Abstract
The effect of intravenous administration of human mesenchymal stromal stem cells (hMSC) has been evaluated by means of large-conductance calcium-dependent potassium channel (BKCa) activity measurements in thoracic aorta smooth muscle cells (SMC) obtained from non-fatal whole-body irradiated rats, using the patch clamp technique in whole-cell modification, and the standard acetylcholine (ACh) test to evaluate functional endothelium integrity using SM contractile recordings. Myofilament calcium sensitivity was estimated using simultaneous contractile recordings versus [Ca2+]i. Arterial blood was measured in intact and irradiated rats before and after hMSC administration. Stimulation of isolated SMC from the control group of animals with depolarizing voltage steps showed that outward K+ currents sensitive to the BKCa inhibitor paxilline were expressed. Outward currents in SMC obtained from irradiated animals were significantly reduced on the 30th day of post-irradiation. Irradiation led to a significant elevation in arterial blood pressure and reduced ACh-induced relaxation responses in irradiated rats as compared with the control group. Simultaneous measurements of contractile force and [Ca2+]i showed that myofilament Ca2+ sensitivity had increased following irradiation. Intravenously injected hMSC effectively restored BKCa current and the amplitude of ACh-induced endothelium-dependent vasodilatation in vascular tissues obtained from post-irradiated rats. SMC obtained from irradiated rats treated with hMSC demonstrated a significantly increased paxilline-sensitive component of outward potassium currents, indicating that BKCa activity had been restored. hMSC administration normalized increased blood pressure and myofilament Ca2+ sensitivity in irradiated animals. When administered to healthy rats, hMSC were without effects on either of these. This study does not provide any immunohistochemical proof of hMSC engraftment in the host rats. PCR analysis showed that hMSCs were negative for hematopoietic cell markers and positive for hMSC markers. There were no clinical signs of graft-versus-host disease throughout the experimental period of 30 days. The data obtained suggest that hMSC demonstrate a clearly expressed ability to normalize vascular function damaged following irradiation, i.e. to reduce an elevated arterial blood pressure and myofilament Ca2+ sensitivity, and to repair BKCa function and endothelium-dependent relaxation in vascular tissues obtained from irradiated animals. Thus, hMSC seem to be worthwhile therapeutic approach in cases of ionizing irradiation accident or radiation beam therapy.
Keywords: Irradiation, Vascular function, Stem cells
Introduction
In the Ukraine, cardiovascular disorders related to consequences of the Chernobyl disaster are a principal cause of disability and one of the main causes of death among so-called liquidators of the catastrophe. It was surprising discovery that arterial hypertension prevails over all those diseases recorded in liquidators of the Chernobyl disaster and population on adjacent territories who were irradiated.
From another point of view, radiation therapy is commonly employed as a primary and adjuvant therapeutic modality in patients with neoplasm, and despite many advances in technology, normal tissues including the blood vessels are often affected. This is manifested as a blunting of endothelium-dependent vasorelaxation, coronary spasm, and development of cardiac arrhythmias that seriously complicate the course and treatment of the main disease. That is why a current challenge in radiation medicine and pharmacology is how cardiovascular cellular function can be preserved or treated during radiation impact.
Vascular endothelium function tested using standard acetylcholine-induced relaxation and large conductance Ca2+-dependent potassium channels (BKCa) activity in endothelial cells seems to be one of the most vulnerable mechanisms of vasodilatation in vascular wall under irradiation [1, 2]. It has recently been shown that both BKCa channels function and expression in smooth muscle cells (SMC) are very sensitive to radiation, suggesting that the vasodilator potential of vascular smooth muscle had decreased following irradiation [3].
Stem/progenitor cells transplantation has been known since the early 1990s to be a novel approach for vascular malfunction therapy [4]. For instance, transplantation of adult peripheral blood CD34+ cells that are endothelial progenitor cells prevents left ventricular dilatation and augments myocardial revascularization and coronary blood flow after myocardial ischemia [5].
Bone marrow mesenchymal stromal stem cells (MSC) are connective tissue progenitor cells that are distinct from hematopoietic stem cells [6]. Whereas MSC can be easily expanded ex vivo from raw bone marrow, there is not yet a generally accepted method for human MSC (hMSC) isolation, propagation, and characterization. As a result, the phenotype of culture-expanded MSC can vary considerably when derived by different methods [7] or from different sources [8]. Recent studies proposed a more extensive differentiation potential of MSC showing phenotypic plasticity that seems to cross the boundaries of the traditional germ layers, including cardiac cells [9], skeletal muscle [10], and neural cells [11]. Whether this apparent plasticity represents transdifferentiation, a pool of persistent pluripotent stem cells, cell fusion, or artefacts of culturing remains controversial [12–14]. Because of their ability to differentiate into a variety of cells, the ease of their isolation and expansion, and their potential use for clinical application, efforts have increased to better understand the biology of MSC.
The objective of this study was to estimate potential efficacy of hMSC to restore vascular function damaged in rats following experimental non-fatal whole-body ionizing irradiation.
Methods
Stem cell separation and culture
Bone marrow was aspirated in heparin from the sternum of 4 human male healthy volunteers of ages 38, 44, 54, and 56 years after informed consent and Institute Hematology and Transfusion Ethics Committee approval. To reduce the burning effect of lidocaine solution, local anesthesia was achieved by use of 1% lidocaine (up to 4 μg/kg) in 1% sodium bicarbonate solution injected subcutaneously and into the periosteum.
hMSC were separated using a negative selection procedure with monoclonal antibodies (Human Rosette Set Mesenchymal Stem Cell Enrichment; StemCell, USA). The isolated cells were resuspended in MesenCult medium (StemCell) supplemented with appropriate Mesenchymal Stem Cell Stimulatory Supplement (StemCell) and cultivated in a 95% humidified incubator at 37°C in 5% CO2 in air. Attached cells developed into colonies within 3–7 days. After this period, non-adherent cells were removed by replacing the medium. The cell density and morphology were monitored under an inverted microscope. Isolated cells were cultivated for 20–32 days in the same medium with additional recombinant human growth factors: EGF, PDGF-AA, and PDGF-BB (CHO-grade; Prospec, USA), which were added to the culture twice a week to obtain a final concentration of 5 ng/ml. When these primary cultures of hMSC had reached 80–90% confluence, the cells were harvested using 0.25% trypsin and subcultured at a ratio of 1:3. At the end of cultivation hMSC were detached from the bottom of the flasks with 0.25% trypsin and 1 mM EDTA. After two passages hMSC were administered to irradiated (6 Gy) rats intravenously on the 7th day of post-irradiation in a single dose of 16–20 × 106 cells per rat. There were no clinical signs of graft-versus-host disease throughout the experimental period of 30 days.
Taking into account that the cell-transplantation procedure in this study does not satisfy all the demands of classical transplantology we considered the influence of hMSC as pharmacological intervention by means of an effect of MSCs. We also clearly understand that for anyone making a new attempt of clinical use of hMSC, prudence, common sense, and regulatory authorities require non-clinical trials with human cells in non-human animals.
Isolation of rat thoracic aorta smooth muscle cells
All animal studies were performed in accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes and approved by Institutional Animal Care and Use Committees.
Each of 4 groups of experimental animals involved in the studies consisted of 20 rats and were allocated to one of the following treatments:
non-irradiated, phosphate-buffered saline-treated, age-matched control group sacrificed on the 30th day of the experimental period;
irradiated, phosphate-buffered saline group with sacrifice on the 30th day post irradiation;
hMSC treated, non-irradiated animals sacrificed on the 30th day of the experimental period; and
hMSC treated, irradiated rats sacrificed on the 30th day post irradiation.
Isolated smooth muscle cells dispersed from the rat thoracic aorta were obtained from 6 to 8-week-old male Wistar rats (250–300 g) by collagenase treatment. Briefly, the rats were anesthetized with ketamine (37.5 mg/kg b.w., IP) and xylazine (5 mg/kg, b.w., IP) and approximately 1.0–1.5 cm of the thoracic aorta was excised and cleaned of connective tissue. The aorta was then cut into small pieces (1.5 × 1.5 mm) in a cold, low-Ca2+ solution containing (mM): 140 NaCl; 6 KCl; 3 MgCl2; 10 glucose; 10 HEPES (pH 7.4) for 15 min. The vascular tissues were transferred to a fresh, low-Ca2+ solution containing: 2 mg/ml collagenase type IA (417 U/mg), 0.5 pronase E type XXV, and 2 mg/ml bovine serum albumin. The tissues were then incubated for 30 min at 37°C. After incubation, the tissues were washed (2–3 min) twice in a fresh low-Ca2+ solution to remove the enzyme. Cells were dispersed by agitation using a glass pipette, and then were placed in normal Krebs bicarbonate buffer. Aliquots of the myocytes were stored at +5°C and remained functional for at least 5 h.
Electrophysiology
The whole-cell patch-clamp technique in the perforated-patch configuration was used to study whole-cell potassium currents (voltage-clamp mode). Data acquisition and voltage control were performed using an Axopatch 200B Patch-Clamp amplifier and Digidata 1200B interface (Axon Instruments, Foster City, CA, USA) coupled to an IBM-compatible computer equipped with pClamp software (version 6.02, Axon Instruments). Membrane currents were filtered at 2 kHz and digitized at a sampling rate of 10 kHz. The reference electrode was an Ag–AgCl plug electrically connected to the bath.
At the beginning of each experiment, the junction potential between the pipette solution and bath solution was electronically adjusted to zero. No leakage current subtraction was performed on the original recordings, and all cells with visible changes in leakage currents during the course of study were excluded from further analysis. Macroscopic current values were normalized as pA/pF. The membrane capacitance of each cell was estimated by integrating the capacitive current generated by a 10-mV hyperpolarizing pulse after electronic cancellation of pipette-patch capacitance using Clampfit software (version 6.02, Axon Instruments). All electrophysiological experiments were carried out at room temperature (20°C).
Patch pipettes were made from borosilicate glass (Clark Electromedical Instruments, Pangbourne Reading, UK) and backfilled with intracellular solution containing (mM): 140 KCl, 10 NaCl, 1.2 MgCl2, 2.5 CaCl2, 10 N-2-hydroxyethylpiperazine-N-2 ethanesulfonic acid (HEPES) 11.5 glucose, adjusted to pH 7.3 with KOH. Pipettes had resistances of 2.5–5.0 MΩ. The standard Krebs-HEPES external solution contained (mM): 140 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 10 HEPES and 11.5 glucose at pH 7.4.
Contractile recording experiments (ACh-test)
Experiments were performed on the tissues of the thoracic aorta obtained from healthy and irradiated adult rats. The vascular tissues were prepared with special care in order to keep the endothelium intact. Vascular tissues were mounted isometrically in a tissue bath between a stationary stainless steel hook and an isometric transducer (AE 801; SensoNor, Norten, Norway) coupled to a AD converter Lab-Trax-4/16 (World Precision Instruments, Sarasota, USA). Experiments were conducted at 37°C in modified Krebs bicarbonate buffer solution of the following composition (mM): 133 NaCl, 4.7 KCl, 16.3 NaHCO3, 1.38 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, 10 HEPES, 7.8 glucose at pH 7.4. To evaluate endothelium function, isolated thoracic aorta rings obtained from experimental animals were preconstricted with 10 μM arterenol and, after contraction reached a plateau level, acetylcholine was added to the bath solution in dose-dependent manner. The relaxation responses of arterenol-preconstricted rings were expressed as a percentage of the contractile response of the aorta to arterenol.
Simultaneous measurement of [Ca2+]i and contractile force (coefficient of myofilament calcium sensitivity)
Segments of thoracic aorta (1.5 cm-long) were obtained as described above, cleaned of both connective and adipose tissue, and cut into rings 1–1.5 mm wide. All procedures were performed at room temperature in a nominally Ca2+-free physiological salt solution.
Experiments for simultaneous measurement of [Ca2+]i and contractile force were carried out in a 500-μl tissue chamber mounted on the stage of a fluorescence microscope LUMAM-2 (Russian Federation) equipped with epifluorescence collection equipment. The aortic rings were mounted isometrically between a stationary stainless steel hook and a force transducer (AE 801; SensoNor). Except for during the Fura-2AM loading procedure, the rings were continuously perfused with Krebs solution preheated to 35°C at a rate of 2.0 ml/min; Ca2+ measurements tended to be more stable at 35°C than at 37°C. The rings were loaded with 10 μM Fura 2-AM in physiological solution of composition (mM): 122 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 11.6 HEPES, 11.5 glucose, and pH 7.3–7.4. The loading solution also contained 2.5% DMSO and 5 mg/ml Pluronic F-127. Loading continued for 2 h at room temperature. The tissues were then allowed to equilibrate with a resting tension of 10–15 mN in normal physiological salt solution for at least 30 min. Following the equilibration period, the tissues were exposed several times to arterenol (10 μM) until reproducible contractile responses were obtained.
Fura-2 fluorescence was excited at 340 and 380 nm wavelength (λ) and recorded at 510 nm emission wavelength from a central region (approximately 0.5 mm in diameter) on the blood surface of the aortic ring. The fluorescence emitted from the tissue was collected by a photomultiplier through a 510-nm filter. The results of [Ca2+]i measurements are presented as the ratio (R) of the 510 nm emission fluorescence intensity [I 510(λ)] at λ = 340 nm and λ = 380 nm excitation signals: R = I 510(340)/I 510(380). At the end of the experiment, the maximum fluorescence ratios were determined. The maximum fluorescence was determined in a phosphate-free, bicarbonate-free, 120 mM KCl, 5 mM CaCl2, salt solution containing 10 μM ionomycin. The minimum fluorescence ratio was determined by adding 10 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA). [Ca2+]i was determined using the formula [Ca2+]i (nM) = K d × [(R − R min)/(R max − R)] × (S f2/S b2), where K d (224 nM) is the dissociation constant of Fura-2 for Ca2+; R is the ratio of fluorescence of the sample at 340 nm to that at 380 nm; R min and R max represent the ratios of fluorescence at the same wavelengths in the presence of zero and saturating Ca2+, respectively; and S f2/S b2 is the ratio of fluorescence of Fura-2 at 380 nm in zero Ca2+ to that in saturating Ca2+. Preliminary experiments indicated that contractions induced by arterenol were not significantly affected by Fura-2 AM loading.
RNA extraction, reverse transcription and PCR analysis
A total of 20 μg RNA from each sample of investigated MSCs was extracted using the RNeasy Mini Kit (Qiagen, USA) followed by DNase I treatment (GE Healthcare–AmershamBioscience, USA). The reverse polymerase transcription was performed using SuperScript III reverse transcriptase (Invitrogen, USA) with random primers, according to the manufacturer’s recommended procedure, and the cDNA product was treated with RNase H (Hoffmann-La Roche, USA). Tfi DNA polymerase (Invitrogen) was used for PCR and reactions were conducted according to the manufacturer’s recommended procedure. The minus-reverse transcriptase template and minus-template PCR were included in PCR experiment as a negative control, and 15S small ribosomal subunit protein RNA and pairs of the needed primers (Ambion, USA) were used as a positive control. Primer-BLAST software was used for finding primers specific to selected PCR templates at a primer melting temperature (T m) of 60°C (Table 1). The products of PCR reactions with this set of primers were treated with the restrictase EarI (Fermentas, USA) according to the manufacturer’s recommended procedure for confirmation of the specificity of the ones used. Aliquots (15 μl) of PCR reaction products were run on native agarose 2% gel, stained by SYBR safe DNA gel stain (Invitrogen) and visualized under UV light.
Table 1.
Primer sequences used for PCR and PCR products
| hMSC and hematopoietic cells markers | GeneBank accession no. | Primer sequences (5′ to 3′) | Size of product (bp) | |
|---|---|---|---|---|
| Non-MSC phenotype of bone marrow cells | ||||
| CD14 | NM_000591.2 | Sense | CACTGCCAGGAGACACAGAA | 692 |
| Antisense | TAGGTCCTCGAGCGTCAGTT | |||
| CD34 | NM_001773.2 | Sense | CAACATCTCCCACTAAACCCTATAC | 561 |
| Antisense | TGCATGTGCAGACTCCTTTC | |||
| CD45 | NM_080921 | Sense | CCGAATCTGACATCATCACCT | 261 |
| Antisense | TAAGGTAGGCATCAGTGGGG | |||
| MSC phenotype of bone marrow cells | ||||
| CD71 | NM_001128148.1 | Sense | CGCGCTAGTGTTCTTCTGTG | 595 |
| Antisense | ACAAAATGTTGATCACGCCA | |||
| CD29 | NM_002211.3 | Sense | AATGAAGGGCGTGTTGGTAG | 679 |
| Antisense | CAACATGAACCATGACCTCG | 144 + 535 | ||
| CD44 | NM_001001392.1 | Sense | GAACTTCCAAAGGCTGCTTG | 859 |
| Antisense | CAATGTTGCAAGGGTTTGTG | 335 + 524 | ||
| CD105 | NM_00111475.1 | Sense | CACTAGCCAGGTCTCGAAGG | 843 |
| Antisense | TGAAGTGAGACAATGCTGGC | 65 + 778 | ||
| CD73 | NM_002526.2 | Sense | GGCACTATCTGGTTCACCGT | 813 |
| Antisense | GTTGCATTCTCTAAAGCGGC | 174 + 639 | ||
| CD166 | NM_001627.2 | Sense | AGCGAAAAGAACCGCTTACA | 491 |
| Antisense | TCCATTTGCCAAACATGAGA | 174 + 639 | ||
| CD90 | NM_006288.3 | Sense | TGCATGCGATTATCTACCCA | 486 |
| Antisense | TTCCACAGTGGTAAGGAGGG | 142 + 344 | ||
Whole-body animal γ-irradiation
Rats were exposed to a 6 Gy dose of ionizing irradiation and were euthanized 30 days after irradiation. Whole-body irradiation was performed with gamma rays delivered at a rate of 0.80 Gy/min from a cobalt60 source (TGT ROCUS M, Russian Federation). During irradiation animals were restrained in a plastic box specifically designed for this study and the radiation beam was focused on the animal’s chest. There was no change in housing, standard food, or drink after irradiation. All animals survived the 30-day experimental period.
Systolic blood pressure measurements
Systolic arterial pressure was measured in non-anesthetized rats by use of a cuff tail sphygmomanometer S-2 (Hugo Sachs Electronic, Germany).
Chemicals
Collagenase, type1A, pronase E, type XXV, bovine serum albumin, arterenol, acetylcholine, and all the constituents of Krebs solution were purchased from Sigma Chemicals (St Louis, MO, USA).
Statistics and analysis
Averaged data from electrophysiological studies and contractile recording data are shown as mean ± SEM. n indicates the number of animals studied. To obtain a final mean value at least 10 current or 8 contractile force measurements from each of animals were recorded.
Half-maximally effective concentration (EC50) values were expressed as pD2 (−Log EC50). If necessary, comparisons between two values were performed using the Student’s t test. Multiple comparisons were performed using one way analysis of variance (ANOVA). If any significant difference was found, Tukey’s multiple comparison test was applied. Differences were considered to be statistically significant when P was less than 0.05.
Results
Analysis of cell surface marker expression in hMSC and hematopoietic cells
Taking into account that the first challenge in stem cell therapy is to assess the quality of the cells, we have provided phenotype analysis of the cells used in our experiments. PCR analysis (Fig. 1) showed that hMSCs were negative for hematopoietic cell markers (CD14, CD34, CD45) and positive for hMSC markers (CD29, CD44, CD71, CD73, CD90, CD105, CD166). Bone marrow progenitors were positive for hematopoietic cell markers in contrast to hMSCs.
Fig. 1.
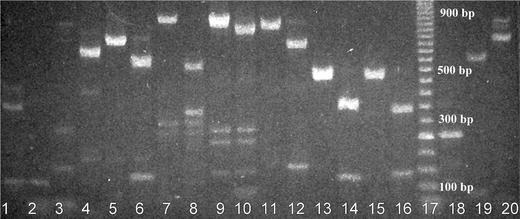
Analysis of expression of cell markers in hMSCs and hematopoietic cells (bone marrow progenitors) by means of PCR. cDNA from the cell mRNA and gene-specific primers were used (an expected PCR product size in base pairs is indicated for each gene). Restrictase EarI was employed in some cases for the verification of PCR products with a single restriction site (expected sizes of both fragments are indicated also). MSCs (1–16), bone marrow progenitors (18–20): 1 CD45, 2 CD34, 3 CD14, 4 CD71, 5 CD29, 6 CD29/EarI, 7 CD44, 8 CD44/EarI, 9 CD105, 10 CD105/EarI, 11 CD73, 12 CD73/EarI, 13 CD166, 14 CD166/EarI, 15 CD90, 16 CD90/EarI, 17 50 base-pair ladder, 18 CD45, 19 CD34, 20 CD14
Our results suggest that the cells used for transplantation correspond to criteria for defining hMSC and have been expanded without loss in their differentiation capacity.
Effect of irradiation on acetylcholine (ACh)-induced vascular relaxation in rat thoracic aorta
To evaluate endothelium-dependent vascular function we compared standard ACh-induced relaxant responses of thoracic aortas obtained from healthy and irradiated (6 Gy) rats on the 30 day after irradiation. ACh-induced dose-dependent relaxation of rat thoracic aortas preconstricted with arterenol (10−5 M) provided data on whether the endothelium remained intact. Figure 2 shows the effects of ACh on intact aortas obtained from healthy animals in comparison with aortas from rats 30 days after exposure to 6 Gy irradiation. In a healthy tissues, ACh produced maximum relaxation of 90 ± 2% (n = 20) at a concentration of 10 μM, and 50% of the maximum response at around 245 nM (pD2 = 7.6 ± 0.13). When the same experiment was repeated on aortas obtained from irradiated animals, ACh induced a smaller maximum relaxation of 56 ± 6% (n = 20, P < 0.05 vs. control value) and pD2 was 6.6 ± 0.03 (P < 0.05 vs. control value).
Fig. 2.
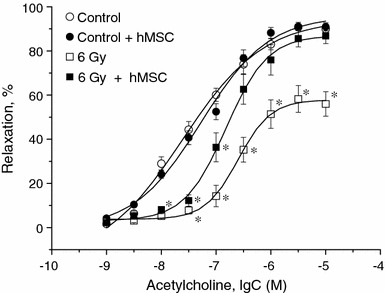
Endothelium-dependent acetylcholine-induced vascular responses in rat thoracic aorta rings obtained from healthy (open circles) and irradiated (open squares) rats and hMSC treated non-irradiated (closed circles) and post-irradiated (closed squares) animals. hMSC were administered intravenously on the 7th day after irradiation (6 Gy). Relaxations are expressed as percentage decrease in tension evoked by 10 μM arterenol. Asterisks, P < 0.05 compared with control responses to the same ACh concentration
Effects of irradiation on outward currents in SMC
The next series of experiments was performed to compare outward K+-channel activity in control and irradiated thoracic aorta SMC. Isolated SMC from healthy rats were stimulated with increasing depolarizing voltage steps. The original traces and current–voltage (I/V) relationship are shown in Fig. 3a–e. The current density in intact SMC was 31 ± 2 pA/pF (n = 20) at +70 mV.
Fig. 3.
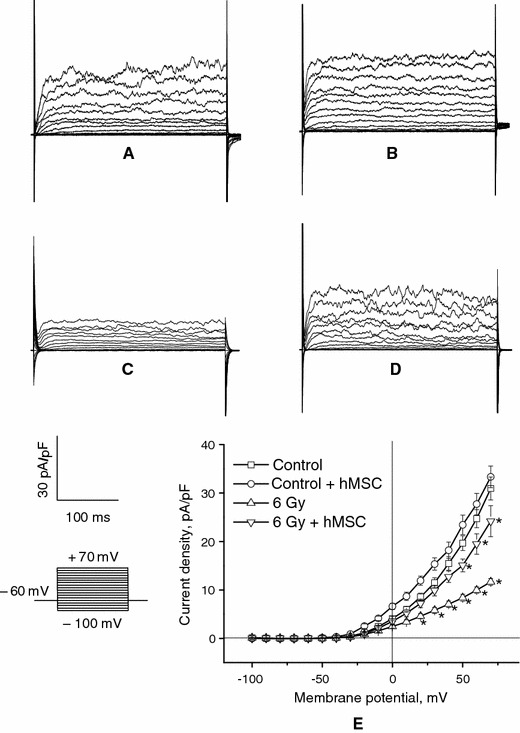
Effects of hMSC administration on outward K+ currents in a healthy SMC and SMC obtained on the 30th day after irradiation (6 Gy). Outward membrane currents were elicited by a series of 10-mV pulses of 300 ms duration to +70 mV from a holding potential of −60 mV. Families of outward K+ currents in intact (a, b) and irradiated (c, d) SMC before (a, c) and after (b, d) treatment with hMSC. e related I/V relationships. Asterisks P < 0.05 compared with control currents
The current–voltage relationship (I/V curve) demonstrates that outward currents in intact cells were absent at potentials below −40 mV and increased steeply in response to repeated more positive potentials. The I/V curve exhibited non-linear behavior at potentials above −40 mV and this can be more clearly seen at potentials more positive than +25 mV (Fig. 3e).
Outward currents in SMC obtained from irradiated animals 30 days after irradiation demonstrated a significant decrease in amplitude from 31 ± 2 to 12 ± 1 pA/pF at + 70 mV (n = 20, P < 0.05 vs. control values).
Effects of paxilline on outward currents in SMC before and after irradiation
To identify outward currents, paxilline (500 nM), a selective inhibitor of BKCa channels, was added to the external bathing solution. Paxilline sharply reduced outward current density from 31 ± 2 to 7 ± 0.4 pA/pF at +70 mV (P < 0.001, n = 20) in cells from control animals (Fig. 4), i.e. the paxilline-sensitive component of total current was equal to 24 ± 2 pA/pF.
Fig. 4.
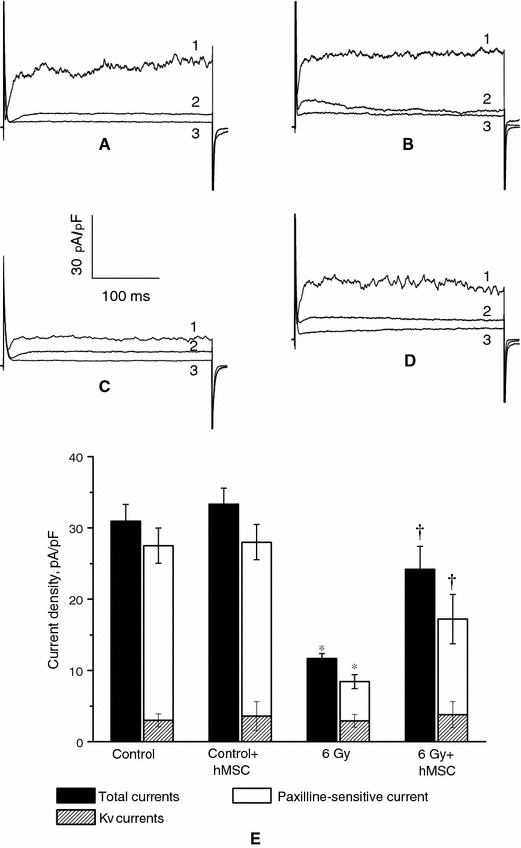
Effects of the BKCa blocker paxilline (500 nM) on outward potassium currents in thoracic aorta SMC obtained at a potential +70 mV from healthy and irradiated rats treated and not treated with hMSC. a, b Original traces for healthy cells before and after hMSC administration; c, d original traces for irradiated cells before and after hMSC administration (1 initial current, 2 current after paxilline treatment, 3 paxilline + tetraethylammonium in the external bath solution); e diagrams showing effects of paxilline and tetraethylammonium on outwards potassium currents in intact and irradiated SMC treated and not treated with hMSC. Asterisks P < 0.05 versus control; daggers P < 0.05, comparison between irradiated SMC and irradiated SMC treated with hMSC
The paxilline-sensitive component in SMC 30 days after irradiation was 5 ± 1 pA/pF only, suggesting the lack of BKCa channel conductance a month after exposure to the radiation. It is important to note that the value of residual paxilline non-sensitive current appeared to be increased compared with non-irradiated cells.
Effect of hMSC on endothelium-dependent vascular relaxation in intact and irradiated vascular tissues
As shown in Fig. 2, hMSC administration effectively restored the endothelium-dependent responses to ACh in irradiated vessels, i.e. the relaxation produced by ACh 30 days after irradiation was without significant differences compared with that measured in control vascular tissues (maximum relaxation in hMSC-treated SMC was 87 ± 3%, and pD2 6.8 ± 0.04 (n = 20, P > 0.05 vs. control values).
When administered to healthy animals, hMSC transplantation was without effect on ACh-induced vascular relaxation in rat thoracic aorta rings.
Effects of hMSC on BKCa channels before and after irradiation
Having established that hMSC effectively restored endothelium-dependent ACh-induced vascular relaxation damaged following irradiation, we next investigated the effect of hMSC on BKCa channels activity. It was shown (Fig. 3d) that outward current was increased by treatment with hMSC to 24 ± 1 pA/pF at +70 mV (n = 20, P < 0.05).
It is important to note that the treatment of irradiated rats with hMSC restored SMC response to paxilline (Fig. 4), i.e, the paxilline-sensitive component had increased to 11 ± 2 pA/pF. This was a twofold increment compared with irradiated cells in the absence of hMSC treatment.
Effect of hMSC administration on myofilament calcium sensitivity
To further investigate the effects of irradiation on aortic muscle cells contractility, we compared myofilament Ca2+ sensitivity in intact and irradiated aortic tissues treated and not treated with hMSC on the 30th day of the experimental period. Figure 5 shows that intact aortic tissue produced 0.022 ± 0.0022 mN/nM Ca2+ whereas smooth muscle obtained from irradiated tissues produced 0.064 ± 0.016 mN/nM Ca2+ (n = 20, P < 0.05). The slope of tension/[Ca2+]i relationship significantly increased in irradiated tissue compared with control, indicating that myofilament calcium sensitivity had increased following irradiation.
Fig. 5.
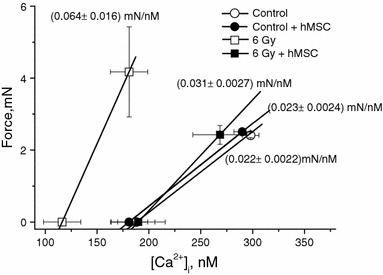
Effect of hMSC administration on tension/[Ca2+]i relationship (i.e. coefficient of myofilament Ca2+ sensitivity defined as the ratio of force change to Δ[Ca2+]i developed in response to arterenol, 10 μM) in rat thoracic aorta smooth muscle obtained from healthy and irradiated rats
hMCS administration effectively normalized increased myofilament Ca2+ sensitivity, the ratio force/[Ca2+]i became equal to 0.031 ± 0.0027 mN/nM Ca2+ (n = 20, P > 0.05 vs. control), and was without effect in healthy tissues.
Effect of hMSC on arterial blood pressure in irradiated rats
Non-invasive measurements of systolic blood pressure (BP) in irradiated rats showed that on the 30th day after irradiation BP had increased from 114 ± 2 in control animals to 152 ± 4 mmHg in irradiated rats not treated with hMSC (P < 0.05, n = 20). In contrast, in irradiated rats treated with hMSC BP was slightly increased at the end of experimental period compared with the control group 121 ± 2 mmHg (P < 0.05, n = 20) and appeared to be significantly reduced as compared with irradiated rats not treated with hMSC. There was no difference in BP in non-irradiated rats and non-irradiated rats treated with hMSC 114 ± 2 and 112 ± 3 mmHg, respectively (P > 0.05, n = 20) (Fig. 6).
Fig. 6.
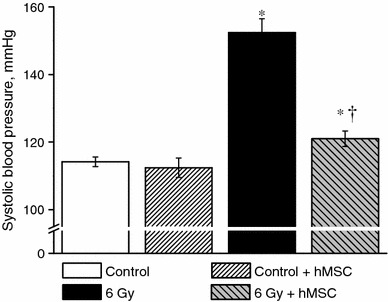
Effect of hMSC administration on arterial blood pressure in irradiated rats. Columns are mean ± SEM from 20 experiments. Asterisks P < 0.05 versus the control values, dagger P < 0.05, comparison between irradiated non-treated and irradiated rats treated with hMSC
Discussion
The main finding of this study is that intravenously administered hMSC were capable of treating vascular abnormalities produced in rats by ionizing irradiation. We have focused our efforts on functional evidence of hMSC effectiveness in irradiated rats using contractile recordings and electrophysiological studies.
We clearly understand that the main limitation of this paper is the absence of the proof of hMSC homing, i.e. mechanisms underlying hMSC engraftment and mechanical and electrical integration with host vascular tissues. This is a typical situation for a so-called “black box”. Our approach to a problem based on a “black box” concept: shifts in membrane potential and ACh (input signals) were applied to smooth muscle cells and endothelium, respectively, and related transmembrane currents and relaxation responses (output signals) were measured before and after irradiation and hMSC administration. Ionizing irradiation and hMSCs administration in this case are appear as a disturbing signals. This approach could not allow clarification of the precise cellular mechanisms of hMSC action but, nevertheless, in principle, enables confirmation or rejection of its possible therapeutic effectiveness after irradiation.
It is well known and has long been firmly established that functional integrity of the vascular endothelium is the main factor in normal blood perfusion, and that calcium-dependent potassium channels in vascular SMC are extremely important for normal vascular function. That is why we decided to base our final conclusion on above-mentioned criteria of vascular functional activity.
In any discussion of irradiation-induced vascular malfunction it is important to bear in mind the underlying mechanisms producing related alterations is multifaceted. Work from our own group has suggested that the loss of vasodilatory potential of the vascular wall under irradiation may be because of the depression of EDRF/NO and the endothelium-dependent component of vasorelaxation [1] and/or may be related to a decrease in large conductance Ca2+-activated K+ channel (BKCa) activity in endothelial cells [2]. Nevertheless, although endothelium is the most radiation-sensitive and vulnerable component of the vascular wall, the studies performed have shown that irradiation has the ability to enhance vascular contractility in an endothelium-independent manner [3, 15]. Another potential mechanism by which vascular tone could be enhanced is through changes in the transmembrane potential of the vascular SMC or myofilament calcium sensitivity.
It is generally accepted that potassium channels play an important role in regulation of the resting membrane potential of smooth muscle cells and, therefore, vascular contractility [16]. Membrane hyperpolarization/depolarization, caused by activation/inhibition of K+ channels leading to closure/opening of L-type voltage-dependent Ca2+ channels is one of the primary factors responsible for blood vessel relaxation/contraction. Although many different K+ channels exist in vascular smooth muscle one relatively important K+ channel is the BKCa channel.
Some years ago we also showed that radiation-induced endothelial dysfunction leads to a significant increase in systolic blood pressure in rats [17], most likely as a direct result of the increase in vascular tone. It is appropriate to mention here that BKCa channels may play a key role in blood pressure elevation, because mice deficient in the BKCa channel beta1 subunit gene showed increased blood pressure [18]. It has also been demonstrated that animals without the BKCa channel β1-subunit have increased mean arterial pressure [19], and that down-regulation of the BKCa channel β1-subunits occurs in rats made hypertensive by chronic exposure to angiotensin II [20]. These observations support the hypothesis that alterations in β1-subunit expression, function, or both, could contribute to the development of hypertension.
The data obtained clearly indicate that irradiation significantly suppresses K+ channel activity in SMC, suggesting that their contribution to vascular relaxation may be diminished following radiation impact. It is important to note that the paxilline-sensitive component of outward K+, i.e. current carried through BKCa channels, is the most vulnerable component of total outward current in SMC. Taking into account our previous experiments [2], in which we demonstrated radiation-induced BKCa suppression in endothelial cells, it became obvious that both of the two main vasorelaxation forces in the vascular wall (endothelium-dependent relaxation and relaxation of SMC related to membrane hyperpolarization) will be defective following radiation treatment.
Thus, relevant pharmacological intervention focused on repair of BKCa channels in the vascular wall and its renewal under irradiation impact may prevent unwanted vascular abnormalities and related cardiovascular disease development.
hMSC are known as a pluripotent cells that differentiate into a variety cells. Bone marrow-derived hMSC represent a stem cells population that can be isolated easily, expanded in culture greatly and efficiently, retain the ability to differentiate to several mesenchymal lineages, express genes encoding a broad spectrum of arteriogenic cytokines, promote in-vitro and in-vivo arteriogenesis, and home to sites of tissue damage [21]. For these reasons, hMSC might be appearing as an alternative for involvement in vascular therapy. However, little information is available regarding the therapeutic potency of systemically delivered hMSC for vascular malfunction related to excess of reactive oxygen species, for instance, under ionizing irradiation.
This study clearly demonstrates that hMSC, administered 7 days after irradiation, have the ability to normalize arterial blood pressure in irradiated rats and related endothelium-dependent vascular relaxation responses. It is seen also that outward potassium conductance in irradiated SMC has been restored as the result of hMSC treatment. It is important to stress that the effect of hMSC on outward current in irradiated SMC manifests as a twofold increase of the paxilline-sensitive component, i.e. current carried through BKCa channels.
A direct effect of irradiation on contractile machinery in vascular smooth muscle may be also important in the vascular anomalies induced by irradiation. The potential involvement of protein kinase C in the mechanism(s) responsible for enhanced myofilament Ca2+ sensitivity is well established. We have shown recently that smooth muscle Ca2+ sensitivity is increased following irradiation and this increment is the result of an increase in protein kinase C activity. Our experiments demonstrated that myofilament Ca2+ sensitivity is increased 9 days after irradiation, the earliest time point measured, and persisted over the 30 day experimental period [15].
This provides an attractive hypothesis to justify the enhanced force development observed in irradiated vascular tissues. The radiation-induced hypercontractility seen after irradiation can, therefore, be attributed to an increase in myofilament Ca2+ sensitivity. Simultaneous measurements of contractile force and [Ca2+]i in this study have confirmed that myofilament Ca2+ sensitivity, defined as the ratio of force change to Ca2+, significantly increased up to the 30th day post-irradiation and was completely normalized as the result of hMSC transplantation. It was a surprising discovery for us and one possible and provisional explanation for that phenomenon may be involvement of protein kinase C in proliferation and apoptosis of stem cells [22].
Molecular mechanisms of hMSC engrafting and their possible electrical and mechanical integration to host vascular tissue, and their contributions to functional recovery are not yet understood precisely. Of course, transplanted hMSC are expected to engraft and differentiate resulting in vascular tissue regeneration and functional repair. The question remains: how do they do it? Are they targeting smooth muscle and endothelial cells and homing in on radiation-induced injury or is their effectiveness a result of unknown indirect effects?
For a long period it was generally accepted that hMSC have the ability to improve heart or vascular function by transdifferentiating into myocytes and endothelial cells. However, other mechanisms that contribute to tissue renovation cannot be excluded. Within the last few years it has become evident that the effects of cell transplantation is in a large part mediated by paracrine effects on the host tissue [23, 24].The authors suppose that strategies to enhance the paracrine effects of cell transplantation may be employed in the next generations of technical approaches to cell therapy. The effect of hMSC in our study is thought to be mediated mainly in a paracrine manner through the paracrine action of growth factors secreted by hMSC. The mechanisms of this paracrine action hMSC remain unknown.
In this paper we have not presented direct morphological and/or immunohistochemical evidence of administered hMSC differentiation into vascular endothelial and smooth muscle cells, because we concentrated our efforts on physiological proof of their therapeutic potential. Nevertheless, taking into account the urgent need in cell therapy to assess the quality of the cells we have reported that the hMSC population we used for transplantation is free from hematopoietic cell contamination and contains its specific markers. It is important to note that PCR analysis showed that the hMSC used in our experiments were negative for hematopoietic cell markers (CD14, CD34, CD45) and positive for hMSC markers (CD29, CD44, CD71, CD73, CD90, CD105, CD166). In contrast with hMSC, bone marrow progenitors were positive for hematopoietic cell markers.
Thus, hMSC show distinct ability to restore endothelial function and BKCa activity in smooth muscle damaged by irradiation. It is important to stress that these positive cellular effects of hMSC have been confirmed by normalization of such integrative cardiovascular properties as arterial blood pressure. A statistically significant improvement of vascular functions clearly demonstrates the therapeutic potential of hMSC under ionized irradiation impact. This study can provide some perspectives for implementation of a new method for treatment of unwanted vascular malfunctions in patients with neoplasm under external beam radiation therapy.
It is important to note that when administered in control experiments to non-irradiated rats, hMSC lost their therapeutic efficacy. It means that hMSC are targeting injured tissue preferentially. Data presented by Mouiseddine et al. [25] clearly indicate that total body irradiation increased hMSC implantation in bone marrow and muscle and further led to engraftment in brain, heart, and liver.
We clearly understand that these results raise a host of additional ethical questions. It is clear that rats could serve as a model organism for non-clinical trials. Before anyone makes a new attempt at clinical use of human stem cells, common sense, prudence, and regulatory authorities require non-clinical trials with human stem cells in non-human animals. The result is likely to be a large number of human/non-human; chimerical cells. Nevertheless, we do hope that our studies will provide a novel platform from which basic non-clinical and clinical investigations can be launched to explore the fate and function of transplanted human hMSC in irradiated vascular tissues more precisely.
In conclusion, the hMSC administration has potential as a new therapeutic strategy for treatment of vascular malfunctions following ionizing irradiation impact, and may be considered as an alternative source of transplant cells to repair vascular tissues damaged by the action of oxidative stress and reactive oxygen species. We suppose that this study is a fundamental basis from which new basic, non-clinical and clinical investigations can be launched to explore the fate and function of administered hMSC in irradiated vascular tissues.
Acknowledgments
This study was supported by The Physiological Society “Centre of Excellence Award Scheme” 2009.
References
- 1.Soloviev A, Tishkin S, Parshikov A, Ivanova I, Goncharov E, Gurney A. Mechanisms of endothelial dysfunction after ionized radiation: selective impairment of the nitric oxide component of endothelium-dependent vasodilation. Br J Pharmacol. 2003;138:837–844. doi: 10.1038/sj.bjp.0705079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tishkin S, Rekalov V, Ivanova I, Moreland R, Soloviev A. Ionizing non-fatal whole-body irradiation inhibits Ca2+-dependent K+ channels in endothelial cells of coronary artery: possible contribution to depression of endothelium-dependent vascular relaxation. Int J Radiat Biol. 2007;83:161–169. doi: 10.1080/09553000601146931. [DOI] [PubMed] [Google Scholar]
- 3.Soloviev A, Tishkin S, Ivanova I, Zelensky S, Dosenko V, Kyrychenko S, Moreland R. Functional and molecular consequences of ionizing irradiation on large conductance Ca2+-activated K+ channels in rat aorta smooth muscle cells. Life Sci. 2009;84:164–171. doi: 10.1016/j.lfs.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Freedman S, Isner J. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med. 2002;136:54–71. doi: 10.7326/0003-4819-136-1-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto A, Gwon H, Iwaguro H, Jamaguchi J, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner J, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger M, Mackay A, Beck S, Jaiswal RK, Douglas R, Mosca J, Moorman M, Simonetti D, Craig S, Marshak D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Phinney D, Kopen G, Righter W, Webster S, Tremain N, Prockop D. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–436. doi: 10.1002/(SICI)1097-4644(19991201)75:3<424::AID-JCB8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Peister A, Mellad J, Larson B, Hall B, Gibson L, Prockop D. Adult stem cells from bone marrow isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 9.Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann N Y Acad Sci. 2003;996:152–157. doi: 10.1111/j.1749-6632.2003.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 10.LaBarge M, Blau H. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fibre in response to injury. Cell. 2002;111:589–601. doi: 10.1016/S0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 11.Kopen G, Prockop D, Phinney D. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 2003;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog F, Chai L, Krause D. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Rao M. Transdifferentiation––fact or artifact. J Cell Biochem. 2003;88:29–40. doi: 10.1002/jcb.10281. [DOI] [PubMed] [Google Scholar]
- 14.Song S, Sanchez-Ramos J. Brain as the sea of marrow. Exp Neurol. 2003;184:54–60. doi: 10.1016/S0014-4886(03)00306-6. [DOI] [PubMed] [Google Scholar]
- 15.Soloviev A, Tishkin S, Zelensky S, et al. Ionizing radiation alters myofilament calcium sensitivity in vascular smooth muscle: potential role of protein kinase C. Am J Physiol Regul Integr Comp Physiol. 2005;289:R762–R775. doi: 10.1152/ajpregu.00748.2004. [DOI] [PubMed] [Google Scholar]
- 16.Jackson W, Blair K. Characterization and function of Ca2+-activated K+ channels in arteriolar muscle cells. Am J Physiol Heart Circ Physiol) 1998;274:H27–H34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- 17.Soloviev A, Stefanov A, Tishkin S, Khromov A, Parshikov A, Ivanova I, Gurney A. Saline containing phosphatidylcholine liposomes possess the ability to restore endothelial function damaged resulting from gamma-radiation. J Physiol Pharmacol. 2002;53:701–712. [PubMed] [Google Scholar]
- 18.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollash M, et al. Mice with disrupted BK channel beta 1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res. 2002;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 19.Brenner R, Perez G, Bonev A, Eckman D, Kosek J, Wiler S, Patterson A, Nelson M, Aldrich R. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 20.Amberg G, Bonev A, Rossow C, Nelson M, Santana L. Modulation of the molecular composition of large conductance Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnaird T, Stabile E, Burnett M, Lee C, Barr S, Fuchs S, Epstein S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 22.Quinlan LR, Faherty S, Kane MT. Phospholipase C and protein kinase C involvement in mouse embryonic stem cell proliferation and apoptosis. Reproduction. 2003;126:121–131. doi: 10.1530/rep.0.1260121. [DOI] [PubMed] [Google Scholar]
- 23.Chehg AS, Yau TM. Paracrine effect of cell transplantation: strategies to augment the efficacy of cell therapies. Semin Thorac Cardiovasc Surg. 2008;20(2):94–101. doi: 10.1053/j.semtcvs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Xu RX, Chen X, Chen JH, Han Y, Han BM. Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol Physiol. 2009;36(2):176–180. doi: 10.1111/j.1440-1681.2008.05041.x. [DOI] [PubMed] [Google Scholar]
- 25.Mouiseddine M, Flancois S, Semont A, Sache A, Allenet B, Mathiev N, Frick J, Therry D, Chapel A. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80:549–555. doi: 10.1259/bjr/25927054. [DOI] [PubMed] [Google Scholar]


