Abstract
Cognitive function declines with age. The underlying mechanisms responsible for the deterioration of cognitive performance, however, remain poorly understood. We hypothesized that an incremental rate of prefrontal oxygenation during a cognitive Stroop test decreases in progress of ageing, resulting in a slowdown of cognitive performance. To test this hypothesis, we identified, using multichannel near-infrared spectroscopy, the characteristics of the oxygenated-hemoglobin concentration (Oxy-Hb) responses of the prefrontal cortex to both incongruent Stroop and congruent word-reading test. Spatial distributions of the significant changes in the three components (initial slope, peak amplitude, and area under the curve) of the Oxy-Hb response were compared between young and elderly subjects. The Stroop interference time (as a difference in total periods for executing Stroop and word-reading test, respectively) approximately doubled in elderly as compared to young subjects. The Oxy-Hb in the rostrolateral, but not caudal, prefrontal cortex increased during the Stroop test in both age groups. The initial slope of the Oxy-Hb response, rather than the peak and area under the curve, had a strong correlation with cognitive performance speed. Taken together, it is likely that the incremental rate of prefrontal oxygenation may decrease in progress of ageing, resulting in a decline in cognitive performance.
Keywords: Prefrontal oxygenation, Cognitive function, Multichannel near-infrared spectroscopy, Incongruent Stroop test, Congruent word-reading test
Introduction
Cognitive function is crucial for coping with changes in environmental contexts in daily life and usually declines with age. Cognitive performance during a task, such as visual discrimination, Erikson flanker task (working memory test), and Stroop test, diminishes in elderly people [1–6]. However, the underlying mechanisms responsible for the deterioration of cognitive performance remain poorly understood.
The cerebral prefrontal cortex plays an important role in cognitive function [1, 2, 7]. Previous studies with functional magnetic resonance imaging (fMRI) reported that the Stroop test increased regional cerebral blood flow (rCBF) in the dorsolateral area of the prefrontal cortex (DLPFC), the middle frontal gyrus, the anterior cingulate (ACC), and the parietal cortex in young subjects [8–10]. The increased rCBF in these cortical areas were smaller in elderly subjects, suggesting that a decline in the cognitive performance with ageing may be due to the lower cortical activation. In several studies, however, higher neural activation has been detected in the inferior, anterior, and ventrolateral subregions of the prefrontal cortex in elderly than those in young individuals [5, 8, 11, 12]. These results suggest the differential changes in cortical activation during a cognitive task in elderly as compared to young individuals. To explore the underlying neural mechanism responsible for the age-related difference in cognitive function, therefore, it is important to compare the dynamic changes in the rCBF associated with a cognitive task over the subregions of the prefrontal cortex. Although the fMRI technique is useful to assess the rCBF with high spatial resolution, it could not detect the dynamic changes in rCBF of the prefrontal areas due to limited temporal resolution.
An imaging technique of near-infrared spectroscopy (NIRS) enables noninvasive real-time monitoring of brain tissue oxygenation with higher time resolution than fMRI, according to absorption of near-infrared light by oxygenated-hemoglobin (Oxy-Hb) and deoxygenated-hemoglobin (Deoxy-Hb) [6, 13–15]. If the concentration of Deoxy-Hb remains constant during a cognitive test, the dynamic changes in the concentration of Oxy-Hb may reflect rCBF in the cortical region in relation to cognitive performance. The concentration of Oxy-Hb in the prefrontal cortex increased during a series of cognitive tests in both young and elderly subjects [6, 16–18]. Interestingly, the temporal profiles of the Oxy-Hb response seemed different between the two groups. The concentration of Oxy-Hb increased quickly as soon as a cognitive task started in young subjects, whereas the Oxy-Hb concentration increased slowly until the end of the task in elderly subjects [6, 16, 17]. However, how the temporal characteristics of the Oxy-Hb response relate to the cognitive performance remained to be studied.
The previous results led us to an idea that not only the peak amplitude or area under the curve of the Oxy-Hb response but also its incremental rate at the initial period of Stroop test may reduce in elderly subjects. The components of peak amplitude and area are strongly influenced by the time spent for a cognitive task, which is known to be prolonged in elderly than young subjects. We hypothesized that the incremental rate of the prefrontal Oxy-Hb response at the initial period (termed as initial slope) decreases with progress of ageing, resulting in a slowdown of cognitive performance. To test this hypothesis, we identified, using multichannel NIRS, the characteristics of the prefrontal Oxy-Hb response to Stroop test in terms of the three components (initial slope, peak amplitude, and area under the curve) and compared the prefrontal distributions of the significant changes in the components of the Oxy-Hb response between young and elderly subjects. The Oxy-Hb response was also examined during a word-reading test, which needed less cognitive process but most likely a simple reaction, as a control trial for the Stroop test. Finally, which component of the dynamic Oxy-Hb response most contributed to cognitive performance was examined by assessing the relationships with cognitive performance speed.
Methods
Subjects
Nine young and nine elderly subjects participated in the study. All subjects had normal or corrected vision and normal color discrimination. They were right-handed native Japanese speakers and identified themselves as being healthy and physically active. None of the young subjects had cardiovascular and autonomic diseases and took any medication. Five elderly subjects intook prescription medication for treatment of hypertension and other diseases, although all elderly subjects had no history of severe neurological disorder (Table 1). Each subject was instructed not to ingest caffeine and alcohol for at least 12 h prior to the experiments. Written informed consent was obtained from all subjects prior to participation. The experimental protocols and procedures were performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethical Committee of Hiroshima University (Permit no. 1308).
Table 1.
Physical characteristics and medical condition of young and elderly subjects
| Age (years) | Height (cm) | Body weight (kg) | BMI (kg/m2) | Subjects taking prescription medicines (medical condition) | |
|---|---|---|---|---|---|
| Young subjects (n = 9) | 23 ± 1 | 165 ± 4 | 56 ± 4 | 20 ± 1 | None |
| Elderly subjects (n = 9) | 64 ± 1 | 164 ± 2 | 63 ± 4 | 23 ± 1 | Subject A |
| Norvasc (for hypertension), Ludiomil, Depromel, Lendrmin (for mood disorder) | |||||
| Subject B | |||||
| Diovan, Coniel (for hypertension) | |||||
| Januvia (for diabetes) | |||||
| Subject C | |||||
| Nu-Lotan (for hypertension) | |||||
| Mevalotin (for hyperlipemia) | |||||
| Subject D | |||||
| Caduet, Blopress (for hypertension) | |||||
| Subject E | |||||
| Norvasc (for hypertension), implantation of cardiac pacemaker | |||||
| Others | |||||
| None |
Values are mean ± SE
BMI body mass index
Stroop and word-reading tests
We used a modified Japanese version of the Stroop test [19] as a cognitive test, which requires attention, response inhibition, interference, and behavioral conflict resolution. The test used four kinds of words (‘red’, ‘blue’, ‘green’, and ‘yellow’), which were displayed in a color different from the word’s meaning. When presenting a series of the color words at random on a TV screen, the subjects were requested to answer the color of a display word as fast as possible, instead of the word’s meaning. After then, the color word was replaced with a new one by clicking a mouse button. Accordingly, the period of presentation of the color word was dependent on a response speed. We used the word-reading test as a control trial for the Stroop test, in which all words were displayed in black ink. The word-reading test is usually used as a simple reaction test; it influences the visual perception and verbal motor response but causes less cognitive response such as attention, processing speed, inhibit cognitive interference. The test was performed in order to examine a difference in the total period between the cognitive task (Stroop test) and simple reaction test (word reading), which could be considered as an interference period necessary for discriminating and judging the color of a displayed word. Before starting the experiment, all subjects received detailed instructions and performed a practice session to become accustomed to the cognitive tests. After taking a rest for 10–15 min on a comfortable chair, the subjects started performing the Stroop and word-reading test. To determine reproducibility and variability of the cognitive performance and NIRS signals, the Stroop and word-reading tests were conducted four times in a randomized manner, respectively. There was no significant difference in the intervals of time between trials as a result (3.9 ± 0.6 and 4.9 ± 1.5 min, respectively with young and elderly groups).
Cardiovascular responses during cognitive test
In an additional experiment, we measured mean arterial blood pressure (MAP) and pulse rate (PR) with a non-invasive automatic blood pressure monitor (HM-762, OMRON, Kyoto, Japan) before and at the end of the Stroop and word-reading tests in five young and five elderly subjects on a separate day. To do this, a blood pressure cuff was wrapped around the left upper arm.
Changes in the concentrations of Oxy-Hb and Deoxy-Hb in the prefrontal cortex
The relative concentrations of the Oxy-Hb and Deoxy-Hb in the bilateral prefrontal cortices were measured using a multichannel NIRS (FOIRE-3000, Shimadzu Corporation, Kyoto, Japan). The concentrations of the Oxy- and Deoxy-Hb at 22 sites in a 15 × 15-cm area were measured with the NIRS probes placed over the frontal surface of the head (referring to the international EEG 10–20 system) as shown in Fig. 1. The probes were mounted on a plastic helmet that was held by adjustable screws and straps over the subject’s scalp. The interprobe distance was 3 cm. Near-infrared light emitted from three laser photodiodes with different wavelengths (780, 805, and 830 nm) penetrated brain tissue. Some of the light was absorbed by Hb, and the remaining light scattered by the brain tissue was picked up with photodetectors, sampled at a rate of 7 Hz, and converted to optical densities. NIRS measured the relative concentrations of the Oxy- and Deoxy-Hb in arterial and venous blood vessels and capillaries of the illuminated tissue, reflecting balance between oxygen supply and demand. When some subjects felt discomfort according to long-term placement of the NIRS probes on the head, the fourth trial of the Stroop and/or word-reading test was cancelled in the subjects.
Fig. 1.
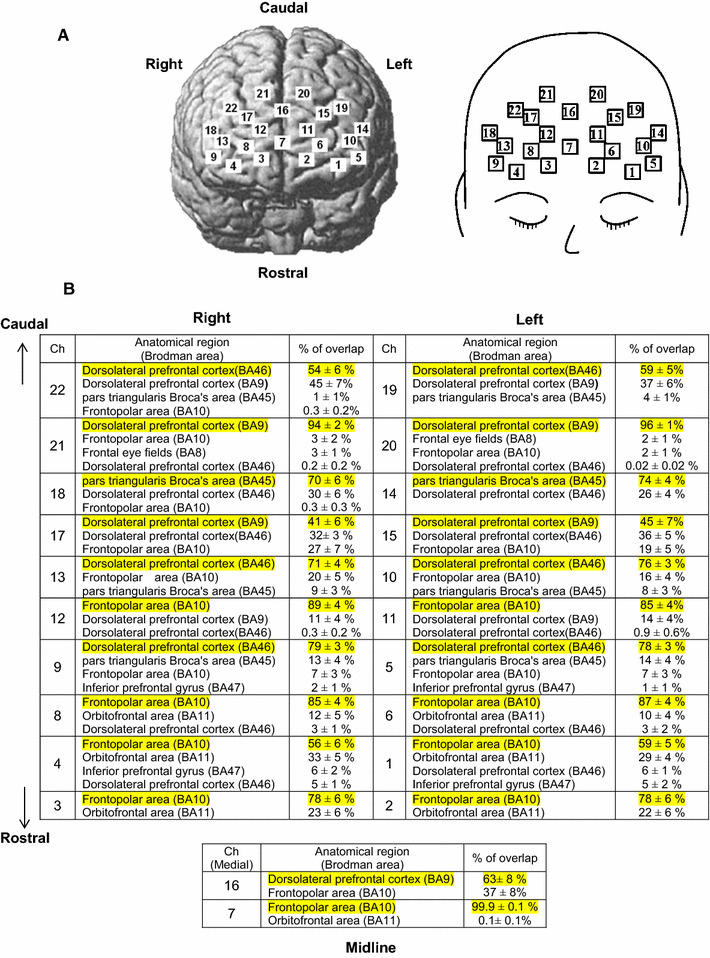
a Schematic representation of multichannel near-infrared spectroscopy (NIRS) probes. The NIRS probes were placed over the frontal surface of the head. b An anatomical brain region [Brodmann area (BA)] for the channels’ coordinates were probabilistically estimated by using a MRIcro. A probabilistic mapping of the 22 channels and its corresponding anatomical brain region are summarized
The three-dimensional location of each NIRS probe was determined using a magnetic space digitizer (FASTRAK, Polhemus, Colchester, VT). Using a probabilistic registration method (NIRS-SPM) [20], we identified the NIRS-channel positions on the Montreal Neurological Institute standard template [21]. The anatomical brain regions (Brodmann area; BA) for the channels’ coordinates were probabilistically estimated by using a MRIcro program [22] (Fig. 1). We detected three distinct subregions of the prefrontal cortical cortex, each of which contained a total of 2–9 channels. The channels 1–4, 6–8, 11, and 12 corresponded to the frontopolar area (BA10 at 56–100% probability); the channels 14 and 18 to the pars triangularis Broca’s area (BA45 at 70–74% probability); the channels 5, 9, 16, 19, and 20–22 to the DLPFC (BA 9 and 46 at 54–96% probability).
Data and statistical analyses
The cardiovascular responses were compared by using a three-way ANOVA with a Dunnett post hoc test between young and elderly groups [main effects = cognitive test (Stroop vs. word-reading) × age group × condition (baseline vs. during)]. We measured the total period required for processing the Stroop or word-reading test, each consisting of 100 questions. We defined the 100 answers/total period as index of response speed, while the number of questions in that the subjects exhibited a wrong answer was measured as index of response accuracy. Moreover, a difference in total periods between the Stroop and word-reading test was considered as an interference period necessary for discriminating and judging the color of a displayed word. The cognitive performance of total period, number of errors, and 100 answers/total period was similarly compared by the three-way ANOVA with the Dunnett post hoc test [main effects = cognitive test (Stroop vs. word-reading) × age group × trial number]. With the post hoc test, a paired t test was used to compare the difference between the tasks, and an unpaired t test between the groups. A Dunnet post hoc test was used to detect the difference in the conditions (trials two to four) from the control (trial one).
The Oxy-Hb and Deoxy-Hb data of the 22 NIRS channels were continuously measured throughout the experiments and were analyzed using a data acquisition system and software (FOIRE-3000, Shimadzu, Kyoto, Japan). The absolute concentrations of the Oxy- and Deoxy-Hb could not be obtained, because the path length of near-infrared light within brain tissue was unknown. The relative changes in the NIRS signals against the baseline were obtained in every trial of a cognitive test and were aligned at the onset of the test. The time course data of the NIRS responses were statistically analyzed by a one-way ANOVA with repeated measures. If either normality or equal variance test failed, a Friedman repeated measures analysis of variance on ranks was performed.
Next, the Oxy-Hb response was assessed by the following three components: (1) initial slope, (2) peak amplitude, and (3) area under the Oxy-Hb curve (as shown in Fig. 2). The initial slope of the Oxy-Hb response was determined as an incremental rate obtained from calculating a ratio between the 50% of the peak Oxy-Hb response and the time at which the Oxy-Hb reached the 50% level. The peak Oxy-Hb response was defined as the greatest change from the baseline during a cognitive test. The area of Oxy-Hb response was defined as a product of the average Oxy-Hb response and the period of the test. To examine which component of the dynamic Oxy-Hb response is most related to cognitive performance speed, the relationship with the 100 answers/total period was analyzed with a linear regression analysis and Pearson’s correlation method. The level of statistical significance was defined at P < 0.05 in all cases. All statistical analyses were performed using SigmaPlot® version 13 (Systat Software, San Jose, CA, USA). All data are expressed as mean ± SE.
Fig. 2.
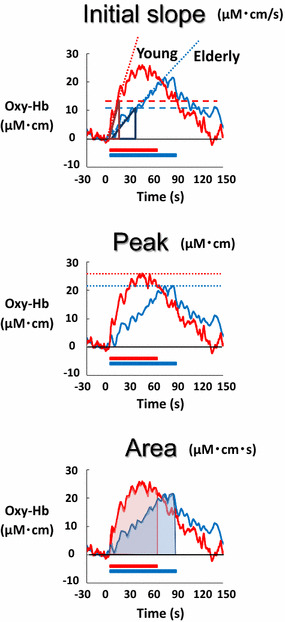
Definition of the initial slope, peak, and area under the curve of the Oxy-Hb response in a young (red line) and an elderly subject (blue line). The initial slope of the Oxy-Hb response was determined as an incremental rate obtained from calculating a ratio between the 50% of the peak Oxy-Hb response and the time at which the Oxy-Hb reached the 50% level. The peak component of the Oxy-Hb response was defined as the greatest change during the cognitive test from the baseline value. The area under the curve of the Oxy-Hb response was defined as a product between the average Oxy-Hb response and the duration of the test
Results
The cardiovascular responses during the Stroop and word-reading tests
There was no significant difference in the baseline PR or MAP between the Stroop test and word-reading test both in young (PR; 61 ± 6 and 60 ± 5 beats/min, MAP; 85 ± 4 and 85 ± 4 mmHg) and elderly subjects (PR; 63 ± 2 and 64 ± 2 beats/min, MAP; 95 ± 4 and 94 ± 3 mmHg). The baseline PR was comparable between the groups, while the baseline MAP was slightly higher (P < 0.05 respectively in Stroop and word-reading test) in elderly than in young subjects. The PR and MAP significantly increased during Stroop and word-reading test, and there was no difference in the amount of the increments between Stroop and word-reading test or between young (ΔPR; 32 ± 3 and 29 ± 4 beats/min, ΔMAP; 16 ± 3 and 12 ± 5 mmHg) and elderly subjects (ΔPR; 23 ± 3 and 23 ± 2 beats/min, ΔMAP; 16 ± 4 and 18 ± 3 mmHg).
Stroop and word-reading test scores
Table 2 summarizes cognitive performance (total period, 100 answers/total period, and number of errors) for the Stroop and word-reading test in young and elderly subjects. Irrespective of the Stroop or word-reading test, the time scores and the number of errors were comparable over the four trials. Regarding the Stroop test, the total period was prolonged (P < 0.05) in elderly than young subjects, and the 100 answers/total period was smaller (P < 0.05) accordingly. The number of errors was greater (P < 0.05) in elderly subjects. The Stroop interference time approximately doubled (P < 0.05) in elderly as compared to young subjects. In the word-reading test, the total period and 100 answers/total period had the similar tendency as those for the Stroop test, while the number of errors was not different (P > 0.05) between the two age groups.
Table 2.
The cognitive performance for the Stroop and word-reading test in young and elderly subjects
| Trial | Stroop test | Word-reading test | Stroop interference time (s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Total time period (s) | 100/total time period (answers/s) | Number of errors | n | Total time period (s) | 100/total time period (answers/s) | Number of errors | ||
| Young subjects | |||||||||
| Tr 1 | 9 | 64.2 ± 4.8# | 1.6 ± 0.1# | 1.7 ± 0.7# | 9 | 47.4 ± 2.0 | 2.1 ± 0.1 | 0 | 16.9 ± 3.0 |
| Tr 2 | 9 | 62.7 ± 3.8# | 1.6 ± 0.1# | 1.4 ± 0.6# | 9 | 45.9 ± 1.4 | 2.2 ± 0.1 | 0 | 16.8 ± 2.8 |
| Tr 3 | 9 | 61.8 ± 3.5# | 1.7 ± 0.1# | 2.0 ± 0.9# | 9 | 46.1 ± 1.1 | 2.2 ± 0.1 | 0 | 15.6 ± 2.6 |
| Tr 4 | 8 | 64.7 ± 4.2# | 1.6 ± 0.1# | 1.6 ± 0.5# | 7 | 45.3 ± 0.7 | 2.2 ± 0.0 | 0.1 ± 0.1 | 19.2 ± 4.5 |
| Elderly subjects | |||||||||
| Tr 1 | 9 | 94.6 ± 4.9†,# | 1.1 ± 0.1†,# | 3.2 ± 0.6†,# | 9 | 63.2 ± 2.8† | 1.6 ± 0.1† | 0.1 ± 0.1 | 31.4 ± 3.2† |
| Tr 2 | 9 | 90.7 ± 4.4†,# | 1.1 ± 0.1†,# | 3.0 ± 0.9†,# | 9 | 59.6 ± 3.3† | 1.7 ± 0.1† | 0.1 ± 0.1 | 31.1 ± 2.3† |
| Tr 3 | 9 | 89.6 ± 4.4†,# | 1.1 ± 0.1†,# | 4.1 ± 0.9†,# | 9 | 58.2 ± 2.5† | 1.7 ± 0.1† | 0 | 31.4 ± 3.3† |
| Tr 4 | 9 | 84.2 ± 3.8†,# | 1.2 ± 0.1†,# | 2.2 ± 0.7†,# | 6 | 61.6 ± 4.5† | 1.7 ± 0.1† | 0.6 ± 0.4 | 26.8 ± 3.1† |
Values are mean ± SE. A difference in total periods between the Stroop and word-reading tests is considered as an interference period (Stroop interference time = total period for Stroop − total period for word-reading). n number of subjects
†Significant difference (P < 0.05 by three-way ANOVA with a Dunnett post hoc test) between young and elderly subjects
#Significant difference (P < 0.05 by three-way ANOVA with the Dunnett post hoc test) between the Stroop and word-reading tests
The prefrontal Oxy-Hb responses during Stroop test
The time courses of the average Oxy-Hb responses during four repetitive trials of the Stroop test are represented at 22 various sites of bilateral prefrontal cortices in young and elderly subjects (Fig. 3). Significant increases in the Oxy-Hb response (P < 0.05 by one-way ANOVA with repeated measures) were found in both age groups at most sites, except the caudal region; the Oxy-Hb responses were greater in the rostral prefrontal cortex (ch1–14, 18) than the caudal prefrontal cortex (ch15–17, 19–22) in both age groups. Furthermore, it was noted that the Oxy-Hb responses in the prefrontal cortex were the greatest in the first Stroop trial than the following trials in both age groups. The Deoxy-Hb at all prefrontal sites did not change significantly (P > 0.05) in any trial of the Stroop test (not shown in Fig. 3).
Fig. 3.
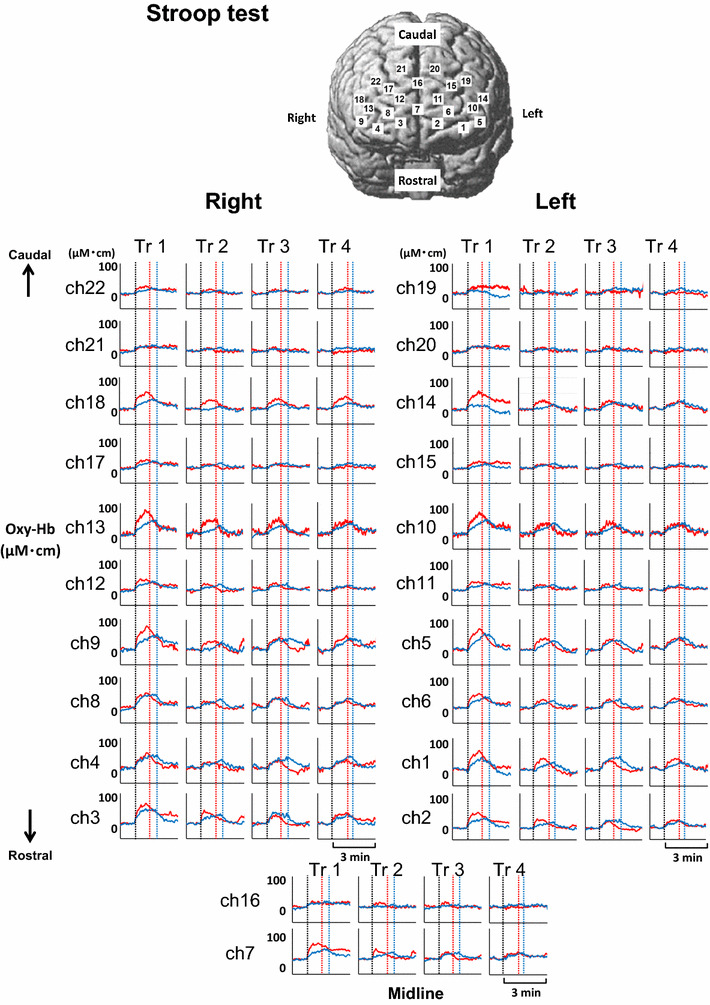
Time courses of the average Oxy-Hb responses in four repetitive trials of the Stroop test are represented at 22 various sites of bilateral prefrontal cortices in nine young (red line) and nine elderly subjects (blue line). Vertical dotted lines indicate the start and end of the Stroop test in both age groups
By using the time course data, Fig. 4 describes the spatial distributions of the prefrontal Oxy-Hb responses over the prefrontal 22 sites, according to the three components of the initial slope, peak, and area under the curve (as defined in Fig. 2). At individual sites, all of the initial slope, peak, and area components of the Oxy-Hb responses were the greatest in the first Stroop trial (P < 0.05 by one-way ANOVA with repeated measure), despite no significant inter-trial differences in cognitive performance. Because other factors different from the cognitive processing (for example, a startle and/or attention response etc.) might influence the NIRS signals for the first Stroop trial, the data in the first Stroop and word-reading trials were excluded from the further analysis. In Fig. 4a, the sites with the significant initial slope of the Oxy-Hb response were predominantly distributed in the rostrolateral prefrontal cortex in both young and elderly subjects, whereas no or only slight changes in the initial slope were observed in the caudal prefrontal cortex. Although the number of the sites with a significant initial slope almost matched between the two age groups (young: 45–82% of the 22 sites vs. elderly: 64–68% of the 22 sites), the average magnitude of the initial slope was greater (P < 0.05) in young [0.85 ± 0.1 (µM cm)/s] than elderly subjects [0.47 ± 0.03 (µM cm)/s].
Fig. 4.
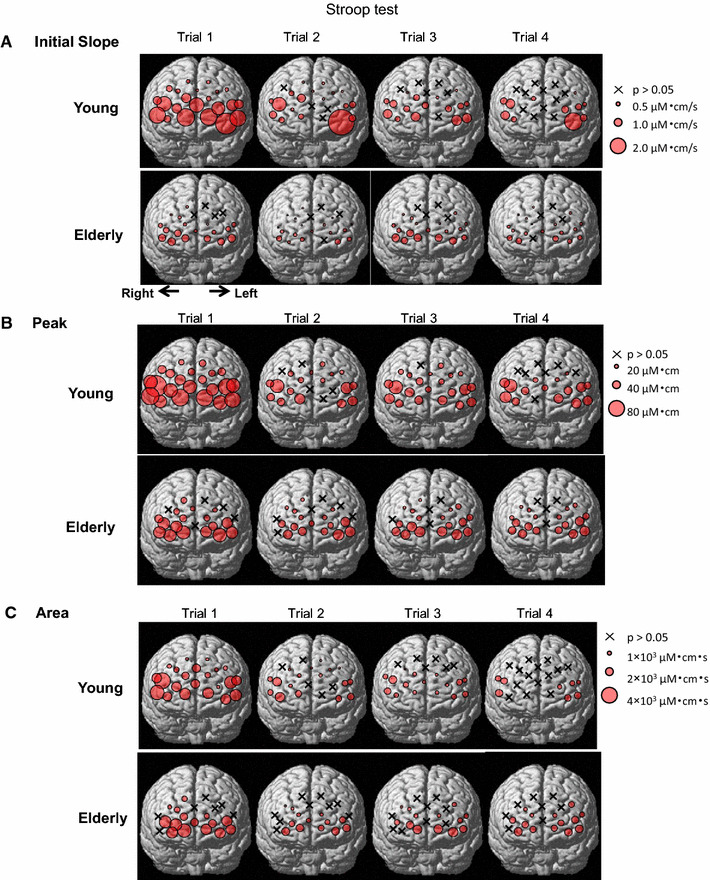
Spatial distributions of the three components [initial slope (a), peak (b), and area under the curve (c)] of the Oxy-Hb response during the Stroop test in nine young and nine elderly subjects. Each circle indicates a significant difference (P < 0.05) from the baseline before the Stroop test. An x indicates no significant difference (P > 0.05) from the baseline. The diameter of each circle is illustrated in proportion to the response magnitude
With respect to the peak and area components of the Oxy-Hb response, the sites with the significant peak and area under the curve were predominantly distributed in the rostrolateral prefrontal cortex in both young and elderly groups (Fig. 4). Both number of the sites and magnitudes of the significant peak and area components had no significant differences (P > 0.05) between the two age groups.
The Oxy-Hb responses during word-reading test
The time courses of the prefrontal Oxy-Hb responses during four repetitive trials of the word-reading test in both young and elderly groups are shown in Fig. 5. Although the Oxy-Hb increased during the word-reading test, the Oxy-Hb responses were smaller as compared to those during the Stroop test in both age groups. The Oxy-Hb increased (P < 0.05 by one-way ANOVA with repeated measures) in most of the rostrolateral prefrontal sites (ch1–6, 8–15), whereas the Oxy-Hb changes in the rostromedial and caudal prefrontal sites (ch2, 11, 14–17, 18–22) were not significant. The prefrontal distributions of the significant Oxy-Hb response were almost similar between young and elderly groups. Differently from the Stroop test, it was not evident that the Oxy-Hb response was the greatest in the first trial of the word-reading test. The Deoxy-Hb did not change significantly during any trial of the word-reading test.
Fig. 5.
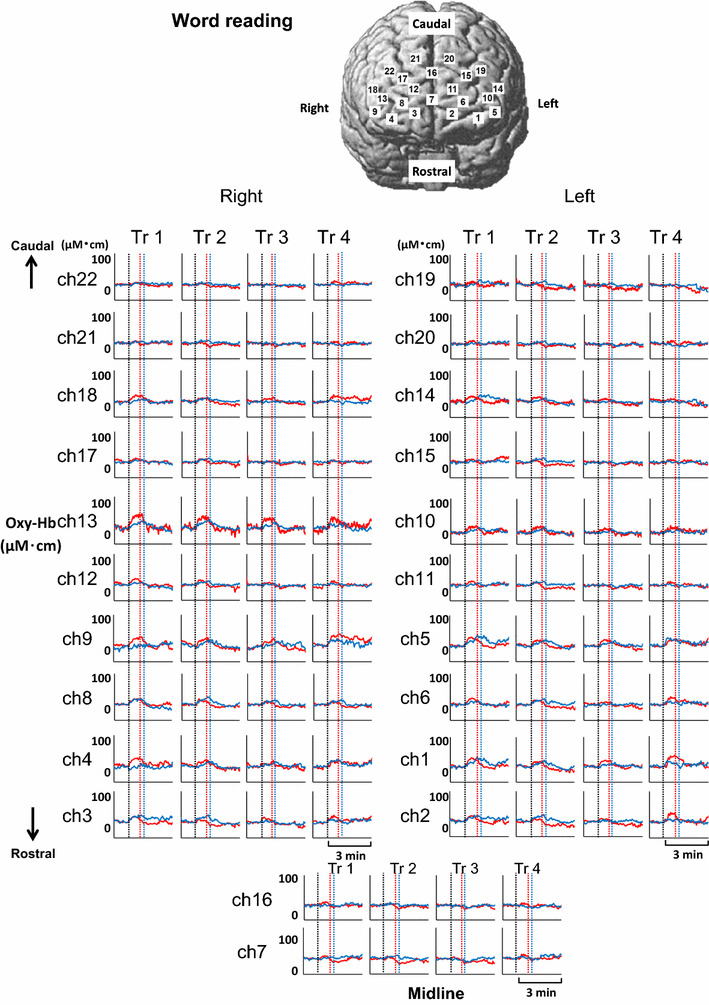
Time courses of the average Oxy-Hb responses in four repetitive trials of the word-reading test are represented at various 22 sites of bilateral prefrontal cortices in young (red line) and elderly subjects (blue line). Vertical dotted lines indicate the start and end of the word-reading test in both age groups
Figure 6 shows schematically the distribution of the Oxy-Hb responses over the bilateral prefrontal cortices during four repetitive trials of the word-reading test. The initial slope, peak, and area components of the Oxy-Hb responses were not different among the four trials of the word-reading (P > 0.05 by one-way ANOVA with repeated measures). In both age groups, the significant initial slope, peak, and area of the Oxy-Hb response were distributed in the rostrolateral prefrontal cortex, but not in the rostromedial and caudal prefrontal cortex. The initial slope and peak of the Oxy-Hb responses were greater in young than elderly subjects [slope, 0.7 ± 0.1 (µM cm)/s for young vs. 0.3 ± 0.1 (µM cm)/s for elderly; peak, 31 ± 3 vs. 21 ± 2 µM cm, respectively], although the area under the curve was comparable between the two groups [young, 834 ± 79 (µM cm) s; elderly, 612 ± 76 (µM cm) s].
Fig. 6.
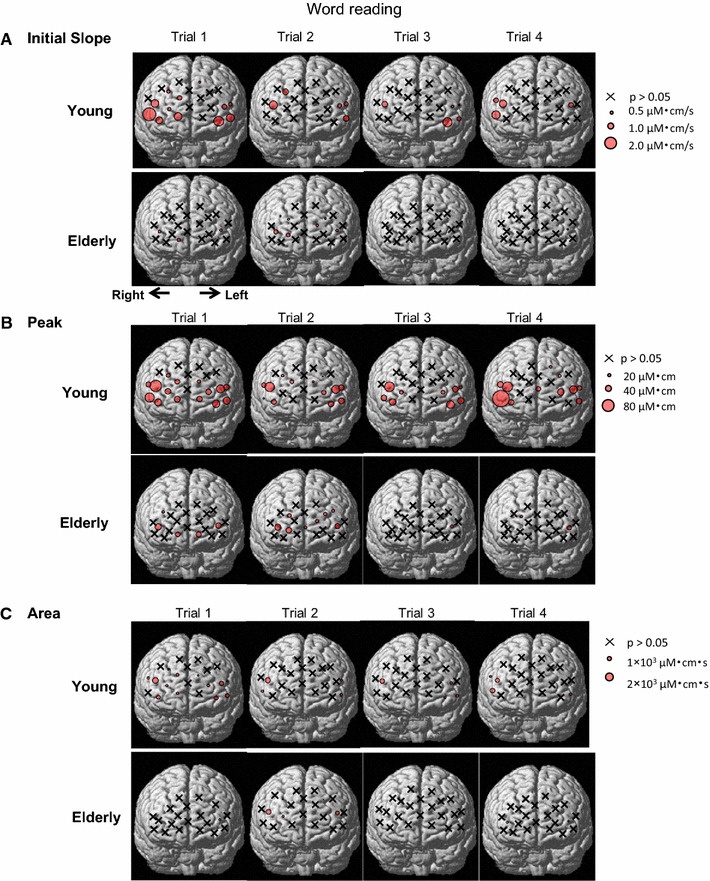
Spatial distributions of the three components [initial slope (a), peak (b), and area under the curve (c)] of the Oxy-Hb response during the word-reading test in nine young and nine elderly subjects. Each circle indicates a significant difference (P < 0.05) from the baseline before the word-reading test. An x indicates no significant difference (P > 0.05) from the baseline. The diameter of each circle is illustrated in proportion to the response magnitude
The initial slope, peak, and area under the curve of the prefrontal Oxy-Hb response
Figure 7a shows the prefrontal distributions of the Oxy-Hb responses (initial slope, peak, and area) that were averaged over the Stroop 2–4 trials. The number of the sites with any of the significant components (initial slope, peak, and area) of the Oxy-Hb response was almost the same between both age groups. Regarding the response magnitude, the age-related significant difference in the initial slope of the Oxy-Hb response was recognized in seven sites [DLPFC (ch5, 10, 13), frontopolar area (ch6, 12), and pars triangularis Broca’s area (ch14, 18)]. In contrast, the age-related difference in the peak of the Oxy-Hb response was detected only in two sites [DLPFC (ch13) and pars triangularis Broca’s area (ch18)] between the two age groups and no differences in the area component were detected.
Fig. 7.
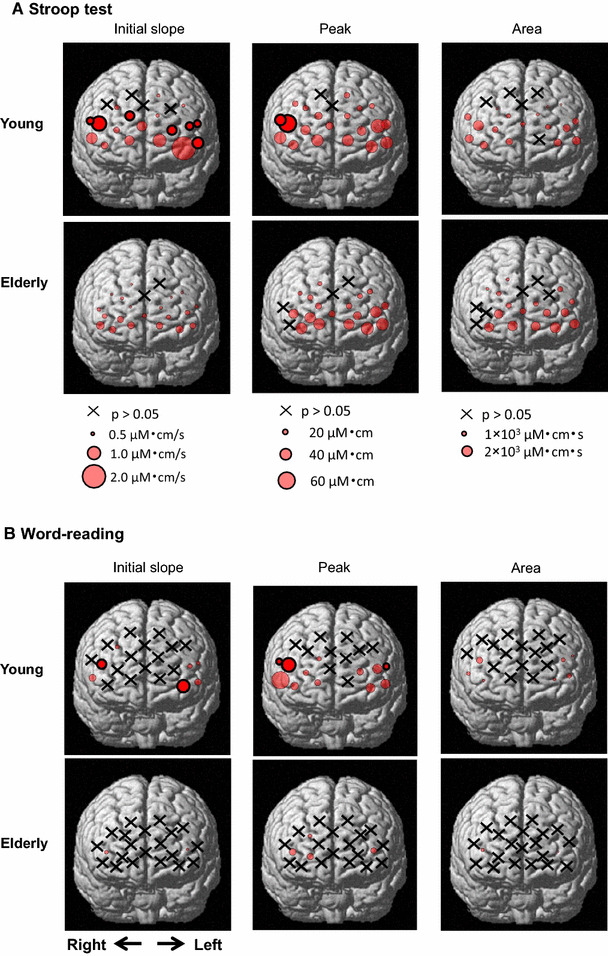
Spatial distributions of the three components (initial slope, peak, and area under the curve) of the Oxy-Hb response averaged over the Stroop 2–4 trials (a) and over the word-reading 2–4 trials (b) in nine young and nine elderly subjects. Each circle with thin lines indicates a significant difference (P < 0.05) from the baseline before the cognitive test. Each circle with thick lines indicates significant differences (P < 0.05) from the baseline and between the two age groups. An x indicates no significant difference (P > 0.05) from the baseline control
In the word-reading test, the distributions of the significant components (initial slope, peak, and area) of the Oxy-Hb response became scantier as compared to the Stroop test (Fig. 7b). The number of the sites for the significant components of Oxy-Hb was smaller in elderly (slope, 9%; peak, 18%; area, 9% of the 22 sites) than young subjects (slope, 32%; peak, 59%; area, 27% of the 22 sites). Regarding the response magnitude, the age-related difference in the initial slope of the Oxy-Hb response was detected in two sites [(DLPFC (ch13) and frontopolar area (ch1)]. The age-related difference in the peak of the Oxy-Hb response was detected in three sites [DLPFC (ch13, 14) and pars triangularis Broca’s area (ch18)]. No differences in the area component were detected between the age groups.
Correlations between the Oxy-Hb response and performance speed (100 answers/total time period)
The relationships between the three components of the Oxy-Hb response and cognitive performance speed (100 answers/total period) in young and elderly subjects are shown in Figs. 8, 9, 10. With the slope of Oxy-Hb responses and cognitive performance (Fig. 8a), significant relationships [average correlation coefficient (γ) = 0.62 ± 0.02, range 0.52–0.72, P < 0.05] were detected at 13 sites [DLPFC (ch5, 10, 13, 16, 17, 22), frontopolar area (ch1, 2, 6, 7, 12), and pars triangularis Broca’s area (ch14, 18)]. The average slope of the significant regression lines was 0.41 ± 0.05 (answers)/(µM cm) and the intersection point was 1.18 ± 0.02 (answers/s). In the word-reading test (Fig. 8b), there were significant correlations (γ = 0.57 ± 0.02, range 0.51–0.68, P < 0.05) between the initial slope of Oxy-Hb and the performance speed at seven sites [DLPFC (ch5, 13, 17) and frontopolar (ch1–3, 6)]. The average slope of the significant regression lines [0.29 ± 0.06 (answers)/(µM cm)] was not different (P > 0.05) from the slope for the Stroop test, while the average intersection point of the regression lines shifted upward (1.83 ± 0.02 answers/s). Importantly, the slopes of the linear approximation were greater in young subjects than those in elderly subjects during Stroop test irrespective of the significance. With the word reading, reversely, the slopes of the linear approximation were greater in elderly subjects than those in young subjects. It is suggested that the greater increase in the Oxy-Hb responses at the initial phase of the cognitive task is associated with the higher cognitive performance (increased performance speed). The blunted slope of the linear approximation in the elderly subjects compared with that in the young subjects might reflect the slower cognitive performance speed along with less responsiveness of the Oxy-Hb responses.
Fig. 8.
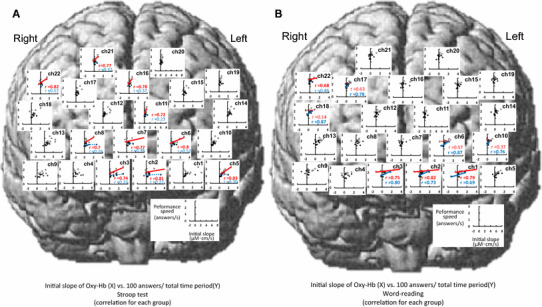
Relationships between the initial slope [(µM cm)/s] of the Oxy-Hb response and cognitive performance speed (answers/s) during the Stroop test (a) and word-reading test (b) across the data consolidated in nine young (white circles) and nine elderly subjects (black circles). If a significant correlation (P < 0.05) is obtained, a solid linear regression line is inserted (red, young, blue, elderly). The correlation coefficients (γ) by a Pearson’s method are inserted in all significant comparisons. The dotted red or blue line was presented, for comparison between young and elderly subjects, in the channels with no significant correlations. The rule of data presentation in Figs. 9 and 10 is the same as this figure
Fig. 9.
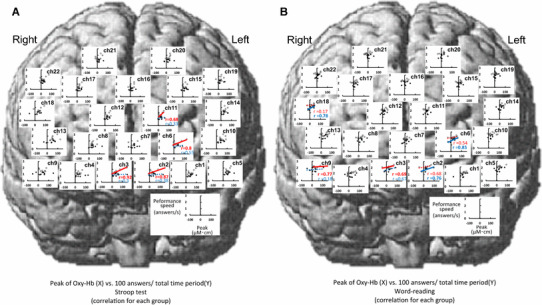
Relationships between the peak (µM cm) of the Oxy-Hb response and cognitive performance speed (answers/s) during the Stroop test (a) and word-reading test (b) across the data consolidated in nine young (white circles) and nine elderly subjects (black circles)
Fig. 10.
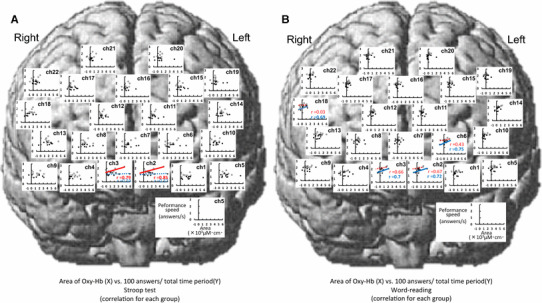
Relationships between the area under the curve [×103 (µM cm) s] of the Oxy-Hb response and cognitive performance speed (answers/s) during the Stroop test (a) and word-reading test (b) across the data consolidated in nine young (white circles) and nine elderly subjects (black circles)
With respect to the peak of the Oxy-Hb response (Fig. 9), the significant relationship between the peak component and performance speed was observed at five sites for the Stroop test [frontopolar area (ch2, 3, 6), DLPFC (ch22), and pars triangularis Broca’s area (ch18)] and at seven sites for the word-reading test [frontopolar area (ch1, 2, 3, 6), DLPFC (ch5, 9), and pars triangularis Broca’s area (ch18)]. The average slope of the significant regression lines [0.01 ± 0.001 (answers/s)/(µM cm)] was the same between the Stroop and word-reading tests and was gentler than in the case of the initial slope component. Similarly, the average intersection point of 1.22–1.83 answers/s was the same between the Stroop and word-reading tests. With respect to the area under the curve of the Oxy-Hb response (Fig. 10), no significant correlations with the performance speed were found at any site of the prefrontal cortices except for two sites [frontopolar area (ch6) and DLPFC (ch18)], irrespective of the Stroop or word-reading test.
Discussion
To test the hypothesis that an incremental rate of the prefrontal oxygenation at the initial period of a cognitive test reduces in elderly subjects, resulting in slowdown of cognitive performance, we compared the dynamic spatiotemporal characteristics of the prefrontal Oxy-Hb response during a Stroop test between elderly and young subjects. The major findings of this study are that (1) the Stroop interference time approximately doubled in elderly as compared to young subjects; (2) the Stroop test caused an increase in Oxy-Hb of the rostral prefrontal cortex, but not in the caudal prefrontal cortex, in both age groups; (3) the initial slope of the Oxy-Hb response significantly reduced in elderly as compared to young subjects, while the peak and area under the curve of the Oxy-Hb response were not different between the two groups; (4) the initial slope of the Oxy-Hb response significantly correlated with performance speed for the Stroop test (as estimated by the 100 answers/total time period), whereas the peak and area component of the Oxy-Hb response had no or slight correlation with the performance speed. Taken together, it is likely that the initial slope of the prefrontal Oxy-Hb response during the Stroop test is a more sensitive indicator of the cognitive performance and that a decrease in the initial slope of the prefrontal Oxy-Hb response suggests age-related deterioration of cognitive performance.
Cognitive performance during the Stroop test
Before quantifying the effect of age on cognitive function, it is important to discuss whether a level of intentional effort was maintained constant among subjects and/or groups. Similar to our previous results [6], the PR and MAP were significantly increased during Stroop test and word-reading test in both groups, which could be assumed as an index of the subjective effort. There were no differences in these cardiovascular responses between Stroop and word-reading test, or between elderly and young subjects, suggesting that the effort the subjects gave during the task was comparable.
Stroop cognitive performance consists of both simple congruent reaction and cognitive incongruent interference. We attempted to discriminate the two factors, referring to the response during the congruent word-reading test. According to the present results, both the time periods for the Stroop and for the word-reading test were prolonged in elderly as compared to young subjects, in agreement with previous studies [3–5]. The age-related increase in the time period for the word-reading test suggested deterioration of the simple reaction involving in visual perception and verbal motor response. Furthermore, the Stroop interference time approximately doubled in elderly as compared to young individuals (Table 2). Our findings demonstrated not only a reduction in simple reaction response but also deterioration of cognitive function in elderly subjects.
Increased oxygenation in the prefrontal cortex during the Stroop test
The Oxy-Hb responses to the Stroop task were the greatest in the first trial among all four trials in both groups, while the performance of the Stroop test (time score and number of errors) was comparable over the four trials. It is plausible that some subjects had more strained feeling for the first Stroop trial, and other factors different from the cognitive processing for example, a startle and/or attention response due to the unfamiliar task, might influence the Oxy-Hb responses. It was likely that the prefrontal Oxy-Hb response in the first Stroop trial could not purely explain the cognitive performance. To exclude the confounding factors, therefore, we omitted the first trial in the final analyses and focused on the trials two to four, which were more likely to reflect the Oxy-Hb responses to the cognitive task. There were no significant differences in the Oxy-Hb response during Stroop test among the trials two to four.
The signal of Oxy-Hb is considered to reflect tissue blood flow as long as the Deoxy-Hb does not change throughout an experimental intervention [6, 8, 13, 23–27]. Since the increase in prefrontal Oxy-Hb accompanied no significant changes in prefrontal Deoxy-Hb in this study, it is likely that the increase in prefrontal oxygenation was attributed to an increase in rCBF. It is possible that the increase in rCBF might be due to an increase in systemic perfusion pressure. However, this possibility seems unlikely because we usually observed that spontaneous fluctuation of MAP (approximately by 5–20 mmHg) had little influence on the Oxy-Hb (unpublished observation). Skin blood flow involved in the illuminated tissue area might influence the Oxy-Hb signal of the NIRS [28]. However, the possibility is also unlikely because we have reported great dissociation between the responses in prefrontal oxygenation and forehead skin blood flow during a cognitive Stroop test and voluntary exercise [6, 13, 29]. Taken together, the increase in rCBF may be derived due to redistribution of blood flow within the cerebral cortex or due to vasodilatation of regional cerebral blood vessels, which may follow augmented neural activity in the prefrontal region.
By recording rCBF with positron emission tomography and fMRI, previous studies have shown that the rostrolateral regions of the prefrontal cortex are involved in the cognitive process for the cognitive incongruent interference [30–32]. A lesion or an artificial inactivation (by repetitive transcranial magnetic stimulation) of the rostrolateral prefrontal cortex diminished the accuracy of a complex cognitive task [30–33]. In line with these studies, the oxygenation response during Stroop test in the present study was greater in the rostrolateral subregion of the prefrontal cortex than the response in the caudal subregion in both age groups. Differently to the caudal prefrontal cortex, which is thought to be responsible for the simple congruent reaction [30], the rostrolateral regions of the prefrontal cortex may play an important role in the complex cognitive processing.
In elderly subjects, age-related structural and functional alterations of the cerebral neurovascular unit may decrease prefrontal oxygenation, because several structural (such as volume reductions of various brain regions [34] and white matter alterations [35]) and pharmacological changes in the dopaminergic system [36] change with aging. Aging may also lead to degeneration of the vascular system, especially a decrease in the regional cerebral blood flow [37, 38], which is accompanied by a reduction in the cerebral metabolic rate for oxygen [39]. Importantly, previous studies also showed that the regional cerebral blood flow negatively correlated with the age of the individuals studied [38, 40–42], suggesting that prefrontal oxygenation decreases in the process of aging. Unfortunately, the absolute value of Oxy- and Deoxy-Hb concentration could not be obtained in the present study, because the limitation of the NIRS technique (the path length of near-infrared light within the tissue was unknown). Although we could not compare directly the baseline value of Oxy- or Deoxy-Hb between young and elderly subjects, it is suggested that the baseline value had no correlation with the cognitive performance during Stroop test [6]. By using NIRS here, therefore, the relative changes in Oxy- and Deoxy-Hb response against the baseline values during a cognitive task, rather than the absolute values of those, are more sensitive to predict the cognitive performance-associated changes in the prefrontal oxygenation.
The initial slope of the prefrontal Oxy-Hb response
The performance of the task might change dynamically as well as Oxy-Hb throughout the task. If it is the case, not only the initial slope but also the peak value, which is often utilized in the literature, cannot fully explain the response during the whole period of a task, because the former takes the rising time and amplitude of the peak response into account and the latter only takes the peak response amplitude into account. In this scenario, it seems the parameter of area may be more sensitive to reflect the response throughout the whole task period because it takes more time and amplitude of response in the task period into account. Taking these issues into consideration, in the present study we adopted all these parameters in the analysis to explore the parameter of prefrontal oxygenation, which is sensitive to reflect the ongoing cognitive performance in a short bout of task (100 questions for Stroop test and word reading, respectively), and the difference in the parameter between young and elderly subjects.
The subjects were asked to perform the Stroop test and word-reading task as fast as possible, and we confirmed online that they did their best throughout the experiment and no dramatically changes in the performance in a particular period of a task. We did not adopt the fixed time for the task period but the number of question, because the analysis with a fixed time window would let us to miss some important information. For example, although the peak or area of the Oxy-Hb response to the Stroop test would be smaller in elderly than young subjects with a fixed time window, the elderly took longer time to complete the cognitive task and these parameters might show comparable or even larger values (later peak) than younger subjects if the parameters were calculated until the end of the cognitive task. We postulate that a parameter that is not influenced by the duration of a cognitive test should be utilized for evaluating the Oxy-Hb response in relation to cognitive function. During the Stroop test in this study, the initial slope of Oxy-Hb was significantly blunted in elderly compared to young subjects, whereas no or slight differences in the peak amplitude and area under the Oxy-Hb curve were detected between the two age groups in the circumstance. As a matter of fact, the initial slope of Oxy-Hb response was independent of the duration of a cognitive task. We therefore considered that the initial slope component of the Oxy-Hb response is a better indicator for evaluating the prefrontal response during cognitive processing. The detection of the initial slope of Oxy-Hb may provide a clear difference in cognitive response between young and elderly subjects. In addition, the age-related reduction in the initial slope of the Oxy-Hb response was observed in the rostrolateral subregion of the prefrontal cortex during the Stroop test, whereas the initial slope failed to show a significant age-related difference in the rostromedial and caudal subregions of the prefrontal cortex. Taken together, it is likely that a reduction in the incremental rate of prefrontal oxygenation may appear in the rostrolateral subregion in association with the age-related deterioration of cognitive performance.
Relationships between initial slope of the Oxy-Hb response and cognitive performace speed
With the Stroop test (Figs. 8a, 9a, and 10a), the performance speed had no significant correlation with the initial slope, peak, and area of Oxy-Hb in elderly subjects, while several channels showed a significant correlation with those in young subjects (ten channels with initial slope, four channels with peak, and zero channels with area). Not only the number of channels but also the slope of linear regression line was larger when plotting the performance speed against the initial slope of the Oxy-Hb response, compared with that plotting the performance speed against the peak or area of the Oxy-Hb response. Furthermore, in these channels which showed a significant correlation between the performance speed and Oxy-Hb response, we could find that the slopes of the regression line were obviously larger in young subjects than those in elderly subjects. In young and elderly subjects, therefore, there was a distinct difference in the Oxy-Hb response and in the relationship between the cognitive performance and Oxy-Hb response during the Stroop test. It is also suggested that the initial slope, but not the peak or area, of the Oxy-Hb response during Stroop test was sensitive to correlate with the cognitive performance.
With word reading (Figs. 8b, 9b, and 10b), only a few channels showed a significant correlation between the cognitive performance and the Oxy-Hb response in young subjects (four channels with initial slope, two channels with peak, and zero channels with area). The slope of linear regression line for the plots of performance speed against the initial slope or peak of Oxy-Hb response during word reading was smaller, and the intercept value of linear regression line was larger than that during Stroop test (Fig. 8a, b), suggesting less contribution of the prefrontal oxygenation to the performance speed during such a simple reaction task of word reading than that during Stroop test. Surprisingly, in elderly subjects, several channels showed significant correlation between the performance speed and Oxy-Hb response during word reading (seven channels with initial slope, three channels with peak, and four channels with area), although no channels showed a significant correlation during Stroop test as mentioned above. Speculatively, in elderly subjects the responsiveness of prefrontal oxygenation decreases in association with the decline in the cognitive function, and that the prefrontal oxygenation may be no longer able to contribute to a cognitive task such as Stroop test, but play a role in a simple reaction task such as word reading.
Limitations
Some substantial problems are involved in this study. First, the NIRS measurement was limited to the regions near the cortical surface and the rCBF changes in deeper cerebral structures could not be measured. Second, the NIRS data in the temporal cortex and occipital cortex were lacking due to a technical limitation in this study, although the rCBF in the cortical regions increased during cognitive tests [9, 11, 17]. Third, the response in the prefrontal Oxy-Hb and cognitive function might be influenced by medical condition and/or taking prescription medicines in some elderly subjects, although in the present study there was no obvious difference in the physical characteristics, cognitive performance, and prefrontal oxygenation response due to the medical condition with our nine elderly subjects (five of them were taking medicines and four were not). Further experiments with more populations are needed to clarify whether the medical condition affects the prefrontal oxygenation response and cognitive performance.
Conclusions
The Oxy-Hb in the rostrolateral, but not caudal, prefrontal cortex increased during the Stroop test in both young and elderly subjects. The initial slope component, rather than the peak or area of the prefrontal Oxy-Hb response, had substantial correlation with the cognitive performance speed. The present study provided evidence that the incremental rate of prefrontal oxygenation may decrease in the progression of ageing, resulting in a decline in incongruent cognitive performance.
Author contributions
KE and KM designed the experiments; KE, KM, NL, MI, and KI performed the experiments; KE analyzed the data; KE, KM, and NL interpreted results of experiments; KE and KM drafted the article; KE, KM, NL, and KI revised the article critically for intellectual content. All authors approved the final version of the manuscript.
Funding
This study was partly supported by a Grant-in-Aid for Scientific Research (B) (K.M.) and Grant-in-Aid for Young Scientists (B) (K.E.) from Japan Society for the Promotion of Science, a Young Researcher Support Program of academia-industry collaboration from Hiroshima University (K.E.), and the Center of Innovation Program from Japan Science and Technology Agency (K.M.).
Compliance with ethical standards
Ethical approval
All procedures and protocols conducted in the present study were performed in accordance with the 1964 Declaration of Helsinki and its later amendments and approved by the Institutional Human Ethical Committee of Hiroshima University (Permit no. 1308). Written informed consent was obtained from all of the participants prior to the experiments.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 2.Kovach CK, Daw ND, Rudrauf D, Tranel D, O’Doherty JP, Adolphs R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. J Neurosci. 2012;32:8434–8442. doi: 10.1523/JNEUROSCI.5468-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West R. Age differences in lapses of intention in the Stroop task. J Gerontol B Psychol Sci Soc Sci. 1999;54:34–43. doi: 10.1093/geronb/54B.1.P34. [DOI] [PubMed] [Google Scholar]
- 4.Schroeter ML, Zysset S, Kruggel F, von Cramon DY. Age dependency of the hemodynamic response as measured by functional near-infrared spectroscopy. Neuroimage. 2003;19:555–564. doi: 10.1016/S1053-8119(03)00155-1. [DOI] [PubMed] [Google Scholar]
- 5.Mathis A, Schunck T, Erb G, Namer IJ, Luthringer R. The effect of aging on the inhibitory function in middle-aged subjects: a function MRI study coupled with a color-matched Stroop task. Int J Geriatr Psychiatry. 2009;10:1062–1071. doi: 10.1002/gps.2222. [DOI] [PubMed] [Google Scholar]
- 6.Endo K, Matsukawa K, Liang N, Nakatsuka C, Tsuchimochi H, Okamura H, Hamaoka T. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J Physiol Sci. 2013;63:287–298. doi: 10.1007/s12576-013-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupfermann I. Localization of higher cognitive and affective functions: the Association Cortices. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of neural science. 3. New York: McGraw-Hill; 1991. pp. 823–829. [Google Scholar]
- 8.Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Lei X, Ding C, Li H, Chen A. The neural mechanisms of semantic and response conflict: an fMRI study of practice-related effects in the Stroop task. Neuroimage. 2013;66:577–584. doi: 10.1016/j.neuroimage.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Erickson KI, Milham MP, Colcombe SJ, Kramer AF, Banich MT, Webb A, Cohen NJ. Behavioral conflict, anterior cingulate cortex, and experiment duration: implications of diverging data. Hum Brain Map. 2004;21:98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Zysset S, Schroeter ML, Neumann J, von Cramon DY. Stroop interference, hemodynamic response and aging: an event-related fMRI study. Neurobiol Aging. 2007;28:937–946. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Matsukawa K, Ishii K, Liang N, Endo K, Ohtani R, Nakamoto T, Wakasugi R, Kadowaki A, Komine H. Increased oxygenation of the cerebral prefrontal cortex prior to the onset of voluntary exercise in humans. J Appl Physiol. 2015;119:452–462. doi: 10.1152/japplphysiol.00406.2015. [DOI] [PubMed] [Google Scholar]
- 14.Hyodo K, Dan I, Suwabe K, Kyutoku Y, Yamada Y, Akahori M, Byun K, Kato M, Soya H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol Aging. 2012;33:2621–2632. doi: 10.1016/j.neurobiolaging.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, Soya H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Hock C, Müller-Spahn F, Schuh-Hofer S, Hofmann M, Dirnagl U, Villringer A. Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near-infrared spectroscopy study. J Cereb Blood Flow Metab. 1995;15:1103–1108. doi: 10.1038/jcbfm.1995.137. [DOI] [PubMed] [Google Scholar]
- 17.Ehlis AC, Herrmann MJ, Wagener A, Fallgatter AJ. Multi-channel near-infrared spectroscopy detects specific inferior-frontal activation during incongruent Stroop trials. Biol Psychol. 2005;69:315–331. doi: 10.1016/j.biopsycho.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Endo K, Liang N, Ishii K, Idesako M, Matsukawa K. Changes in prefrontal oxygenation associated with the Stroop interference task in elderly subjects. J Physiol Sci. 2014;64(Suppl):S128. [Google Scholar]
- 19.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 20.Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 22.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 23.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshi Y, Tamura M. Dynamic multichannel near-infrared optical imaging of human brain activity. J Appl Physiol. 1993;75:1842–1846. doi: 10.1152/jappl.1993.75.4.1842. [DOI] [PubMed] [Google Scholar]
- 25.Elwell CE, Cope M, Edwards AD, Wyatt JS, Delpy DT, Reynolds EO. Quantification of adult cerebral hemodynamics by near-infrared spectroscopy. J Appl Physiol. 1994;77:2753–2760. doi: 10.1152/jappl.1994.77.6.2753. [DOI] [PubMed] [Google Scholar]
- 26.Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol. 2001;90:1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- 27.Rostrup E, Law I, Pott F, Ide K, Knudsen GM. Cerebral hemodynamics measured with simultaneous PET and near-infrared spectroscopy in humans. Brain Res. 2002;954:183–193. doi: 10.1016/S0006-8993(02)03246-8. [DOI] [PubMed] [Google Scholar]
- 28.Miyazawa T, Horiuchi M, Komine H, Sugawara J, Fadel PJ, Ogoh S. Skin blood flow influences cerebral oxygenation measured by near infrared spectroscopy during dynamic exercise. Eur J Appl Physiol. 2013;113:2841–2848. doi: 10.1007/s00421-013-2723-7. [DOI] [PubMed] [Google Scholar]
- 29.Asahara R, Matsukawa K, Ishii K, Liang N, Endo K. The prefrontal oxygenation and ventilatory responses at start of one-legged cycling exercise have relation to central command. J Appl Physiol. 2016;121:1115–1126. doi: 10.1152/japplphysiol.00401.2016. [DOI] [PubMed] [Google Scholar]
- 30.Azuar C, Reyes P, Slachevsky A, Volle E, Kinkingnehun S, Kouneiher F, Bravo E, Dubois B, Koechlin E, Levy R. Testing the model of caudo-rostral organization of cognitive control in the human with frontal lesions. Neuroimage. 2014;84:1053–1060. doi: 10.1016/j.neuroimage.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 32.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 33.Bahlmann J, Beckmann I, Kuhlemann I, Schweikard A, Münte TF. Transcranial magnetic stimulation reveals complex cognitive control representations in the rostral frontal cortex. Neuroscience. 2015;300:425–431. doi: 10.1016/j.neuroscience.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 34.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.WNL.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 35.Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 36.Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59:898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Reich T, Rusinek H. Cerebral cortical and white matter reactivity to carbon dioxide. Stroke. 1989;20:453–457. doi: 10.1161/01.STR.20.4.453. [DOI] [PubMed] [Google Scholar]
- 38.Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol. 1999;172:213–218. doi: 10.2214/ajr.172.1.9888770. [DOI] [PubMed] [Google Scholar]
- 39.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- 40.Tachibana H, Meyer JS, Kitagawa Y, Rogers RL, Okayasu H, Mortel KF. Effects of aging on cerebral blood flow in dementia. J Am Geriatr Soc. 1984;32:114–120. doi: 10.1111/j.1532-5415.1984.tb05850.x. [DOI] [PubMed] [Google Scholar]
- 41.Vriens EM, Kraaier V, Musbach M, Wieneke GH, van Huffelen AC. Transcranial pulsed Doppler measurements of blood velocity in the middle cerebral artery: reference values at rest and during hyperventilation in healthy volunteers in relation to age and sex. Ultrasound Med Biol. 1989;15:1–8. doi: 10.1016/0301-5629(89)90125-7. [DOI] [PubMed] [Google Scholar]
- 42.Schultz SK, O’Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. NeuroReport. 1999;10:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]


