Abstract
Hyperalgesia and allodynia are commonly observed in patients with diabetic neuropathy. The mechanisms responsible for neuropathic pain are not well understood. Thus, in this study, we examined the role played by purinergic P2X3 receptors of the midbrain periaqueductal gray (PAG) in modulating diabetes-induced neuropathic pain because this brain region is an important component of the descending inhibitory system to control central pain transmission. Our results showed that mechanical withdrawal thresholds were significantly increased by stimulation of P2X3 receptors in the dorsolateral PAG of rats (n = 12, P < 0.05 vs. vehicle control) using α,β-methylene-ATP (α,β-meATP, a P2X3 receptor agonist). In addition, diabetes was induced by an intraperitoneal injection of streptozotocin (STZ) in rats, and mechanical allodynia was observed 3 weeks after STZ administration. Notably, the excitatory effects of P2X3 stimulation on mechanical withdrawal thresholds were significantly blunted in STZ-induced diabetic rats (n = 12, P < 0.05 vs. control animals) as compared with control rats (n = 12). Furthermore, the protein expression of P2X3 receptors in the plasma membrane of the dorsolateral PAG of STZ-treated rats was significantly decreased (n = 10, P < 0.05 vs. control animals) compared to that in control rats (n = 8), whereas the total expression of P2X3 receptors was not significantly altered. Overall, data of our current study suggest that a decrease in the membrane expression of P2X3 receptors in the PAG of diabetic rats is likely to impair the descending inhibitory system in modulating pain transmission and thereby contributes to the development of mechanical allodynia in diabetes.
Keywords: P2X receptor, Diabetic neuropathy, Midbrain PAG
Introduction
Diabetes mellitus is one of the most common chronic medical problems affecting millions of people worldwide and is the most dominant cause of neuropathy [19]. A frequent complication of diabetes is unremitting pain, which leads to a reduced quality of life. Patients with diabetes often experience various aberrant sensations including spontaneous pain and hypersensitivity to mechanical or thermal stimuli (namely hyperalgesia and allodynia) followed by the long-term paradoxical loss of stimulus-evoked sensation [3, 9]. Neuropathic pain is likely to result from disorders of the peripheral nervous system or the central nervous system (brain and spinal cord) [13, 17]. Treatment options for these abnormal sensations have been limited, partly because of our poor understanding of the underlying mechanisms by which neuropathic pain is induced by diabetes.
The midbrain periaqueductal gray (PAG) is a substantial component of the descending pain modulatory network and exerts inhibitory or excitatory control on pain transmission via the rostral ventromedial medulla, which in turn projects to the spinal dorsal horn [2, 6], the first synaptic site of sensory nerve inputs. These findings are of particular interest because of the pivotal role of the PAG in integrating an animal’s somatomotor, autonomic and behavioral responses to pain [1], most of which are associated with diabetes. Among regions of the PAG, the dorsolateral region also receives abundant afferent inputs from the spinal cord and sends descending neuronal projections to the medulla in integrating those physiological functions [6]. Importantly, accumulated evidence from recent studies has shown that the PAG plays a role in regulating neuropathic pain [13, 16]. Nevertheless, the underlying molecular/receptor mechanisms responsible for the PAG-mediated activities in the engagement of neuropathic pain remain unclear.
Adenosine triphosphate (ATP) released from numerous regions of the brain has been reported to be a mediator involved in synaptic transmission and functional integration in the central nervous system [5, 15]. As a fast neurotransmitter [15], the action of ATP in the brain is mediated via purinergic P2X receptors [14]. Also, P2X receptors exist in the PAG [21, 23], and a crucial study has previously demonstrated that activation of subtype P2X3 receptors increases excitatory glutamatergic synaptic transmission within the dorsolateral PAG [23]. Given that the PAG has a regulatory effect on neuropathic pain [13, 16], P2X3 receptors within the dorsolateral PAG neurons are likely engaged in regulating the transmission of the neuropathic pain observed in diabetes. On the basis of the evidence, in this report, we examined mechanical withdrawal thresholds in streptozotocin (STZ)-induced diabetic rats and control rats after stimulation of P2X3 receptors within the dorsolateral PAG. We further examined the total and membrane protein expressions of P2X3 receptors in the PAG of diabetic and control rats. We hypothesized that diabetes induced by STZ decreases the membrane expression of P2X3 receptors in the PAG, thereby leading to a blunted descending inhibitory system in modulating pain transmission, and this plays a role in the development of mechanical allodynia in diabetes.
Methods
All experimental procedures were in accordance with the guidelines of the International Association for the Study of Pain and were approved by the Animal Research Committee of Liaocheng People’s Hospital. Forty-two male Sprague–Dawley rats weighing 150–200 g were used in this study. STZ was freshly dissolved in 0.9 % sterile saline, and diabetes was induced by a single injection of STZ (70 mg/kg i.p., Sigma Co., St. Louis, MO) as described previously [24]. Diabetes was confirmed by measurements of blood glucose concentrations in samples obtained from the tail vein 3 weeks after injection of STZ. It should be noted that rats whose blood glucose concentration was >350 mg/dl and mechanical paw withdrawal threshold (PWT) was <5 g were included in the study. Age- and body weight-matched rats with saline injection were used as controls.
Rats were implanted with a stainless-steel guide cannula (0.8 mm o.d.) under anesthesia with 4 % chloral hydrate (10 ml/kg body weight, i.p.). The guide cannula was fixed to the skull using dental zinc cement and a jewelers’ screw. Stereotaxic coordinates for the dorsolateral PAG were 7.6 mm posterior to the bregma, 0.65 mm lateral to the midline and 4.2 mm ventral to the brain surface. A dummy cannula inserted into the guide cannula at the time of surgery was used to reduce the incidence of occlusion. Five days were allowed before the experiments.
The mechanical threshold was determined before and after STZ or saline injection. To quantify the mechanical sensitivity of the hindpaw, rats were placed in individual plastic boxes and allowed to acclimate for >30 min. Rat hindpaw PWTs in response to the stimulation of von Frey filaments were determined. A series of calibrated von Frey filaments (ranging from 0.4 to 15.0 g) was applied perpendicularly to the plantar surface of the hindpaw with sufficient force to bend the filaments for 60 s or until paw withdrawal. In the presence of a response, the filament of next lower force was applied. In the absence of a response, the filament of next greater force was applied. To avoid injury during tests, the cutoff strength of the von Frey filament was 15 g. The tactile stimulus producing a 50 % likelihood of withdrawal was determined using the “up-down” method [7]. Each trial was repeated twice at approximately 2-min intervals. The mean value was used as the force producing a withdrawal response. All the studies were performed in a blinded manner.
A-317491 (a P2X3 receptor antagonist) and α,β-methylene-ATP (α,β-meATP, a P2X3 receptor agonist) were obtained from Sigma Co. and prepared freshly on the day of the experiment. A-317491 and α,β-meATP were dissolved in artificial cerebrospinal fluid (aCSF), which thereby was used as a control vehicle in this study. A prior study suggested that a microinjection of 100 nmol/μl (in 0.3 μl of vehicle) of α,β-meATP into the lateral PAG resulted in distinct antinociceptive effects, and 5 nmol/μl (in 0.3 μl of vehicle) of A-317491 significantly attenuated the antinociception evoked by α,β-meATP [22]. According to these prior findings [22], the concentrations of α,β-meATP selected in this study were 2–50 nmol/μl, and the concentration of A-317491 was 10 nmol/μl. For drug and aCSF injection, an injection cannula was inserted into the guide cannula and was connected to a 1-μl Hamilton microsyringe. A total volume of 0.25 μl of drugs or aCSF was injected over a 2-min period, and the injector remained in place for an additional 2 min to ensure complete diffusion of the drug. At the end of the experiments, 2 % Evans blue in 0.25 μl was infused through the microinjection cannula. Then, the animals were deeply anesthetized by 4 % chloral hydrate (20 ml/kg body weight, i.p.) and intracardiacally perfused with physiological saline followed by 4 % paraformaldehyde solution. The midbrain was sectioned, and under a microscope the location of administration sites was verified by histological examination of blue dye according to the atlas of Swanson [20]. A histological section showing the location of the injection cannula is presented in Fig. 1a. Only those rats with a microinjection site localized within the dorsolateral PAG were used for data analysis.
Fig. 1.
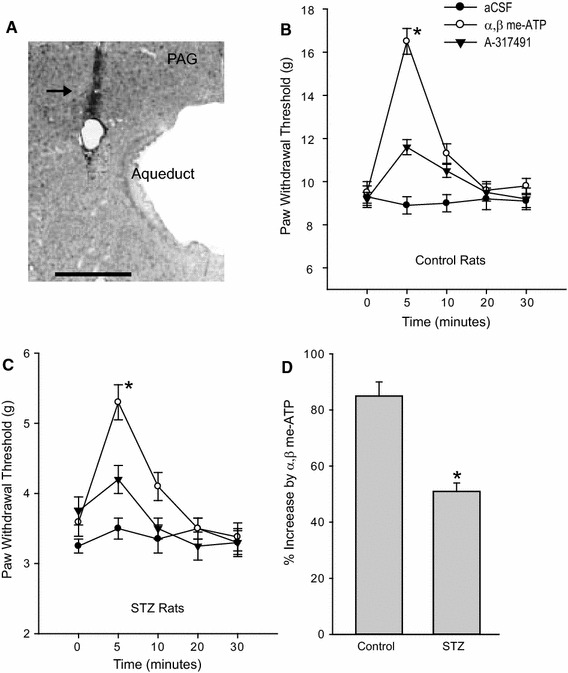
Effects of P2X3 stimulation in the dorsolateral PAG on the paw withdrawal potential (PAW) in control rats and STZ rats. A histological section shows the location of the injection cannula track in the PAG (a). An arrow indicates the cannula tract. Scale bar = 0.5 mm. Microinjection of α,β-meATP (50 nmol/µl) into the dorsolateral PAG increased the PAW in control rats (b) and STZ rats (c) compared with aCSF injection. Also, blocking P2X3 with the prior administration of A-317491 (10 nmol/µl) attenuated P2X3-enhanced PAW in both groups. The percentage increase of PAW evoked by stimulation of P2X3 was smaller in STZ rats than in control rats (d). Data are expressed as mean ± SEM. b and c *P < 0.05 vs. aCSF and α,β-meATP with prior injection of A-317491. d *P < 0.05 vs. control rats. The number of control rats = 12; the number of STZ rats = 12
In another group of experiments, the protein expression of P2X3 receptors in the dorsolateral PAG of STZ-treated rats and control rats was determined using Western blot analyses. The rats were first euthanized by 4 % chloral hydrate (20 ml/kg body weight, i.p.), and then the regions of the PAG were dissected under an anatomical microscope. To determine the expression of P2X3 on the cell surface, PAG tissues were incubated with Sulfo-NHS-LC-Biotin (1 mg/ml, Pierce) for 30 min on ice as described previously [25]. Because biotin is impermeable to the cell membrane, only proteins on the cell surface were biotinylated. The unbound biotin in the solution was removed by 5× wash of PAG tissues. PAG tissues were then homogenized in buffer A and centrifuged at 13,500g (4 °C) for 12 min. A sample (200 μg protein) was incubated with streptavidin beads (20 μl, Sigma Co.) for 3 h at 4 °C. The beads were washed 3× with RIPA buffer and precipitated by centrifugation and collected. Sample buffer (50 μl) was added to the collected beads and boiled for 3 min. Beads were pelleted again by centrifugation, and the supernatant was collected. The supernatant was diluted to the same volume as the starting material (i.e., 200 μg total protein). An equal volume of total and membrane samples was applied to SDS-PAGE. Membranes were incubated with the rabbit anti-P2X3 primary antibody (1:1000, Neuromics, Edina, MN, USA) and goat anti-rabbit secondary antibody (1:200, Neuromics, Edina, MN, USA). Immunoreactive proteins were detected by enhanced chemiluminescence (ECL kit, Amersham-Pharmacia Biotech). The membrane was also incubated with the mouse anti-β-actin primary antibody and goat anti-mouse secondary antibody (Sigma Co.). This was used to show equal protein loading control in the Western blot analysis.
The densities of protein bands were analyzed using NIH image software, and the ratio for densities of P2X3 immunoreactive bands/β-actin band densities from the same sample was determined and then normalized to a control sample [8].
All data were analyzed using a two-way repeated-measures analysis of variance. Values were presented as mean ± SEM. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SAS for Windows, version 9.13 (SAS Institute, Cary, NC, USA).
Results
First, we examined the effects of STZ injection on blood glucose, body weight and PWT in this study. Rats developed hyperglycemia 3 weeks after STZ injection. Blood glucose was 135 ± 7 mg/dl in control rats (n = 20) and 425 ± 8 mg/dl in STZ rats (n = 22, P < 0.05 vs. control rats). In addition, an increase in body weight (255 ± 14 g in control rats and 205 ± 15 g in STZ rats, P < 0.05 vs. control rats) was significantly attenuated, and the PWT significantly decreased in STZ rats (3.5 ± 0.2 g, n = 22, P < 0.05 vs. control rats) as compared with control rats (9.5 ± 0.5 g, n = 20).
Then, we examined the effects of stimulation of P2X3 receptors in the PAG on the PWT in STZ rats (n = 12) and in control rats (n = 12). Figure 1b, c shows that the PWT was significantly increased 5 min after microinjection of 50 nmol/μl of α,β-meATP into the dorsolateral PAG of both control and STZ rats (P < 0.05 vs. aCSF control), whereas the PWT was not altered by aCSF. In addition, the PWT returned to the prior level 20 min after administration of α,β-meATP. In particular, the percentage increase of the α,β-meATP-evoked PWT was significantly smaller in STZ rats (P < 0.05 vs. control rats) than in control rats (Fig. 1d). In this subset of the experiment, the PWT was also examined after 2 and 10 nmol/μl of α,β-meATP were microinjected into the PAG, respectively. Five minutes after injection of 2 nmol/μl of α,β-meATP, the PWT was 10.5 ± 0.5 g in control rats (n = 8) and 3.6 ± 0.3 g in STZ rats (n = 10). There was no significant difference in the PWT between this dose of α,β-meATP and aCSF control (P > 0.05 for control rats and STZ rats). Five minutes after injection of 10 nmol/μl of α,β-meATP, the PWT was significantly increased (P < 0.05 vs. aCSF control in both groups). They were 13.5 ± 0.5 g in control rats (n = 8) and 4.6 ± 0.3 g in STZ rats (n = 10). Also, the percentage increase of the PWT induced by 10 nmol/μl of α,β-meATP was significantly smaller in STZ rats (P < 0.05 vs. control rats) than in control rats.
Also, the effects of P2X3 receptor stimulation on the PWT were examined with the prior injection of the P2X3 antagonist, A-317491 (10 nmol/μl, Fig. 1b, c). Our results showed that blocking P2X3 significantly attenuated increases of the PWT induced by microinjection of 50 nmol/μl of α,β-meATP into the dorsolateral PAG in both control and STZ rats (P < 0.05 vs. α,β-meATP alone).
Moreover, we employed Western blot analysis to determine total and membrane protein expressions of P2X3 receptors in the dorsolateral PAG of control (n = 8) and STZ rats (n = 10). Figure 2 demonstrated that STZ injection did not significantly alter the total P2X3 expression in the PAG, but significantly decreased membrane P2X3 expression. Optical density of membrane protein expression of P2X3 was 0.98 ± 0.15 in control rats and 0.71 ± 0.10 in STZ rats (P < 0.05 vs. control rats).
Fig. 2.
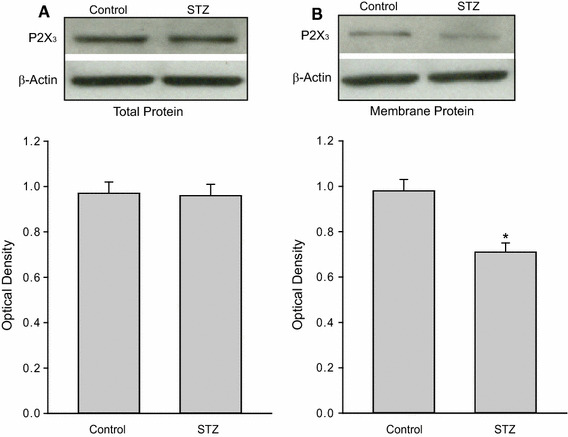
Effects of STZ treatment on the protein expression of P2X3 receptors in the dorsolateral PAG. a Total expression of P2X3. Top and bottom panels are typical bands and averaged data obtained from control and STZ rats. b Membrane expression of P2X3 of control rats and STZ rats is presented as typical bands and averaged data. Data are expressed as mean ± SEM. *P < 0.05 vs. control rats. The number of control rats = 8; the number of STZ rats = 10. β-Actin was used as equal loading control
Discussion
Prior studies have shown that a single injection of STZ can induce diabetes and mechanical allodynia in rats 3 weeks later [24]. The abnormalities were maintained for at least 7 weeks. Using the same interventions, we observed significantly elevated blood glucose levels and a decreased threshold to evoke mechanical withdrawal 3 weeks after STZ injection in our current study. Results of our current study demonstrated that microinjection of α,β-meATP into the dorsolateral PAG significantly increased the PWT in control rats and STZ rats, and the effects of α,β-meATP were smaller in STZ rats (Fig. 1). Our results also demonstrated that cell membrane expression of P2X3 protein is downregulated in the dorsolateral PAG of STZ rats as compared with control animals (Fig. 2). It should be acknowledged that the P2X3 receptor expression in the PAG is unlikely to be limited to PAG neuronal cells. There is the possibility that P2X3 receptors are expressed in glial cells in the PAG. To the best of our knowledge, this is the first report to suggest that a decrease in the membrane expression of P2X3 receptors in the PAG of diabetic rats is likely to impair the descending inhibitory system in modulating pain transmission and thereby contributes to the development of mechanical allodynia in diabetic neuropathy.
P2X receptors are ligand-gated cationic channels, and there are seven P2X receptor subtypes (P2X1–7) with different tissue distributions [15]. Among these subunits, P2X3 receptors are abundantly expressed in peripheral afferent nerves in rats and engaged in pain regulation after tissue inflammation and nerve injury [5, 15]. Moreover, it has been established that ATP and purinergic receptors are involved in peripheral signaling in diabetic rats [11, 24]. The mRNA and protein levels of P2X3 receptors in peripheral afferent neurons are increased in STZ-induced diabetes, and P2X3 receptor antagonists inhibit the STZ-induced mechanical allodynia in animal models [11, 24].
In addition to their localization at the terminals of the primary afferent neurons, P2X3 receptors were found at the central nerve system as well. A considerable amount of evidence has been established showing that antinociception is mediated in part by descending pathways arising from the midbrain PAG [10, 12]. Early studies have shown that electrical stimulation or opioids microinjected into the PAG produced profound long-lasting antinociception [10, 12]. Previous studies have shown that P2X receptors appear in the PAG [21], and activation of the P2X receptor in the PAG plays a role in pain modulation via the descending transmission pathways [22]. Particularly, a prior study has demonstrated that the P2X3 receptors were dominantly localized in the dorsolateral PAG [23]. In addition, stimulation of P2X3 receptors within the dorsolateral PAG increases excitatory glutamatergic synaptic transmission, indicating that activation of P2X3 in this region of the PAG has an inhibitory effect on the descending pathways [23].
Nevertheless, it is largely unknown how P2X3 receptors in the PAG can influence mechanical allodynia in rats with STZ-induced diabetes. Our data of the current study demonstrated that a threshold to cause mechanical stimulation withdrawal is significantly increased in both control rats and STZ rats when α,β-meATP is microinjected into the dorsolateral PAG to stimulate P2X3. We further assured that P2X3 receptors are specifically activated because a prior administration of P2X3 antagonist significantly attenuates the effects of α,β-meATP. Interestingly, our data showed that the facilitating effects of P2X3 stimulation on mechanical withdrawal thresholds are significantly blunted in STZ-induced diabetic rats compared with control rats. Consistent with this result, we further demonstrated that cell membrane expression of P2X3 protein is downregulated in the dorsolateral PAG of STZ-induced diabetic rats. However, the total protein expression of P2X3 was not significantly altered in the PAG of STZ rats, suggesting that P2X3 trafficking to the cell membrane of PAG is specifically decreased in diabetic rats. The underlying mechanism for the decrease in trafficking of P2X3 receptors following diabetes needs to be determined. It is speculated that the decrease in PAG activities is likely a result of neuronal loss within the PAG as apoptosis has been reported in the brains of diabetic rats in the hippocampus and frontal cortex [4, 18].
Interestingly, a prior report showed that α,β-meATP injected into the lateral PAG caused the antinociceptive effects, and the expression of P2X3 receptor was increased in this region of the PAG in rats with neuropathic pain [22]. This also supports the notion that activation of P2X3 receptor within the PAG plays an inhibitory role in regulating the descending pain pathways. Nonetheless, some discrepancies should be noticed in the results obtained from the two studies. In this prior study, the neuropathic pain model was made by a chronic constriction injury of the rats’ sciatic nerve, and P2X3 receptors in the lateral region of PAG were examined. In general, the PAG is divided into several subregions (i.e., distinct dorsolateral and ventrolateral PAG) having different effects on pain [1, 6]. We speculate that alterations in P2X3 expression are possibly varied within subregions of PAG in the same neuropathic pain model or different models.
In summary, we have demonstrated that the membrane expression of P2X3 receptors was decreased in the dorsolateral PAG of STZ rats, which thereby was likely to deactivate P2X3-mediated descending inhibitory regulation in pain transmission. These abnormalities are likely to contribute to the development of mechanical allodynia in diabetic neuropathy. The results may offer promising clues for the development of new therapeutic strategies for managing intractable neuropathic pain in patients with diabetes.
Acknowledgments
Conflict of interest
None.
References
- 1.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-K. [DOI] [PubMed] [Google Scholar]
- 2.Benarroch EE. Periaqueductal gray: an interface for behavioral control. Neurology. 2012;78:210–217. doi: 10.1212/WNL.0b013e31823fcdee. [DOI] [PubMed] [Google Scholar]
- 3.Benbow J, Chan AW, Bowsher D, MacFarlane IA, Williams G. A prospective study of painful symptoms, small-fibre function and peripheral vascular disease in chronic painful diabetic neuropathy. Diabet Med. 1994;11:17–21. doi: 10.1111/j.1464-5491.1994.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt MP, Lim Y-C, Hwang JY, Na SH, Kim Y-M, Ha K-S. C-peptide prevents hyperglycemia-induced endothelial apoptosis through inhibition of reactive oxygen species-mediated transglutaminase 2 activation. Diabetes. 2013;62:243–253. doi: 10.2337/db12-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15:1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- 6.Carrive P, Morgan MM. Periaqueductal gray. In: Mai JK, Paxinos G, editors. The human nervous system. 3. San Diego: Academic Press; 2012. pp. 367–400. [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Staikopoulos V, Furness JB, Jennings EA. Inflammation induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience. 2009;162:453–461. doi: 10.1016/j.neuroscience.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 9.Gooch C, Podwall D. The diabetic neuropathies. Neurologist. 2004;10:311–322. doi: 10.1097/01.nrl.0000144733.61110.25. [DOI] [PubMed] [Google Scholar]
- 10.Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–RA273. [PubMed] [Google Scholar]
- 11.Migita K, Moriyam T, Koguchi M, Hond K, Katsuragi T, Takano Y, Ueno S. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett. 2009;452:200–203. doi: 10.1016/j.neulet.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 12.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/S0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 13.Morgado C, Terra PP, Tavares I. Neuronal hyperactivity at the spinal cord and periaqueductal grey during painful diabetic neuropathy: effects of gabapentin. Eur J Pain (London, England) 2010;14:693–699. doi: 10.1016/j.ejpain.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Norenberg W, Illes P. Neuronal P2X receptors: localisation and functional properties. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:324–339. doi: 10.1007/s002100000311. [DOI] [PubMed] [Google Scholar]
- 15.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 16.Paulson PE, Wiley JW, Morrow TJ. Concurrent activation of the somatosensory forebrain and deactivation of periaqueductal gray associated with diabetes-induced neuropathic pain. Exp Neurol. 2007;208:305–313. doi: 10.1016/j.expneurol.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva M, Amorim D, Almeida A, Tavares I, Pinto-Ribeiro F, Morgado C. Pronociceptive changes in the activity of rostroventromedial medulla (RVM) pain modulatory cells in the streptozotocin-diabetic rat. Brain Res Bull. 2013;96:39–44. doi: 10.1016/j.brainresbull.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Sima AAF, Li Z-G. The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes. 2005;54:1497–1505. doi: 10.2337/diabetes.54.5.1497. [DOI] [PubMed] [Google Scholar]
- 19.Spruce MC, Potter J, Coppini DV. The pathogenesis and management of painful diabetic neuropathy: a review. Diabet Med. 2003;20:88–98. doi: 10.1046/j.1464-5491.2003.00852.x. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LW. Brain maps: structure of the rat brain. New York: Elsevier; 1998. [Google Scholar]
- 21.Worthington RA, Arumugam TV, Hansen MA, Balcar VJ, Barden JA. Identification and localisation of ATP P2X receptors in rat midbrain. Electrophoresis. 1999;20:2077–2080. doi: 10.1002/(SICI)1522-2683(19990701)20:10<2077::AID-ELPS2077>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Z, Ou S, He WJ, Zhao YD, Liu XH, Ruan HZ. Role of midbrain periaqueductal gray P2X3 receptors in electroacupuncture-mediated endogenous pain modulatory systems. Brain Res. 2010;1330:31–44. doi: 10.1016/j.brainres.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Xing J, Lu J, Li J. Purinergic P2X receptors presynaptically increase glutamatergic synaptic transmission in dorsolateral periaqueductal gray. Brain Res. 2008;1208:46–55. doi: 10.1016/j.brainres.2008.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu G-Y, Li G, Liu N, Huang L-Y. Mechanisms underlying purinergic P2X3 receptor mediated mechanical allodynia induced in diabetic rats. Mol Pain. 2011;7:60. doi: 10.1186/1744-8069-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu GY, Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA. 2004;101:11868–11873. doi: 10.1073/pnas.0401490101. [DOI] [PMC free article] [PubMed] [Google Scholar]


