. Each subject sent a photograph of the results of the ovulation prediction kit to the researcher each day, and the researcher assessed the first positive result based on the photographs.
Experiments were performed in Japan during the summer (from September to October) to avoid seasonal acclimation to a cold environment. It was difficult to collect data during both the F and L phases of one menstrual cycle in this 2-month period of each year. For example, sometimes the experiment in the F or L phase could not be performed because the subject was ill or had obligations and was therefore not available for testing in that particular phase. Thus, it took 2 years to collect data on six subjects in the period from September to October; we therefore limited the study population to these six subjects.
All experiments were performed in the morning to avoid the effects of activity performed before the experiment. The experimental room was maintained at 27.9 ± 0.1 °C with a relative humidity of 56.3 ± 3.0%. Subjects were allowed to drink water ad libitum before the experiment, but water intake was limited during the experiment. Subjects rested in a seated position, and Tsk and cutaneous blood flow were measured using infrared thermography (Thermo Shot F30; Nihon Avionics, Tokyo, Japan) and laser Doppler flowmetry (LDF; Omegaflo FLO-C1 HP; OMEGAWAVE, Inc., Tokyo, Japan), respectively. Tympanic temperature was measured using a tympanic thermometer (Kenonkun C-510; OMRON HEALTHCARE Co., Ltd., Kyoto, Japan). Mild local cooling (24.7 ± 0.4 °C) was then applied for 30 min by wrapping the right foot in a cooling medium (Shirokuma’s Summer Scarf [regular]; Bigwing Co. Ltd., Osaka, Japan) (Fig. 1B). The cooling medium was filled with water. The local cooling temperature was set based on our own pre-experimental results. The difference between ambient temperature and cooling temperature was 3 °C. In our previous study, the dorsum of the foot of young women was stimulated by direct skin cooling from 27.5 to 25 °C; the difference was thus 2.5 °C [7]. These moderate decreases (2.5 or 3 °C) were considered to constitute mild cooling. After 30 min of cooling, the cooling medium was removed and the foot was maintained for 30 min in the same position for recovery. Prior to the experiment, we ensured that the cooling medium could maintain a temperature of 24.7 ± 0.4 °C for 30 min. Blood pressure was not measured, as a previous study had shown that the mean arterial pressure of young women did not differ between F and L phases at ambient temperature of 29, or 23.5 °C for 80 min [11]. Additionally, we believed that the blood pressure response under the conditions of the present study would not change due to mild local cooling (cooling medium 24.7 °C for 30 min) of foot because the temperature was higher than that of previous study [11]. The skin temperature of the cooled foot was not measured because it was covered with cooling medium.
Fig. 1.
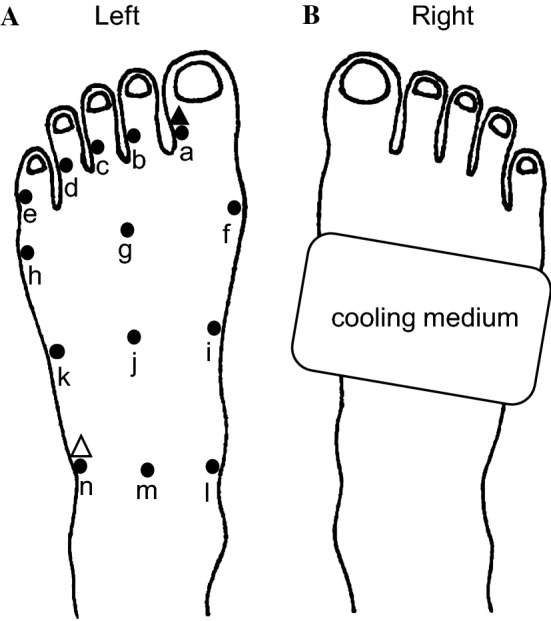
Schema of the left (A) and right (B) feet. A Left foot. Filled circles denote locations at which skin temperature (Tsk) was measured; specifically, at the large (a), second (b), third (c), fourth (d), and little toe (e), respectively; at the medial (f), central (g), and lateral surfaces (h) of the head of the metatarsal bone areas; at the medial (i), central (j), and lateral surfaces (k) of the tarsometatarsal joint areas; at the medial (l), central (m), and lateral (n) surfaces of the transverse tarsal joint areas in the dorsum of the foot. Filled and white triangles denote places at which the laser Doppler flowmetry probe was placed. B Right foot. The rectangle denotes application of the cooling medium
Measurements
The Tsk of all toes (specifically, the big, second, third, fourth, and little toes) of the left foot and of the dorsum of the left foot (including the head of the metatarsal bone area, the tarsometatarsal joint area, and the transverse tarsal joint area) was measured using infrared thermography at 1-min intervals (Fig. 1A). LDF of the lateral surface of the big toe and lateral surface of the transverse tarsal joint area of the left foot was measured as an index of cutaneous blood flow at 15-s intervals, and was averaged every 10 min (Fig. 1A). The two measurement points of LDF were selected because the change in Tsk of the big toe was the greatest among all toes, while the change in the Tsk of the lateral surface of the transverse tarsal joint area was blunted during mild local cooling in our pre-experiment assessments.
Statistics
Data were presented as the mean ± standard error. The Tsk values from the surface of the head of the metatarsal bone areas, tarsometatarsal joint areas, and transverse tarsal joints in the dorsum of the foot were averaged from the Tsk obtained from three points in each area (Figs. 1A: f, g, and h; i, j, and k; and l, m, and n, respectively). The standard value to calculate the LDF ratio was the 10-min average of the LDF during the rest phase. The LDF in the F and L phases in the cool and recovery phases was under 150% (Fig. 5A). We took an increase in the LDF of 200% to indicate a marked rise. Therefore, a spike in the LDF was defined as an averaged LDF per minute that was > 200% of the LDF and peak of waveform in the LDF; the number of spikes was counted. Differences between the F and L phases were assessed by a repeated-measures two-way analysis of variance (ANOVA) using the SPSS statistics package version 21 (IBM Corp., Armonk, NY, USA). A post hoc t test was performed to identify significant differences at specific time points between the F and L phases. The Tukey–Kramer method was used to identify significant differences between the rest, cool, and recovery phases in the number of spikes in LDF in the big toe of the left foot (hereafter referred to as left big toe). The null hypothesis was rejected at a level of p < 0.05.
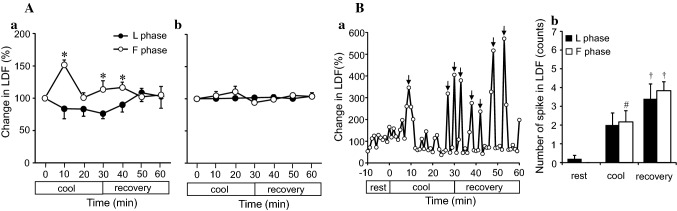
Fig. 5.
Laser Doppler flow in the left lateral surface of the big toe and lateral surface of the transverse tarsal joint (A-a and b) A typical example of spikes in LDF in the left big toe in the L phase. (B-a) and the number of LDF spikes in the lateral surface of the left big toe (B-b), during mild local cooling of the right foot. Values are presented as the mean ± SE (F phase, n = 6 measurements; L phase, n = 6 measurements). Asterisk indicates a significant difference between the F and L phases, hashtag indicates a significant difference between the resting (rest) and cooling (cool) periods, dagger indicates a significant difference between the rest and recovery periods, all at p < 0.05. Arrows in B-a indicate spikes
Results
Skin temperature
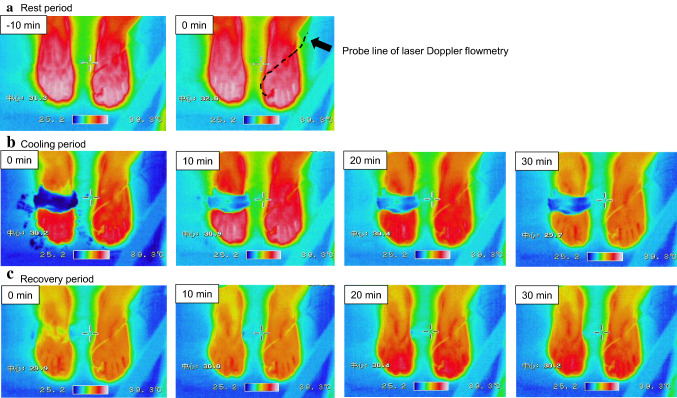
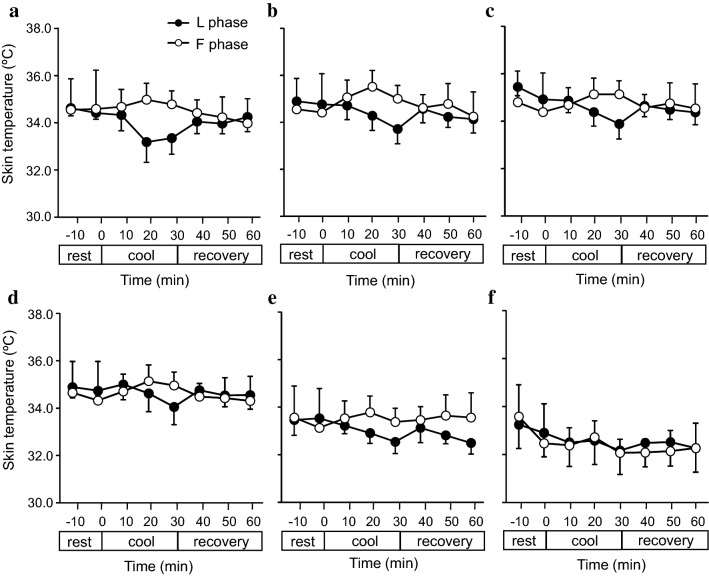
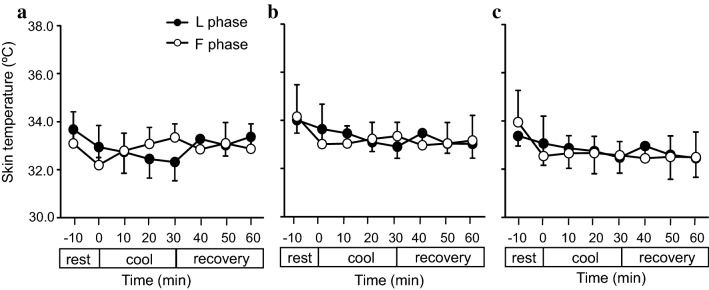
Typical thermograms of feet during the resting (Fig. 2a), cooling (Fig. 2b), and recovery (Fig. 2c) periods. No significant differences were observed in the Tsk of all subjects’ toes of the left foot (Fig. 3a–e), at the lateral surface of the transverse tarsal joint (Fig. 3f), and in the head of the metatarsal bones, the tarsometatarsal joints, and the transverse tarsal joints (Fig. 4a–c) between the F and L phases during both the cooling and recovery.
Fig. 2.
Typical thermograms of feet. The thermograms were obtained from one subject in the pre-ovulatory (F) phase during the resting (rest; a), cooling (cool; b), and recovery (c) periods. Dashed line indicates the probe line of the laser Doppler flowmetry (LDF)
Fig. 3.
Skin temperature (Tsk) in the lateral surface of the big (a), second (b), third (c), fourth (d), and little (e) toes and at the transverse tarsal joint in the dorsum of the foot (f). Values are presented as the mean ± standard error (SE) (F phase, n = 6 measurements ; L phase, n = 6 measurements). L phase Late luteal phase
Fig. 4.
Skin temperature (Tsk) in the surface of the left head of the metatarsal bone (a), tarsometatarsal joint (b), and transverse tarsal joint (c) in the dorsum of the foot. Values are presented as the mean ± SE (F phase, n = 6 measurements; L phase, n = 6 measurements)
Laser Doppler flow
Two-way ANOVA indicated a significant interaction between menstrual cycle and time [F(3,15) = 4.08, p < 0.05] regarding the LDF of the left big toe, with the LDF in the F phase being greater (p < 0.05) than that in the L phase during the cooling and recovery (Fig. 5A-a) phases. There were no differences in the LDF of the lateral surface of the transverse tarsal joint between the F and L phases (Fig. 5A-b). Figure 5B-a shows a typical example of a spike in LDF in the left big toe in the L phase. Two-way ANOVA indicated a significant main effect of time [F(1,8) = 40.36, p < 0.01] on the spikes in LDF in the left big toe. The spikes in the F phase in the cooling period were greater (p < 0.01) than those in the resting period. The big waveforms were counted as spikes, and these were defined as an averaged LDF per minute that was > 200% of the LDF and peak of waveform in the LDF. There was a higher number of spikes in the F and L phases in the recovery period than in the resting period (p < 0.01) (Fig. 5B-b).
Tympanic temperature
Tympanic temperature is shown in Fig. 6. No significant difference was observed in the tympanic temperature between the F and L phases during the cooling and recovery periods.
Fig. 6.
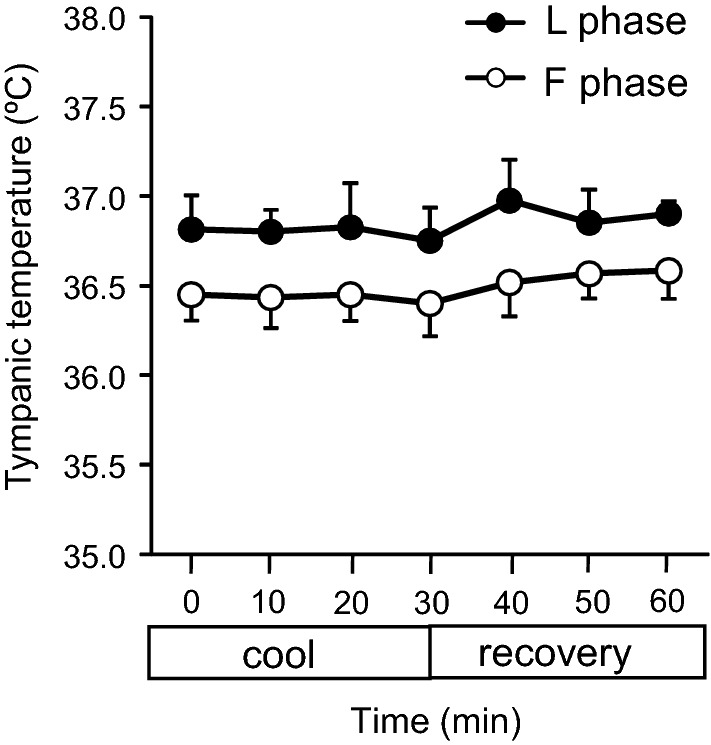
Tympanic temperature. Values are presented as the mean ± SE (F phase, n = 6 measurements, L phase, n = 6 measurements)
Discussion
The results of this study revealed that the menstrual cycle phase did not affect the Tsk of the toes or the dorsum of the foot of the young women participating in the study, but it did affect the LDF of the big toe during mild local cooling of the contralateral foot.
The authors of a previous study reported that the mean Tsk of the whole body was not different between the F and L phases in young women at an ambient temperature of 23.5 °C [5]. In another study, the Tsk of the whole body was found not to be different between the F and L phases in women aged 18–30 years at an ambient temperature of 5 °C, while the Tsk of the whole body in the L phase was higher than that in the F phase during cooling by water at 15 °C [8]. Thus, the effect of menstrual cycle phase on the Tsk of the whole body in cold ambient temperatures is controversial. Our finding that the menstrual cycle phase did not influence the foot Tsk suggests that there are no regional differences between the F and L phases in each area in the dorsum of the foot or the toes. Arteriovenous anastomoses, which have a deep orientation and large luminal diameters in the skin, are important for heat dissipation, and affect Tsk. They are also are more abundant at the tip of toe than in the dorsum of the foot [9]. Thus, arteriovenous anastomoses might affect the Tsk of toes, but their effect may be small in the Tsk of dorsum of the foot. Taken together, it is possible that the foot Tsk is controlled by vasoconstriction of the cutaneous microvascular network under the condition of mild local cooling. Unfortunately, the Tsk in the present study could not be compared with that in previous studies since the Tsk in each area of the dorsum of the foot was not measured in previous studies. In one prior study, the foot Tsk in female subjects (age 20–39 years) increased in the F phase but not in the L phase at an ambient temperature of 22 °C [10]. Another study found the Tsk of the big toe of young women to be higher in the F phase than in the L phase during a change of ambient temperature from 29 to 23.5 °C [5]. These results are not consistent with the results of the present study, and the differences may partly be due to the mild local cooling, which was just 3 °C lower than the ambient temperature. Our results indicate that the response of Tsk to the menstrual cycle during mild local cooling was not different regionally in the toes and areas of the dorsum of the foot.
In visually, the Tsk in the great toe in the F phase seemed to be greater than that in the L phase; however, it was not different between the F and L phases statistically. The increased LDF in the great toe in the F phase during mild local cooling did not increase the Tsk in the great toe in the F phase, because the magnitude of the increased LDF was small. The LDF in the lateral surfaces of the transverse tarsal joint area was not different between in the F and L phases, respectively. The majority of the dorsum of the foot including the lateral surfaces of the transverse tarsal joint area receives innervation from the superficial fibular nerve; however, the area facing the great toe and second toe receives innervation from the deep fibular nerve [11]. The cooling medium was placed at the dorsum area of the foot that receives innervation from the superficial fibular nerve. In addition, arteriovenous anastomoses in the tip of toe are more abundant than in the dorsum of the foot [9]. It was speculated that the increased E2 in the F phase might dilate arteriovenous anastomoses in the tip of toe during mild local cooling. In consequence, the LDF might increase in the great toe in the F phase. These results may have influenced the different response of the LDF for menstrual cycle between the great toe and the lateral surfaces of the transverse tarsal joint area.
The LDF in the present study could not be compared with that in previous studies since the LDF in the big toe during local cooling was not measured in previous studies. Hands resemble feet in terms that both are peripheral parts of the body that utilize arteriovenous anastomosis to control heat dissipation. Whether or not the phase of the menstrual cycle has an effect on blood flow in the hands under cold conditions is still open to debate. In one study, blood flow in the middle finger, as assessed by venous occlusion plethysmography, and fingertip temperature were not different between the preovulatory phase and midluteal phase in healthy women exposed to cold (ambient temperature of 15 °C for 20 min) [12]. On the other hand, the oral administration of 17ß-estradiol (9 mg) to healthy women has been found to increase blood flow in the fingertip, as assessed by LDF and not to affect the skin temperature of the fingertip during local cold exposure of the finger (15 °C water immersion for 5 min) [13]. Taken together, these results suggest that the higher E2 concentration in the F phase may increase the LDF in the great toe during mild local cooling. It is possible that we did not observed this phenomenon in our study in the L phase due to the low E2 concentration in the L phase. Our results indicate that the different menstrual phases affected the LDF in the great toe via cooling stimulus.
We observed notable large waveforms in the LDF that reflected the increased blood flow during local cooling. This appeared to be the “hunting phenomenon”, in which the Tsk in fingers and feet dramatically increases due to cold-induced vasodilation after temporal subjection to cold water [9], although we did not measure Tsk at the same time. The mechanism of cold-induced vasodilation has not been clarified; however, a possible mechanism is the decrease in norepinephrine from the adrenergic nerve endings due to cold [14]. The hunting phenomenon has not been reported during local heating, and it has also been found to occur in both men and women [15]. The number of LDF spikes in the big toe was found not to differ between in the L and F phases during mild local cooling; however, the number of spikes increased by application of a cooling stimulus. It was unclear if the phenomenon was the hunting phenomenon or not, because the hunting phenomenon usually occurs only during the application of a severely cold stimulus. Cold-induced vasodilation of the fingers during cold water immersion at 0 and 8 °C did not differ between in men and women [15]; however, the effect of the menstrual cycle phase on the hunting phenomenon during local cooling is not yet known.
The present study reveal that the phase of the menstrual cycle did not affect the Tsk in the dorsum of the foot or in the toes during the application of mild local cooling in young women. However, our results that an increased LDF occurred in the big toe in the F phase as compared to the L phase suggests that the menstrual cycle phase may influence vasomotion of the toe during mild local cooling. The present study may contribute to an enhancement in the quality of life of women by improving our understanding of thermal responses of the feet in women.
Acknowledgements
We are grateful to Yuri Mizukami, Koko Kano (Nara Women’s University), Dr. Ken Tokizawa (National Institute of Occupational Safety and Health), and to Dr. Mayumi Matsuda-Nakamura (International University of Health and Welfare) for support of this research. The present research was partially supported by the Ministry of Education, Science, Sports, and Culture; Grant-in-Aids for Scientific Research (B), No. 17K17882; Nara Women’s University; Intramural and Mental and Physical Health Project Research Grants.
Funding
This study was funded by the Ministry of Education, Science, Sports, and Culture; Grant-in-Aids for Scientific Research (B), No. 17K17882; Nara Women’s University; Intramural and Mental and Physical Health Project Research Grants.
Compliance with Ethical Standards:
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Takatori A. Assessment of diagnostic criterion of coldness in women with thermography (Japanese ARTICLE) Acta Obst Gynaec Jpn. 1992;44:559–565. [PubMed] [Google Scholar]
- 2.Kondo E, Kurazumi Y, Horikoshi T. Human physiological and psychological reactions to thermal transients with air temperature step changes: a case of climacteric aged females in summer. J Hum Living Environ. 2014;21:75–84. [Google Scholar]
- 3.Uchida Y, Kano M, Yasuhara S, Kobayashi A, Tokizawa K, Nagashima K. Estrogen modulates central and peripheral responses to cold in female rats. J Physiol Sci. 2010;60:151–160. doi: 10.1007/s12576-009-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchida Y, Nagashima K, Yuri K. Systemic estradiol administration to ovariectomized rats facilitates thermoregulatory behavior in a cold environment. Brain Res. 2017;1670:125–134. doi: 10.1016/j.brainres.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda-Nakamura M, Yasuhara S, Nagashima K. Effect of menstrual cycle on thermal perception and autonomic thermoregulatory responses during mild cold exposure. J Physiol Sci. 2015;65:339–347. doi: 10.1007/s12576-015-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagashima K, Yoda T, Yagishita T, Taniguchi A, Hosono T, Kanosue K. Thermal regulation and comfort during a mild-cold exposure in young Japanese women complaining of unusual coldness. J Appl Physiol. 2002;92:1029–1035. doi: 10.1152/japplphysiol.00399.2001. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki F. The cutaneous vasoconstrictor response in lower extremities during whole-body and local skin cooling in young women with a cold constitution. J Physiol Sci. 2015;65:397–405. doi: 10.1007/s12576-015-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman-Weiss EL, Cheatham CC, Caine N, Blegen M, Marcinkiewicz J. Influence of gender and menstrual cycle on a cold air tolerance test and its relationship to thermosensitivity. Undersea Hyperb Med. 2000;27:75–81. [PubMed] [Google Scholar]
- 9.Nakayama T. Onnetsu Seirigaku. Tokyo: Rikogakusya; 2005. [Google Scholar]
- 10.Hassan AA, Carter G, Tooke JE. Postural vasoconstriction in women during the normal menstrual cycle. Clin Sci (Lond) 1990;78:39–47. doi: 10.1042/cs0780039. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Takano H. Human anatomy. 3. Tokyo: Nanzando; 2014. [Google Scholar]
- 12.Greenstein D, Jeffcote N, Ilsley D, Kester RC. The menstrual cycle and Raynaud’s phenomenon. Angiology. 1996;47:427–436. doi: 10.1177/000331979604700501. [DOI] [PubMed] [Google Scholar]
- 13.Bartelink ML, Wollersheim H, Vemer H, Thomas CM, de Boo T, Thien T. The effects of single oral doses of 17 beta-oestradiol and progesterone on finger skin circulation in healthy women and in women with primary Raynaud’s phenomenon. Eur J Clin Pharmacol. 1994;46:557–560. doi: 10.1007/BF00196115. [DOI] [PubMed] [Google Scholar]
- 14.Daanen HAM. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411–426. doi: 10.1007/s00421-003-0818-2. [DOI] [PubMed] [Google Scholar]
- 15.Tyler CJ, Reeve T, Cheung SS. Cold-induced vasodilation during single digit immersion in 0 and 8° C water in men and women. PLoS One. 2015;10:e0122592. doi: 10.1371/journal.pone.0122592. [DOI] [PMC free article] [PubMed] [Google Scholar]