Abstract
Although “teeth clenching” induces pressor response, the reflex tracts of the response are unknown. In this study, dantrolene administration inhibited teeth clenching generated by electrical stimulation of the masseter muscles and completely abolished the pressor response. In addition, trigeminal ganglion block or hexamethonium administration completely abolished the pressor response. Local anesthesia of molar regions significantly reduced the pressor response to 27 ± 10%. Gadolinium (mechanoreceptor blocker of group III muscle afferents) entrapment in masticatory muscles also significantly reduced the pressor response to 62 ± 7%. Although atropine methyl nitrate administration did not change the pressor response, a significant dose-dependent augmentation of heart rate was observed. These results indicate that both periodontal membrane and mechanoreceptors in masticatory muscles are the receptors for the pressor response, and that the afferent and efferent pathways of the pressor response pass through the trigeminal afferent nerves and sympathetic nerves, respectively.
Keywords: Teeth clenching, Pressor response, Periodontal membrane, Mechanoreceptor, Trigeminal afferent nerve, Sympathetic nerve
Introduction
It is known that teeth clenching or mastication induces a variety of physiological responses related to circulatory alterations, particularly increasing cerebral blood flow and oxygen levels in the brain [1–4]. Hirano et al. [5] showed that chewing exercise significantly increases blood oxygen levels in the middle frontal gyrus, right premotor cortex, precuneus, thalamus, hippocampus, and inferior parietal lobe in humans. Kawanishi et al. [6] demonstrated that a solid diet is more effective for functional recovery than a liquid diet in rats with ischemic brain injury after permanent middle cerebral artery occlusion. Based on these previous studies, we propose that increases in cerebral blood flow and oxygen level in the brain during jaw movement are caused by the pressor response.
The pressor response induced by teeth clenching has been reported previously. Ferella et al. [7] reported that arterial pressure (AP) and heart rate (HR) were significantly increased in parallel with the activity of the masticatory muscles in humans. Ishiyama and Suzuki [8] showed that AP, HR, and cerebral blood flow were significantly increased by masticatory exercise in humans, and presumed that the pressor response was induced by the interaction between sympathetic and parasympathetic nerve activation. Moreover, Okada et al. [9] reported that local anesthesia of molar regions attenuated the teeth clenching-induced pressor response in humans, and suggested that the sensory system of the periodontal tissue was involved in the pressor response. Based on these results, it has been confirmed that the pressor response occurs during teeth clenching exercise. However, the pressor response mechanism of teeth clenching has not yet been elucidated. Consequently, we aimed to elucidate the reflex arc of the pressor response. We hypothesized that the pressor response is induced by a neural reflex, and that both the periodontal membrane and mechanoreceptors in masticatory muscles are the pressor receptors, while the pressor response is mediated by the trigeminal afferent nerves (afferent pathway) and the autonomic nerves (efferent pathway). Consequently, we confirmed the effects of ryanodine receptor inhibition, sinoaortic denervation (SAD), local anesthesia of molar regions, block of group III muscle afferents in masticatory muscles, trigeminal ganglion block (TRG-block), and autonomic ganglion block or muscarinic receptor block on teeth clenching-induced pressor response.
Methods
Experimental subjects
One hundred and eleven male Sprague–Dawley rats (age 12–16 weeks, weight 423 ± 34 g; Japan SLC, Hamamatsu, Shizuoka, Japan) were randomly divided into eight groups for the following procedures: varying the stimulus frequency (n = 5 rats), dantrolene sodium (ryanodine receptor inhibitor, muscle relaxant) administration (n = 5 rats), SAD (n = 11 rats), local anesthesia of molar regions (n = 17 rats), gadolinium chloride entrapment in masticatory muscles (n = 23 rats), trigeminal ganglion block (TRG-block) (n = 21 rats), and hexamethonium chloride (n = 22 rats) or atropine methyl nitrate administration (n = 7 rats). All experimental procedures were performed according to the Guidelines for Proper Conduct of Animal Experiments issued by the Science Council of Japan. The experimental protocols of the present study were also reviewed and approved by the committee of Research Facilities for Laboratory Animal Science, National Defense Medical College (approval numbers: 12086 and 15046).
Equipment and procedures
Rats were anesthetized with urethane (1.2 g kg−1, intraperitoneal; Sigma–Aldrich, St. Louis, MI, USA). With a microscope, an arterial catheter (PE-50; Becton–Dickinson, Franklin Lakes, NJ, USA) was inserted into the left femoral artery to measure AP, and a venous catheter (PE-10; Becton–Dickinson) was inserted into the left femoral vein for administration of additional anesthetics or drugs. All catheters were heparinized (2 U ml−1). Electrodes (EKC-20VSBL; Eiko-Kagaku, Tokyo, Japan) were placed subcutaneously at the midchest region to record electrocardiograms (ECG). Stimulating electrodes were also fixed on the bilateral masseter muscles to induce teeth clenching movement (except for the masseter muscle stretch test and the vasomotor response test). A 0.65-mm-thick pressure sensor (PSM-type; Kyowa Electronic Instruments, Tokyo, Japan) was placed on the right lower molar teeth of the rat to measure the force of teeth clenching. The arterial catheter was connected to a pressor transducer (DX-360; Nihon Kohden, Tokyo, Japan). AP and ECG were recorded using amplifiers of the polygraph system (AP-641G for AP, AB601G for ECG, Nihon Kohden). HR was measured with the R wave of the electrocardiogram as the trigger using a rate meter (AT601G; Nihon Kohden). A pressor sensor to detect the force of teeth clenching was connected to a bioamplifier (AP601G; Nihon Kohden). All analog signals were digitalized using an analog–digital converter (PowerLab; ADInstruments, Dunedin, New Zealand). Digitalized values were then obtained at a sampling rate of 1000 samples per second. The observed values and stimulation signals were displayed and recorded on a personal computer (Satellite223; Toshiba, Tokyo, Japan) and recorded on a thermal arraycorder (WR1000; Graphtec, Kanagawa, Japan). Teeth clenching was generated by electrical stimulation of the bilateral masseter muscles of rats at 18–20 mA using electrical stimulators (Electronic stimulator and Isolator SS-102J; Nihon Kohden). Electrical stimulation of the masseter muscles was delivered for periods of 30 s at 1 Hz using a 0.4-s pulse duration (except for stimulus frequency experiment). Stimulation induced a clenching force of about 4 N.
Varying the stimulus frequency
Electrical stimulation was randomly applied to the bilateral masseter muscles of five rats at a frequency of 0.2, 0.25, 0.33, 0.5, or 1.0 Hz or continuously for periods of 30 s. Continuous electrical stimulation was applied for periods of 30 s at 50 Hz using 1-ms pulse durations. We then compared changes in mean arterial pressure (ΔMAP) and HR (ΔHR) in response to electrical stimulation of the bilateral masseter muscles at various frequencies to confirm the most effective frequency as a pressor response.
Dantrolene sodium administration
Five rats were administered dantrolene sodium (6 mg kg−1; Astellas Phama, Tokyo, Japan) intravenously to inhibit the muscle contraction during the electrical stimulation of masseter muscles. Dantrolene sodium is a postsynaptic skeletal muscle relaxant that abolishes the excitation–contraction coupling by inhibiting the release of calcium ions via an action on the ryanodine receptor of the skeletal muscle sarcoplasmic reticulum [10]. Several researchers have reported that 10–30 mg kg−1 dantrolene sodium administration has little effect on circulation or respiration [11–13]. We administered the minimum dose of dantrolene sodium to inhibit muscle contraction.
SAD
We performed SAD (n = 6 rats) in accordance with previous studies [14, 15]. An incision was made on the cervical midline, and the aortic nerves were cut bilaterally. The carotid sinuses were completely isolated from the surrounding tissue, and a 5% phenol solution was applied. After completion of recording, 0.1 ml of phenylephrine hydrochloride (40 μg ml−1; Sigma-Aldrich) was administered intravenously to confirm no baroreflexive HR response with temporal hypertension. In sham-SAD rats (n = 5 rats), we performed an incision on the cervical midline and the isolation of bilateral carotid sinuses, but did not sever the nerves.
Local anesthesia of molar regions
Into the bilateral upper and lower molar regions of rats, 0.1 ml of 0.4% (n = 6 rats) or 0.8% lidocaine (Xylocaine, Astra Zeneca, Osaka, Japan, n = 5 rats) was injected, respectively. Meanwhile, in control rats (n = 6 rats), 0.1 ml of saline was injected into the same points.
Gadolinium chloride entrapment in masticatory muscles
A catheter (PE-50, Becton–Dickinson) was inserted into the right ventricle through the right external jugular vein of rats, and as previously reported [16–20], gadolinium chloride HEPES-buffered solution (20 mM, 2.1 ml kg−1, pH 7.3–7.4, Sigma–Aldrich, n = 12 rats) or HEPES-buffered solution (2.1 ml·kg−1, pH 7.3-7.4, n = 5 rats) was administered into the right ventricle of the rats. Subsequently, the right and left external jugular veins were ligated for 10 min to trap the solution in the bilateral masticatory muscles. In the masseter muscle stretch test (n = 6 rats), which was conducted to confirm the effect of gadolinium entrapment, the unilateral masseter muscle was cut at the distal side and stretched at 1000 g-tension for 2 min before and after entrapment of the gadolinium chloride HEPES-buffered solution (20 mM, 2.1 ml kg−1, pH 7.3–7.4) in the bilateral masticatory muscles. Hayes and Kaufman [17] reported that entrapment of gadolinium chloride (5 mM, 1 ml) in the lower limbs attenuated pressor responses to contraction and to tendon stretch; however, higher concentrations (10–25 mM, 1 ml) caused sufficient attenuation of the responses in both decerebrate and α-chloralose-anesthetized cats. Furthermore, Nakamoto and Matsukawa [19] showed that the entrapment of gadolinium chloride HEPES-buffered solution (20 mM, 2.1 ± 0.3 ml kg−1, pH 7.3–7.4) in the lower limbs blunted the increases in mean arterial pressure (MAP) to 74–78% in anesthetized rats. Based on these previous studies, we determined the dose of gadolinium chloride in the experiment.
TRG-block
Rats were fixed on the stereotaxic apparatus (SR-6R; Narishige, Tokyo, Japan), and two holes of approx. 1 mm in diameter were formed at the surface of the parietal bone. Then, cannulae for implantation in the brain (C315ISPCL/C315GSPCL; Plastic One, Roanoke, VA, USA) were inserted into the right and left trigeminal ganglia (TRG) in accordance with the description of Paxinos and Watson’s rat brain atlas [21]. Subsequently, in the TRG-block rats, 1 μl of 2% (n = 8 rats) or 8% lidocaine (n = 7 rats) was injected into the bilateral trigeminal ganglia. Meanwhile, the control rats (n = 6 rats) were injected with 1 μl of saline.
Hexamethonium chloride administration
A venous catheter (PE-10; Becton–Dickinson) was inserted into the right femoral vein. Then, through the venous catheter, the continuous infusion of phenylephrine hydrochloride (4–5 μg kg−1 ·min−1, or 12–13 μg kg−1 min−1) was started at the intravenous administration of hexamethonium chloride (1 or 10 mg kg−1; MP Biochemicals, Santa Ana, CA, USA) (each group, n = 8 rats) using a syringe pump (Fusion 200; CHEMYX, Stafford, TX, USA) to maintain AP. We then compared ΔMAP or ΔHR in response to electrical stimulation of the bilateral masseter muscles of rats before and at 10 min after hexamethonium administration. In the vasomotor response test (n = 6 rats), continuous infusion of phenylephrine hydrochloride (12–13 μg kg−1 min−1) was started at hexamethonium chloride administration (10 mg kg−1) to maintain AP. Subsequently, we compared ΔMAP before and 10 min after hexamethonium administration at a bolus injection of phenylephrine (40 μg ml−1, 0.1 ml) to confirm the contractility changes of the systemic arteriole.
Atropine methyl nitrate administration
Seven rats were administered atropine methyl nitrate (ChromaDEX, Irvine, CA, USA) at doses of 10, 100, and 1000 μg kg−1 intravenously at 30-min intervals. We then compared ΔMAP and ΔHR in response to electrical stimulation of the bilateral masseter muscles before and at 10 min after each administration of atropine methyl nitrate.
Statistical analysis
All measured values are expressed as mean ± SE. The statistical significance of observed changes was assessed using the paired or unpaired Student’s t test, or analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test. Significant differences were determined at p < 0.05.
Results
There were no significant differences in baseline MAP or HR in any of the experimental groups in the present study (Table 1). However, SAD treatment significantly increased baseline MAP from 90 ± 4 to 99 ± 4 mmHg (p < 0.01) (Fig. 4a).
Table 1.
Baseline MAP and HR in the experiments
| Protocol | n | MAP (mmHg) | HR (beats/min) |
|---|---|---|---|
| Stimulus frequency | 5 | 91 ± 4 | 397 ± 8 |
| Dantrolene sodium | 5 | 101 ± 1 | 410 ± 10 |
| SAD | |||
| Sham | 5 | 88 ± 2 | 404 ± 14 |
| SAD | 6 | 99 ± 4 | 382 ± 16 |
| Local anesthesia of molar regions | |||
| Vehicle (saline) | 6 | 85 ± 5 | 384 ± 14 |
| 0.4% Lidocaine | 6 | 82 ± 3 | 356 ± 16 |
| 0.8% Lidocaine | 5 | 90 ± 2 | 369 ± 11 |
| Gadolinium chloride entrapment in masticatory muscles | |||
| Vehicle (HEPES-buffered solution) | 5 | 93 ± 2 | 358 ± 7 |
| Gadolinium chloride | 12 | 91 ± 3 | 358 ± 12 |
| Masseter muscle stretch test | 6 | 86 ± 3 | 379 ± 9 |
| TRG-block | |||
| Vehicle (saline) | 6 | 90 ± 4 | 392 ± 14 |
| 2% Lidocaine | 8 | 94 ± 4 | 393 ± 14 |
| 8% Lidocaine | 7 | 95 ± 2 | 374 ± 13 |
| Hexamethonium chloride | |||
| 1 mg kg−1, iv | 8 | 93 ± 3 | 393 ± 8 |
| 10 mg kg−1, iv | 8 | 91 ± 3 | 379 ± 8 |
| Vasomotor response test | 6 | 87 ± 2 | 403 ± 23 |
| Atropine methyl nitrate | 7 | 90 ± 2 | 427 ± 5 |
All values are expressed as mean ± SE. Baseline MAP and HR were measured and averaged for 30 s in the experiments
n number of rats, MAP mean arterial pressure, HR heart rate, SAD sinoaortic denervation, TRG-block trigeminal ganglion block
Fig. 4.
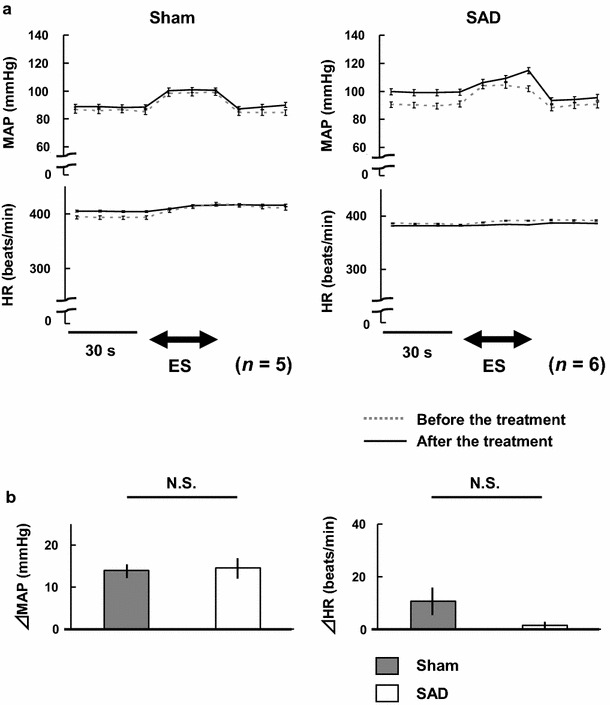
a Comparisons of mean arterial pressure (MAP) and heart rate (HR) in response to electrical stimulation of the bilateral masseter muscles before and at 30 min after sham-operation (n = 5) or bilateral sinoaortic denervation (SAD) (n = 6) in rats. ES electrical stimulation. b Comparisons of changes in MAP (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles in sham and SAD rats. N.S. not statistically significant (unpaired Student’s t test)
Figure 1 shows the results of the stimulus frequency experiment. Pressor response tended to increase with stimulus frequency. However, there were no significant differences in ΔHR. The results showed that 1.0 Hz is the most effective frequency of electrical stimulation for the pressor response. Figure 2 shows representative recordings of AP and HR in a rat in response to electrical stimulation of the bilateral masseter muscles at 1.0 Hz.
Fig. 1.
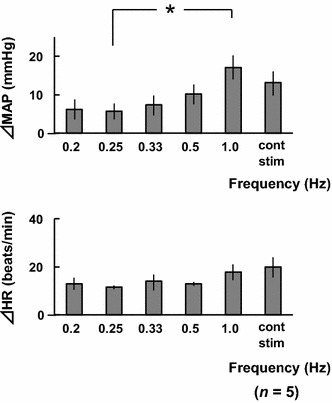
Comparisons of changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles at various frequencies in five rats. cont stim continuous electrical stimulation. *p < 0.05 (one-way ANOVA, followed by the Tukey–Kramer post hoc test)
Fig. 2.
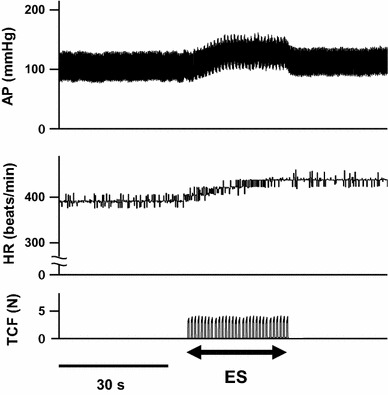
Typical recordings of arterial pressure (AP) and heart rate (HR) of rats in response to electrical stimulation of the bilateral masseter muscles at 1 Hz. TCF teeth clenching force expressed in Newtons (N), ES electrical stimulation
Figure 3 shows the results of the dantrolene sodium administration experiment. Dantrolene sodium almost completely suppressed the masticatory muscle contraction (Fig. 3a), and completely abolished the pressor response (Fig. 3b). However, there were no significant differences in ΔHR.
Fig. 3.
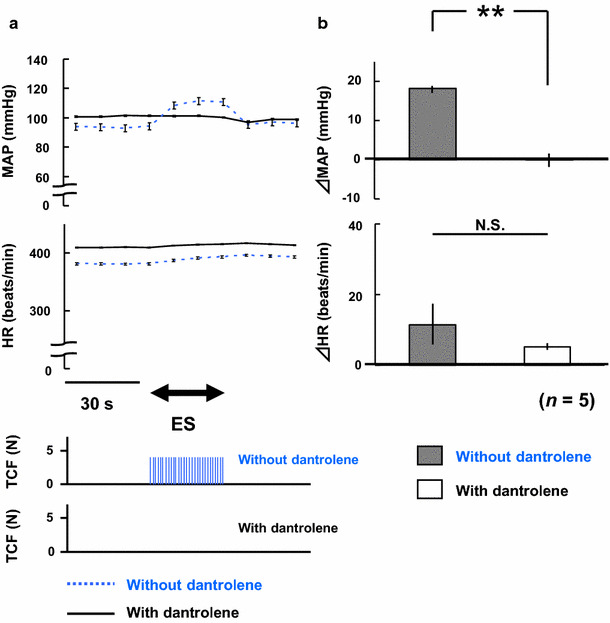
a Comparisons of mean arterial pressure (MAP) and heart rate (HR) in response to electrical stimulation of the bilateral masseter muscles before and at 10 min after dantrolene sodium administration in five rats. ES electrical stimulation, TCF teeth clenching force expressed in Newtons (N). b Comparisons of changes in MAP (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles before and at 10 min after dantrolene sodium administration in five rats. **p < 0.01, N.S. not statistically significant (paired Student’s t test)
Figure 4 shows the results of the SAD experiment. SAD treatment significantly increased baseline MAP. However, it did not enhance the pressor response (Fig. 4a). There were no significant differences in the pressor response between sham-operation and SAD treatment (Fig. 4b). There were also no significant differences in ΔHR.
Figure 5 shows the results of the local anesthesia of molar regions. In the 0.4% lidocaine group, the pressor response was significantly reduced to 27 ± 23% at 30 min after treatment. However, there were no significant differences in ΔHR. In the 0.8% lidocaine group, the pressor response was also significantly reduced to 27 ± 10% at 30 min after treatment. However, there were no significant differences in ΔHR. In the control group, there were no significant differences in ΔMAP or ΔHR.
Fig. 5.
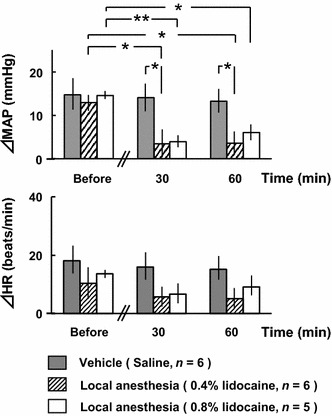
Comparisons of changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles before and after administration of local anesthesia to the bilateral upper and lower molar regions. *p < 0.05, **p < 0.01 (two-way ANOVA, followed by the Tukey–Kramer post hoc test)
Figure 6a shows the results of the gadolinium chloride entrapment in masticatory muscles experiment. In the gadolinium chloride group, the pressor response was significantly reduced to 62 ± 7% of the non–trapped state at 30 min post-entrapment. However, there were no significant differences in ΔHR. Furthermore, there were no significant differences in the teeth clenching force of the rats before and 30, 60, and 90 min after the gadolinium chloride entrapment (3.8 ± 0.3 vs. 3.9 ± 0.3 vs. 4.1 ± 0.3 vs. 4.0 ± 0.2 N). On the other hand, in the control group, there were no significant differences in ΔMAP or ΔHR. In the masseter muscle stretch test, the pressor response during the masseter muscle stretch was significantly reduced to 21 ± 8% at 30 min post-entrapment (Fig. 6b). However, there were no significant differences in ΔHR.
Fig. 6.
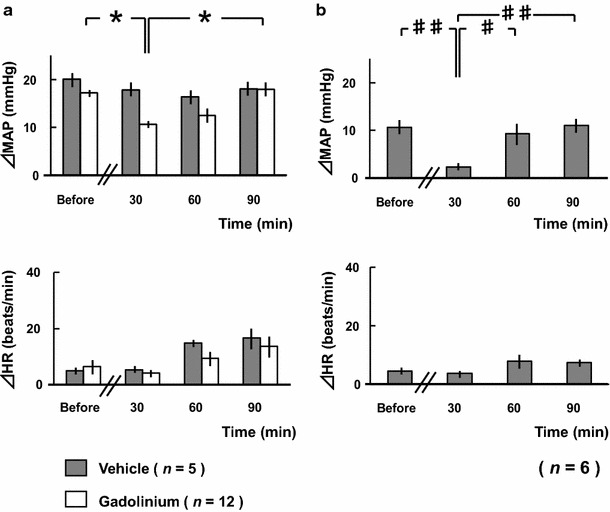
a Comparisons of changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles before and after entrapment of gadolinium chloride in masticatory muscles. *p < 0.05 (two-way ANOVA, followed by the Tukey–Kramer post hoc test). b Comparisons of ΔMAP and ΔHR in response to stretching of the unilateral masseter muscles before and after gadolinium chloride entrapment in masticatory muscles in six rats (the verification test for the effect of gadolinium chloride to the mechanoreceptors in masticatory muscles). # p < 0.05, ## p < 0.01 (one-way ANOVA, followed by the Tukey–Kramer post hoc test)
Figure 7 shows the results of the TRG-block experiment. In the 2% lidocaine group, the pressor response was significantly reduced to 26 ± 9% at 10 min post-lidocaine injection. However, there were no significant differences in ΔHR. In the 8% lidocaine group, the pressor response was completely abolished at 10 min post-lidocaine injection. Furthermore, ΔHR was also almost completely abolished at 10 min post-lidocaine injection. On the other hand, in control group, there were no significant differences in ΔMAP or ΔHR. In order to confirm the spread of the injected solution into the trigeminal ganglia, after completion of recording, 1 μl of hematoxylin solution (Mayers hematoxylin solution; Wako Chemicals, Osaka, Japan) was bilaterally injected through each cannula. The ganglia were then enucleated to observe the solution spread in the ganglion area (Fig. 8).
Fig. 7.
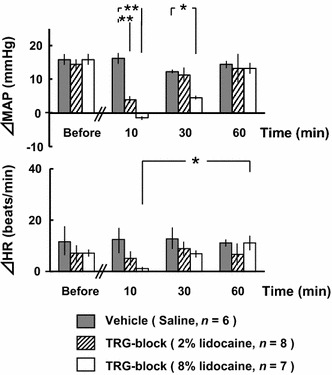
Comparisons of changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles before and after the bilateral trigeminal ganglia block (TRG-block). *p < 0.05, **p < 0.01 (two-way ANOVA, followed by the Tukey–Kramer post hoc test)
Fig. 8.
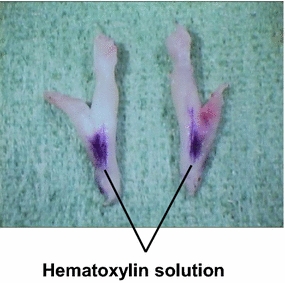
Spread of hematoxylin solution on the surfaces of trigeminal ganglia enucleated after completion of recording
Figure 9 shows the results of the hexamethonium chloride administration experiment. In the hexamethonium chloride (1 mg kg−1) group, the pressor response was significantly reduced to 31 ± 11% at 10 min post-hexamethonium administration. However, there were no significant differences in ΔHR (Fig. 9a). On the other hand, in the hexamethonium chloride (10 mg kg−1) group, the pressor response was completely abolished at 10 min post-hexamethonium administration. Furthermore, ΔHR was also almost completely abolished at 10 min post-hexamethonium administration (Fig. 9b). In the vasomotor response test, there were no significant differences in the increase in MAP with bolus injection of phenylephrine hydrochloride (40 μg ml−1, 0.1 ml) before and at 10 min post-administration of hexamethonium chloride (10 mg kg−1) under the continuous intravenous administration of phenylephrine hydrochloride (Fig. 9c, d). However, baroreflexive HR response was significantly attenuated at 10 min post-administration of hexamethonium chloride. These results show that there were no changes in vasomotor activity, even in the hexamethonium—(10 mg kg−1) and phenylephrine infused rats.
Fig. 9.
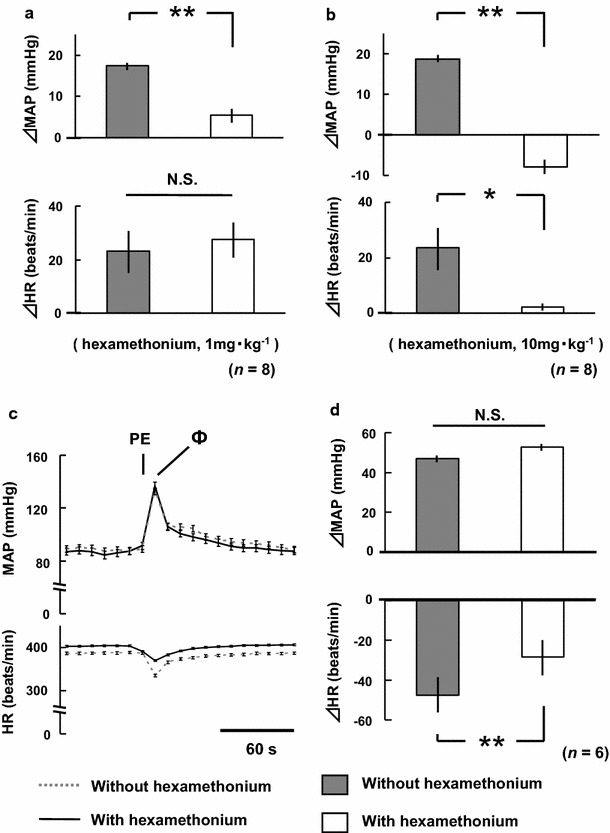
a, b Comparisons of changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles before and at 10 min after hexamethonium chloride administration (1, 10 mg kg−1, each n = 8). Decreases in basal MAP in response to hexamethonium administration were compensated to control levels by the continuous infusion of phenylephrine hydrochloride (see “Methods”). c, d Comparisons of MAP and HR following phenylephrine hydrochloride (40 μg ml−1, 0.1 ml) injection before and at 10 min after hexamethonium chloride (10 mg kg−1) administration, and the comparisons of ΔMAP and ΔHR at the peak pressor response in six rats (the vasomotor response test). Decreases in basal MAP in response to hexamethonium administration were compensated to control levels by the continuous infusion of phenylephrine (see “Methods”). PE additional test administration of phenylephrine hydrochloride, Ф peak of the pressor response following phenylephrine hydrochloride injection, *p < 0.05, **p < 0.01, N.S. not statistically significant (paired Student’s t test)
Figure 10 shows the results of the atropine methyl nitrate administration experiment. There were no significant differences in ΔMAP, regardless of the variations in the dose of atropine methyl nitrate. However, ΔHR increased significantly in proportion to the dose of atropine methyl nitrate.
Fig. 10.
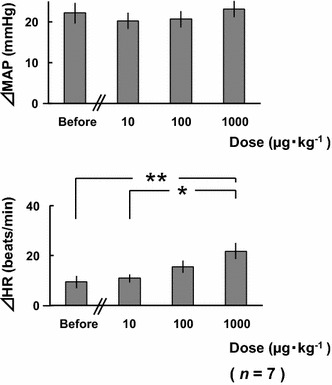
Comparisons of changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) in response to electrical stimulation of the bilateral masseter muscles before and at 10 min after atropine methyl nitrate (10–1000 μg kg−1) administration in seven rats. *p < 0.05, **p < 0.01 (one-way ANOVA, followed by the Tukey–Kramer post hoc test)
Discussion
Teeth clenching and muscle contraction
In the present study, the pressor response was dependent on the teeth clenching force generated by electrical stimulation of masseter muscles. However, the pressor response was abolished by muscle relaxation with dantrolene sodium. These results indicate that muscle contraction and the teeth clenching force are necessary for the pressor response, and other electrical effects scarcely contribute to the pressor response. Based on these results, we proposed that the contraction of masticatory muscles directly causes a pressor response that is proportionate to the teeth clenching force and have designated this response the “teeth clenching-induced pressor response” [22–25]. The neural pathways or the mechanisms of the pressor response have not yet been elucidated. In the present study, we aimed to analyze the reflex arcs, the neural mechanisms and the reflex characteristics of the pressor response.
Before analyzing the neural reflexive tracts, we determined the most effective frequency for electrical stimulation of masseter muscles. Figure 1 shows a comparison of the increases in MAP in response to various stimulus frequencies, and the most effective frequency was 1 Hz. Based on our previous studies [22, 23], we used the most effective electrical stimulation (18–20 mA, 1 Hz) for muscle contraction to analyze the neural reflexive pathways.
Exercise pressor response
Static contraction of skeletal muscles is known to evoke efferent sympathetic responses, resulting in what is termed the exercise pressor response [26–29]. It is presumed that the afferent pathway of the exercise pressor response may be group III and group IV afferent nerve fibers [30]. The terminals of group III fibers are mainly located in the adventitia of arteries, and the terminals of group IV fibers are also located in vascular wall, endoneurium, and perimysium in skeletal muscles [31]. Hayes and Kaufman [17] showed that the entrapment of gadolinium, as a mechanoreceptor blocker of group III muscle afferents in the lower limbs, significantly attenuated the exercise pressor reflex during static contraction of triceps surae muscle or stretching of calcaneal (Achilles) tendon, and that gadolinium had no effect on the responses of group IV muscle afferents, many of which are metabolically sensitive, in both decerebrate and α-chloralose-anesthetized cats. Group III muscle afferents, many of which are mechanically sensitive, terminate within mainly the surrounding tissues of vessels around the myotendinous junctions of skeletal muscles [31–35]. Nakamoto and Matsukawa [19] showed that systemic injection of gadolinium or lidocaine injection into the myotendinous junction of triceps surae muscle blunted the stretch-induced increases in HR and MAP in anesthetized rats. These observations suggest that group III muscle afferents primarily monitor mechanical events during muscle exercise. However, Matsukawa et al. [18] showed that intravenous administration of gadolinium affected neither the baseline values nor the initial increases of HR and MAP at the onset of voluntary static exercise in conscious cats, and concluded that the initial cardiovascular adaptation at the onset of voluntary exercise is predominantly induced by feed-forward control of central command descending from higher brain centers, but not by a muscle mechanoreflex. It is likely that feed-forward regulation of the cardiovascular system is somewhat involved with teeth clenching-induced pressor response in conscious rats. Abe et al. [36] showed that intravenous administration of gadolinium significantly reduced the water drinking-induced pressor response to approx. 48% and also significantly reduced the pressor response by electrical stimulation to the hypoglossal nerve to approx. 43% in conscious rats. Based on these studies, we blocked the muscle mechanoreceptors using gadolinium and assessed the teeth clenching-induced pressor response. Gadolinium suppressed the pressor response to approx. 62%. We therefore propose that the pressor response is partly evoked by muscle mechanoreceptors.
Receptors and afferent pathway of the pressor response
The present study showed that the pressor response was completely abolished by dantrolene administration or TRG-block (Figs. 3, 7, respectively). These results indicate that the pressor response originates in the muscle contraction and propagates through the trigeminal afferent nerves. This evidence suggests that the stimuli of teeth clenching exercise could be input to the muscle mechanosensitive receptors or chemoreceptors [18, 19, 26, 30, 31, 36], and pressure on the teeth may be sensed by periodontal membrane [37–39]. We blocked neural activity in periodontal membrane with local anesthesia (0.4 or 0.8% lidocaine), resulting in approx. 73% blocking of the pressor response. The blocking effects were not enhanced with a higher concentration of lidocaine. These results indicate that the afferent fibers from the molar regions are responsible for the pressor response. We then blocked the mechanoreceptors in masticatory muscles with gadolinium, resulting in approx. 38% blocking of the pressor response. We confirmed the effect of gadolinium chloride by the masseter muscle stretch test. These results indicate that teeth clenching stimulates periodontal membrane, and the mechanoreceptors in masticatory muscles, and deliver afferent signals through the trigeminal afferent nerves to the cardiovascular center in brain. We believe that the trigeminal efferent nerves (motor nerves of the masticatory muscles) were not responsible for the pressor response, because rats were anesthetized and the voluntary jaw movements completely disappeared.
Efferent pathway of the pressor response
We next aimed to determine the efferent arc of the pressor response. Autonomic ganglion block with 10 mg kg−1 hexamethonium completely abolished both the pressor and HR responses (Fig. 9b). Hexamethonium did not reduce α1 receptor-mediated vasomotor response (Fig. 9d). These results indicate that the efferent pathway for the pressor response is mediated through sympathetic nerves.
In contrast to the pressor response, the HR response to teeth clenching appeared somewhat complicated. Teeth clenching did not significantly affect HR (Fig. 1). Dantrolene administration or local anesthesia of the molar regions did not produce significant changes in ΔHR. However, both the TRG-block and the autonomic ganglion block completely abolished the teeth clenching-induced HR response (Figs. 7, 9b, respectively), showing that the interception of afferent or efferent tract abolishes the HR responses. These results indicate that teeth clenching activates the afferent tracts of the reflex and that the efferent responses are mediated through the autonomic nervous system. To clarify the effects of the parasympathetic efferent pathway, we blocked the muscarinic receptors using atropine methyl nitrate, resulting in the significant increases in HR (Fig. 10). We therefore conclude that teeth clenching may activate both the sympathetic and parasympathetic pathways, and the HR response is dependent on the predominance of the sympathetic or parasympathetic nerve activity.
Baroreflex function on the pressor response
To avoid baroreflex-mediated suppression of the pressor response, we bilaterally denervated the sinoaortic nerves. However, SAD did not enhance the pressor response. The data showed that the baroreflex does not suppress the teeth clenching-induced pressor response. Although further examinations are needed, the pressor response might not be suppressed by the baroreflex. The baseline MAP and the increases in MAP were within the operating range of the normal baroreflex. This result indicates that the baroreflex did not function during teeth clenching exercise. While we did not analyze the mechanism of the baroreflex suppression, there are several pertinent examples. It is known that resetting the operating point of the baroreflex to a higher pressure induces increased AP during exercise [40–44]. On the other hand, various diseases, such as hypertension, cardiovascular disease, obesity, and obstructive sleep apnea, decrease baroreflex sensitivity [45–49]. We speculate that the teeth clenching-induced pressor response might not disrupt the blood pressure control system in brain.
Central tracts of the pressor response
Trigeminal mesencephalic nucleus (Vmes) neurons are the primary afferents of the masticatory muscle spindles or periodontal membrane, and are crucial for the control of jaw movements [50–54]. On the other hand, Koeda et al. [55] reported that electrical stimulation of lingual nerve, a branch of the trigeminal nerve, or the spinal trigeminal nucleus (Vsp) evoked elevation in blood pressure and hypothesized that these elevations of blood pressure are induced by the sympathetic pressor response via Vsp, the inter mediolateral cell column of spinal cord, and sympathetic trunks. It is possible that both Vmes and Vsp neurons are involved with the teeth clenching-induced pressor response. In the present study, the central tracts of the pressor response, which comprise trigeminal ganglia to the sympathetic and parasympathetic center containing the spinal tracts, have not yet been analyzed. Consequently, these points will be the focus of future investigations.
Conclusions
We confirmed that both periodontal membrane and the mechanoreceptors in masticatory muscles are the receptors for the teeth clenching-induced pressor response, and that the afferent and efferent pathways of the pressor response pass through the trigeminal afferent nerves and sympathetic nerves, respectively.
Acknowledgements
The authors would like to thank Dr. Kohsuke Hagisawa and Dr. Hiroyuki Ohta for helpful discussion. The authors are also grateful to the staff of the Department of Oral and Maxillofacial Surgery, and Associate Professor Takaichi Fukuda and the staff of the Center for Laboratory Animal Science, National Defense Medical College for technical assistance.
Compliance with ethical standards
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving animals performed in this study were in accordance with the ethical standards of the institution or practice at which the study was conducted.
Funding
None.
References
- 1.Miyake S, Wada-Takahashi S, Honda H, Takahashi S, Sasaguri K, Sato S, Lee M. Stress and chewing affect blood flow and oxygen levels in the rat brain. Arch Oral Biol. 2012;57:1491–1497. doi: 10.1016/j.archoralbio.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Momose T, Nishikawa J, Watanabe T, Sasaki Y, Senda M, Kubota K, Sato Y, Funakoshi M, Minakuchi S. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch Oral Biol. 1997;42:57–61. doi: 10.1016/S0003-9969(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 3.Shibusawa M, Takeda T, Nakajima K, Ishigami K, Sakatani K. Functional near-infrared spectroscopy study on primary motor and sensory cortex response to clenching. Neurosci Letters. 2009;449:98–102. doi: 10.1016/j.neulet.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Miyamoto T, Terao A, Yokoyama A. Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience. 2007;145:791–794. doi: 10.1016/j.neuroscience.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Hirano Y, Obata T, Kashikura K, Nonaka H, Tachibana A, Ikehira H, Onozuka M. Effect of chewing in working memory processing. Neurosci Letters. 2008;436:189–192. doi: 10.1016/j.neulet.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Kawanishi K, Koshino H, Toyoshita Y, Tanaka M, Hirai T. Effect of mastication on functional recoveries after permanent middle cerebral artery occlusion in rats. J Stroke Cerebrovasc. 2010;19:398–403. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Ferella M, Bakke M, Michelitti A, Marotta G, Martina R. Cardiovascular responses in humans to experimental chewing of gums different consistencies. Arch Oral Biol. 1999;44:835–842. doi: 10.1016/S0003-9969(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 8.Ishiyama I, Suzuki M. Blood pressure and cerebral blood flow volume responses to mastication of food. J Jpn Soc Mast Sci Health Promot. 2005;15:24–36. [Google Scholar]
- 9.Okada Y, Kamijo Y, Okazaki K, Masuki S, Goto M, Nose H. Pressor responses to isometric biting are evoked by somatosensory receptors in periodontal tissue in humans. J Appl Physiol. 2009;107:531–539. doi: 10.1152/japplphysiol.91199.2008. [DOI] [PubMed] [Google Scholar]
- 10.Fruen BR, Mickelson JR, Louis CF. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J Biol Chem. 1997;272:26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- 11.Lynch C, 3rd, Durbin CG, Jr, Fisher NA, Veselis RA, Althaus JS. Effects of dantrolene and verapamil on atrioventricular conduction and cardiovascular performance in dogs. Anesth Analg. 1986;65:252–258. [PubMed] [Google Scholar]
- 12.Bowman WC, Houston J, Khan HH, Rodger IW. Effects of dantrolene sodium on respiratory and other muscles and on respiratory parameters in the anesthetized cat. Eur J Pharmacol. 1979;55:293–303. doi: 10.1016/0014-2999(79)90197-3. [DOI] [PubMed] [Google Scholar]
- 13.Ellis KO, Wessels FL, Carpenter JF. Effects of intravenous dantrolene sodium on respiratory and cardiovascular functions. J Pharmacentical Sci. 1976;65:1359–1364. doi: 10.1002/jps.2600650925. [DOI] [PubMed] [Google Scholar]
- 14.Chen QH, Nishida Y, Zhou MS, Murakami H, Morita H, Hosomi H, Kosaka H. Sinoaortic denervation produces sodium retension in Dahl salt-sensitive rats. J Auton Nerv Syst. 1998;69:56–63. doi: 10.1016/S0165-1838(98)00008-3. [DOI] [PubMed] [Google Scholar]
- 15.Nishida Y, Chen QH, Zhou MS, Horiuchi J. Sinoaortic denervation abolishes resetting for daily physical activity in rabbits. Am J Physiol Regul Integr Comp Physiol. 2002;282:R649–R657. doi: 10.1152/ajpregu.00160.2001. [DOI] [PubMed] [Google Scholar]
- 16.Hajduczok G, Chapleau M, Ferlic R, Mao H, Abboud FM. Gadolinium inhibits mechanoelectrical transduction in rabbit carotid baroreceptors. J Clin Invest. 1994;94:2392–2396. doi: 10.1172/JCI117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol. 2001;280:H2153–H2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- 18.Matsukawa K, Nakamoto T, Inomoto A. Gadolinium does not blunt the cardiovascular responses at the onset of voluntary static exercise in cats: a predominant role of central command. Am J Physiol Heart Circ Physiol. 2007;292:H121–H129. doi: 10.1152/ajpheart.00028.2006. [DOI] [PubMed] [Google Scholar]
- 19.Nakamoto T, Matsukawa K. Muscle mechanosensitive receptors close to the myotendinous junction of the Achilles tendon elicit a pressor reflex. J Appl Physiol. 2007;102:2112–2120. doi: 10.1152/japplphysiol.01344.2006. [DOI] [PubMed] [Google Scholar]
- 20.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 22.Shoji I, Maruyama S, Nishida Y. The role of alveolar nerves on the pressor response-induced teeth clenching in rats (in Japanese) Jpn J Aero Environ Med. 2011;48:82. [Google Scholar]
- 23.Takahata T, Shouji I, Maruyama S, Sato Y, Nishida Y, Ueno T. Teeth clenching and positive acceleration—induced cerebral arterial hypotension in rats. Aviat Space Environ Med. 2011;82:442–447. doi: 10.3357/ASEM.2741.2011. [DOI] [PubMed] [Google Scholar]
- 24.Nishida Y, Maruyama S. Animal studies of effects of +Gz acceleration stress on cardiovascular and autonomic function. J Natl Def Med Coll. 2013;38:16–31. [Google Scholar]
- 25.Shoji I, Kemuriyama T, Hiruma M, Maruyama S, Tashiro A, Hagisawa K, Ohta H, Yokoe H, Nishida Y. The tracts of neural reflex for the teeth clenching-induced pressor response in rats. J Physiol Sci. 2016;66(Supplement 1):S122. doi: 10.1007/s12576-016-0513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsukawa K, Sadamoto T, Tsuchimochi H, Komine H, Murata J, Shimizu K. Reflex responses in plasma catecholamines caused by static contribution of skeletal muscle. Jpn J Physiol. 2001;51:591–597. doi: 10.2170/jjphysiol.51.591. [DOI] [PubMed] [Google Scholar]
- 27.Crayton SC, Aung-Din R, Fixler DE, Mitchell JH. Distribution of cardiac output during induced isometric exercise in dogs. Am J Physiol Heart Circ Physiol. 1979;236:H218–H224. doi: 10.1152/ajpheart.1979.236.2.H218. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. Am J Physiol Heart Circ Physiol. 1977;233:H374–H378. doi: 10.1152/ajpheart.1977.233.3.H374. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JH. Neural control of the circulation during exercise. Med Sci Spor Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- 30.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von During M, Andres KH. Topography and ultrastructure of group and nerve terminals of cat’s gastrocnemius—soleus muscle. In: Zenker W, Neuhuber WL, editors. The primary afferent neuron: a survey of recent morpho-functional aspects. New York: Plenum Press; 1990. pp. 35–41. [Google Scholar]
- 32.Stacey MJ. Free nerve endings in skeletal muscle of cat. J Anat. 1969;105:231–254. [PMC free article] [PubMed] [Google Scholar]
- 33.Andres KH, von During M, Schmidt RF. Sensory innervation of the Achilles tendon by group III and IV afferent fibers. Anat Embryol. 1985;172:145–156. doi: 10.1007/BF00319597. [DOI] [PubMed] [Google Scholar]
- 34.Mense S, Meyer H. Different types of slowly conduction afferent units in cat skeletal muscle and tendon. J Physiol. 1985;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrahams VC. Group III and IV receptors of skeletal muscle. Can J Physiol Pharmacol. 1986;64:509–514. doi: 10.1139/y86-083. [DOI] [PubMed] [Google Scholar]
- 36.Abe C, Iwata C, Morita H. Water drinking-related muscle contraction induces the pressor response via mechanoreceptors in conscious rats. J Appl Physiol. 2013;114:28–36. doi: 10.1152/japplphysiol.00923.2012. [DOI] [PubMed] [Google Scholar]
- 37.Hannam AG, Farnsworth TJ. Information transmission in trigeminal mechanosensitive afferents from teeth in the cat. Arch Oral Biol. 1997;22:181–186. doi: 10.1016/0003-9969(77)90152-2. [DOI] [PubMed] [Google Scholar]
- 38.Nagata K, Itoh S, Tsuboi A, Takafuji Y, Tabata T, Watanabe M. Response properties of periodontal mechanosensitive neurons in the trigeminal ganglion of rabbit and neural activities during grinding-like jaw movement induced by cortical stimulation. Arch Oral Biol. 2008;53:1138–1148. doi: 10.1016/j.archoralbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Tabata T, Takahashi Y, Hayashi H. Physiological properties of molar-mechanosensitive periodontal neurons in the trigeminal ganglion of the rat. Arch Oral Biol. 2006;51:729–735. doi: 10.1016/j.archoralbio.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 40.O’Leary DS. Heart rate control during exercise by baroreceptors and skeletal muscle afferents. Med Sci Sports Exerc. 1996;28:210–217. doi: 10.1097/00005768-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Potts JT, Mitchell JH. Rapid resetting of carotid baroreceptor reflex by afferent input from skeletal receptors. Am J Physiol Heart Circ Physiol. 1998;275:H2000–H2008. doi: 10.1152/ajpheart.1998.275.6.H2000. [DOI] [PubMed] [Google Scholar]
- 42.Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- 43.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto K, Kawada T, Kamiya A, Takaki H, Miyamoto T, Sugimachi M, Sunagawa K. Muscle mechanoreflex induces the pressor response by resetting the arterial baroreflex neural arc. Am J Physiol Heart Circ Physiol. 2004;286:H1382–H1388. doi: 10.1152/ajpheart.00801.2003. [DOI] [PubMed] [Google Scholar]
- 45.Choe K, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. 2015;85:549–560. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang CC, Lin WC, Chen HL, Friedman M, Lin MC, Lin HC, Lu CH. Improvement of baroreflex sensitivity in patients with obstructive sleep apnea following surgical treatment. Clin Neurophysiol. 2016;127:544–550. doi: 10.1016/j.clinph.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Indumathy J, Pal GK, Pal P, Ananthanarayan PH, Parija SC, Balachander J, Dutta TK. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, body fat mass and altered cardiometabolic profile in pre-obesity and obesity. Metabolism. 2015;64:1704–1714. doi: 10.1016/j.metabol.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Narayan J, Amatoury J, Verma M, Kairaitis K, Wheatley JR, Amis TC. Resetting the baroreflex during snoring: role of resistive loading and intra-thoracic pressure. Res Physiol Neurobiol. 2013;185:489–496. doi: 10.1016/j.resp.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Pinter A, Horvath T, Toth A, Kadar K, Kollai M. Impaired baroreflex function is related to reduced carotid artery elasticity in patients with tetralogy of Fallot. Auton Neurosci. 2014;183:94–99. doi: 10.1016/j.autneu.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Jerge CR. Organization and function of the trigeminal mesencephalic nucleus. J Neurophysiol. 1963;26:379–392. doi: 10.1152/jn.1963.26.3.379. [DOI] [PubMed] [Google Scholar]
- 51.Matesz C. Peripheral and central distribution of fibres of the mesencephalic trigeminal root in the rat. Neurosci Lett. 1981;27:13–17. doi: 10.1016/0304-3940(81)90198-1. [DOI] [PubMed] [Google Scholar]
- 52.Byers MR, O’Connor TA, Martin RF, Dong WK. Mesencephalic trigeminal sensory neurons of cat: axon pathways and structure of mechanoreceptive endings in periodontal ligament. J Comp Neurol. 1986;250:181–191. doi: 10.1002/cne.902500205. [DOI] [PubMed] [Google Scholar]
- 53.Luo PF, Wang BR, Peng ZZ, Li JS. Morphological characteristics and terminating patterns of masseteric neurons of the mesencephalic trigeminal nucleus in the rat: an intracellular horseradish peroxidase labeling study. J Comp Neurol. 1991;303:286–299. doi: 10.1002/cne.903030210. [DOI] [PubMed] [Google Scholar]
- 54.Ohara H, Tachibana Y, Fujio T, Takeda-Ikeda R, Sato F, Oka A, Kato T, Ikenoue E, Yamashiro T, Yoshida A. Direct projection from the lateral habenula to the trigeminal mesencephalic nucleus in rats. Brain Res. 2016;1630:183–197. doi: 10.1016/j.brainres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Koeda S, Ishii H, Kuchiiwa S, Izumi H. Role of the spinal trigeminal nucleus in the rat autonomic reflex. Arch Oral Biol. 2009;54:1136–1142. doi: 10.1016/j.archoralbio.2009.08.008. [DOI] [PubMed] [Google Scholar]


