Abstract
Preterm twins have a higher morbidity rate of patent ductus arteriosus (PDA) than do singletons. However, the effect of multiple births on maturation of the ductus arteriosus (DA) has not been reported. Because intimal thickening (IT) is required for DA anatomical closure, we examined IT development in the DA of preterm twins and singletons. Sheep DA tissues obtained from preterm fetuses were subjected to elastica van Gieson staining to evaluate IT. The total IT score in each DA was the sum of the IT scores obtained from six evenly divided parts of the DA, which was positively correlated with gestational ages in singletons. Total IT scores were smaller in preterm twins than in singletons, although no difference in gestational age, birth weight, or gender ratio was observed. These data suggest that IT development of the DA is attenuated in sheep preterm twins, which may affect the higher morbidity of PDA.
Keywords: Ductus arteriosus, Intimal thickening, Preterm infant, Multiple births
Introduction
The ductus arteriosus (DA) is an arterial shunt connecting the pulmonary artery and the aorta. Although the DA is necessary for maintaining fetal circulation, persistent patent DA (PDA) after birth causes systemic hypo-perfusion and pulmonary congestion, and thus affects morbidity and mortality in newborns [1–3]. Very preterm infants frequently suffer from PDA, and more than 60% of extremely preterm infants (<28 weeks gestation) exhibit PDA [4].
Recent studies have suggested that preterm multiple births have a higher ratio of morbidity and mortality than preterm singletons [5, 6]. Specifically, some studies comparing the clinical outcome of singleton and multiple births have demonstrated that multiple births carry a higher risk for PDA [7, 8]. Kirkby et al. examined a total of 5507 infants including singletons, twins, and higher-order multiples and found that multiples had higher rates of PDA than singletons did [7]. Nielsen et al. reported that infants of multiple births from 27 to 29 weeks gestation were more likely to have PDA [8]. Although some studies have demonstrated no association between PDA and multiple births [9, 10], a European population-based cohort study also recently demonstrated that a high risk of PDA treatment was associated with multiple births in preterm infants born at 24–31 weeks of gestation [11].
Closure of the DA requires two mechanisms: functional closure induced by postnatal muscle contraction and anatomical closure achieved via morphological remodeling [1, 2]. Morphological DA remodeling normally starts even before 22 weeks of gestation and continues to develop throughout the fetal period [1]. DA remodeling is associated with intimal thickening (IT) formation, which is characterized by duplication and/or interruption of internal elastic lamina, deposition of extracellular matrix in the subendothelial region, and migration of medial smooth muscle cells (SMCs) into the subendothelial space [1]. We therefore hypothesized that morphological DA remodeling was attenuated in multiple births.
To investigate whether multiple births affect morphological DA remodeling, we chose sheep because they are a valuable model for comparative studies between twins and singletons. Sheep usually have one or two offspring per pregnancy in contrast to other animal models, which often have litters.
Materials and methods
Animals and materials
DA tissues of preterm sheep fetuses (term age: 147 days) were obtained at Tohoku University School of Medicine. To compare DA remodeling in preterm singletons and twins, we utilized DA tissues of singletons (gestational age: 103–117; n = 8) and twins (gestational age: 103–112; n = 4) who underwent an operation to connect an artificial placenta system [12, 13] because it provided a more efficient use of resources. Physiological conditions were stably maintained during 59–72 h in all preterm sheep fetuses used in this study [12, 13]. We used blood from the respective maternal sheep for the extracorporeal circulation device. We administrated lipo-prostaglandin E1 (10–20 ng/kg/min) and milrinone (0.5 ng/kg/min) to both groups to maintain physiological circulatory condition and tissue homeostasis during the use of an artificial placenta system [12, 13]. Laminar flow of right-to-left shunting via the DA was detected by Doppler echocardiography in all preterm sheep. Two-way ANOVA analysis showed no difference in blood pressure during the experiment between singletons and twins (range of average blood pressure from 0 to 48 h under the artificial placenta system: 41.4–48.2 vs. 37.2–48.1 mmHg, respectively). There was no difference in the duration for connecting the artificial placenta system in fetuses of singletons and twins (65.1 ± 4.8 vs. 64.8 ± 6.3 h, p = 1.00). To define the stages of IT formation, we used fetuses at 82, 100, and 147 days gestation, in addition to the preterm sheep as described above.
This study was conducted with the approval of the institutional animal care and use committees of the Tohoku University School of Medicine (reference number: 2013 MdA-008, 2014MdA-219, 2015MdA-209). Characteristics of preterm sheep singletons and twins are shown in Table 1.
Table 1.
Characteristics of the two groups
| Variables | Singleton (n = 8) | Twin (n = 4) | P value |
|---|---|---|---|
| Gestational age (days) | 106 ± 6.02 | 106 ± 4.27 | 0.525 |
| Birth weight (kg) | 1.59 ± 0.55 | 1.16 ± 0.29 | 0.202 |
| Male (%) | 4 (50%) | 2 (50%) | 1.000 |
Continuous variables are expressed as mean ± SD, and were statistically analyzed between the two groups using Mann–Whitney U test. Categorical variables are shown as the number of positive cases, with the percentages indicated in parentheses, and were statistically analyzed using Fisher’s exact test. There were no significant differences in the scores on any of the items between the two groups
Tissue staining
The mid portions between the pulmonary artery and the aorta of sheep DA tissues were used for histological analysis. Paraffin-embedded tissue sections were subjected to elastica van Gieson staining (Muto Pure Chemicals, Tokyo, Japan), as previously described [16, 23]. Each sample image was obtained using BIOREVO bz-9000 (KEYENCE, Osaka, Japan).
Evaluation of IT development in sheep DA
We defined the developmental stages of IT formation in sheep DA using singleton sheep fetuses at 82–147 days of gestation as follows (Fig. 1): stage 0 (score 0), internal elastic laminae are continuously aligned between endothelial cells and SMCs, and no intimal thickening is observed; stage 1 (score 1), duplication or partial disruption of internal elastic laminae are observed, and a few SMCs have migrated into the subendothelial region; stage 2 (score 2), internal elastic laminae are obviously disrupted and intimal thickening consisting of SMCs are readily recognized; stage 3 (score 3), internal elastic laminae are extremely disrupted and a substantial number of SMCs have migrated into the luminal region.
Fig. 1.
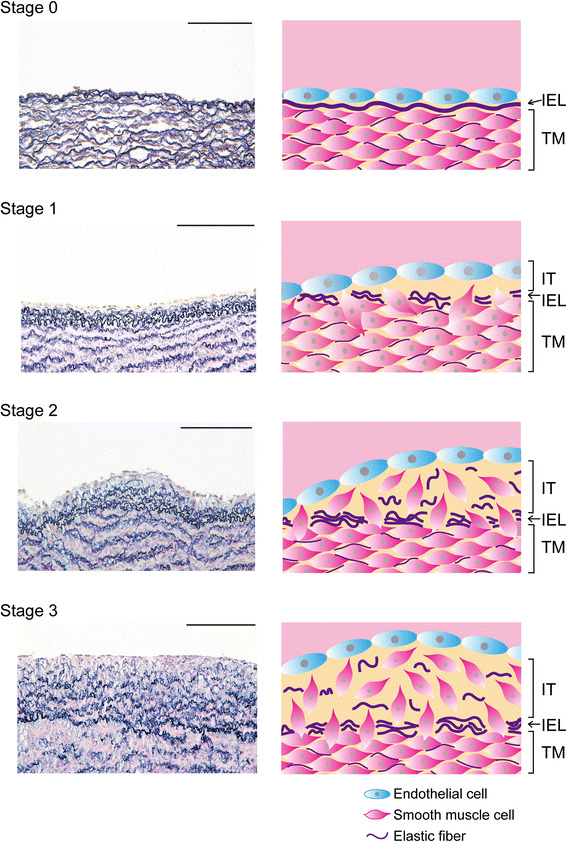
Staging of IT in sheep DA. The left column demonstrates the elastica van Gieson staining of singleton sheep fetuses. All parts of the DA of sheep at 82 days gestation exhibited a single internal elastic lamina and no IT formation (stage 0). The DA of sheep at 100 days gestation contained a part showing the duplication or partial disruption of internal elastic laminae with very modest IT formation (stage 1). The DA of sheep at 103 days gestation showed modest IT formation accompanied by apparent SMC migration (stage 2). All parts of the DA at 147 days gestation exhibited prominent IT formation (score 3). The right panels show schematic models corresponding to histological changes in each stage. IEL internal elastic lamina, TM tunica media, IT intimal thickening. Scale bars 100 µm
Each DA tissue section was evenly divided into six parts, and an individual IT score was determined for all six parts. The total IT score of each DA was the sum of the IT scores for each of the parts (maximum score: 18).
Statistical analysis
In the analysis of sheep characteristics, continuous variables are expressed as mean ± SD, and were analyzed by Mann–Whitney U test. Categorical variables were analyzed using Fisher’s exact test. Spearman’s rank correlation coefficient was used to test the association between IT scores and gestational ages. The difference in IT formation between singletons and twins was analyzed using Mann–Whitney U test. A value of p < 0.05 was considered significant.
Results
Time-dependent IT development in preterm singleton sheep
To investigate whether IT formation is attenuated in preterm twins, we analyzed sheep DA of preterm singletons and twins, in which no significant difference in gestational age, birth weight, or gender ratio was observed between these two groups (Table 1). Table 2 showed IT scores of preterm sheep singletons and twins. All DA tissues were sampled as short-axis slices from the central portions between the pulmonary artery and the aorta. The most prominent IT area of each section was defined as area 1, and subsequent areas were analyzed in the circumferential direction.
Table 2.
Intimal thickening score in sheep fetuses
| Sheep no. | Day of gestation | Intimal thickening score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | Area 4 | Area 5 | Area 6 | Total | |||
| Singleton | 1 | 117 | 3 | 3 | 3 | 3 | 2 | 2 | 16 |
| 2 | 113 | 3 | 3 | 2 | 1 | 1 | 2 | 12 | |
| 3 | 103 | 2 | 2 | 2 | 2 | 1 | 1 | 10 | |
| 4 | 103 | 3 | 2 | 2 | 2 | 1 | 1 | 11 | |
| 5 | 103 | 2 | 2 | 2 | 2 | 2 | 1 | 11 | |
| 6 | 103 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
| 7 | 101 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
| 8 | 101 | 2 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Twin | 1 | 112 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| 2 | 107 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
| 3 | 103 | 2 | 2 | 1 | 0 | 0 | 0 | 5 | |
| 4 | 103 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |
This table contains all of the raw data from sheep DA tissues
Representative histological images of DA tissues from singleton are shown in Fig. 2a. In preterm singletons, IT score was correlated with gestational age (Fig. 2b), which appears to correspond to histological studies of human DA [14–16]. These data suggest that IT formation develops time-dependently in preterm singleton sheep as in humans [1, 14–16].
Fig. 2.
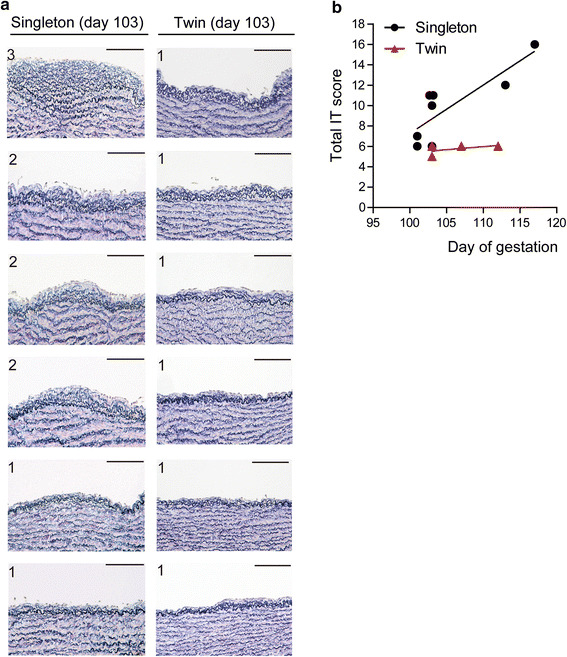
IT formation in the DA in preterm sheep singletons and twins. a Representative DA images of elastica van Gieson staining in a preterm singleton and a twin. The IT score in each tissue section is indicated in the top left of each image. Scale bars 100 µm. b Correlation between total IT score and gestational age in singletons (n = 8, Spearman r = 0.827, p = 0.015) and twins (n = 4, Spearman r = 0.544, p = 0.417)
IT formation in the DA was attenuated in preterm twin sheep
An association of total IT score with gestational age was unable to be detected in preterm twins when we analyzed four fetuses at 103–112 days gestation (Fig. 2b). Total IT scores in the preterm twin group were significantly lower than those in the singleton group (5.8 ± 0.5 vs. 9.9 ± 3.4, p = 0.03). Although we examined only the central portion of the DA, these data suggest that preterm twins have impaired IT formation in the DA compared to that of singletons.
Discussion
In the present study, histological analysis revealed that the beginning of IT formation in sheep DA was detectable at around 100 days of gestation, and IT continued to develop toward the late gestation period. These processes were attenuated in preterm twin sheep compared to singletons, making ours the first study demonstrating the difference in DA remodeling between singletons and twins.
The extensive morphological remodeling of the human DA wall, i.e., IT formation, that begins during mid-gestation leads to the permanent closure of the DA after birth [1, 16–19]. This DA-specific histological change has also been reported in other mammalians including sheep [20, 21], dogs [22], and rodents [23–25]. In this study, we analyzed IT developmental change via a grading score and found in the DA of sheep singletons that duplication and interruption of the internal elastic laminae are readily observed at 101 days of gestation. Migration of SMCs into the subendothelial region occurred at 103 days of gestation, and thicker IT formation was detectable after 113 days of gestation. We were unable to determine the exact time course of IT formation because our data contain a smaller sample number and there were gaps between the gestational ages examined. However, these data suggested that sheep DA exhibited modest but similar IT formation process to human DA, at least in singletons.
Our previous reports suggest that prostaglandin E2 (PGE2) promotes SMC migration through EP4 receptor signaling pathways including protein kinase A, phospholipase C, and exchange protein activated by cyclic AMP (Epac), which leads to IT formation in the DA [1, 2, 23, 24, 26, 27]. As in humans, plasma PGE2 concentrations in sheep increase progressively in the fetal circulation toward term [28], and PGE2-EP4 signaling contributes to vascular tone of the DA [29, 30]. The present study demonstrated that IT development progressed between 103 and 117 days of gestation. It has been reported in sheep that fetal plasma PGE2 concentration was 1.90 ± 0.23 nmol/l at 75 to 95 days gestation and increased after 95 days gestation to exceed 11.7 nmol/l by 125 days gestation [28]. These changes in plasma PGE2 concentration in sheep seem to correspond to the time-course of IT development. As in humans, it has been reported that EP4 is a primary receptor among PGE receptors in sheep DA [30, 31]. PGE2-EP4 signaling may play a role in IT formation as well as vascular tone in sheep.
We demonstrated that IT formation was less developed in preterm twin sheep. We do not have DA samples of near-term twins with artificial placenta. However, our preliminary data of two samples of near-term twins without connecting artificial placenta exhibited marked IT formation (day 141: score 12, and day 146: score 18) (data not shown). These data suggest that IT development in the DA of sheep preterm twins advances late. We currently do not know the cause of poorly developed IT in preterm twins. Some studies in sheep have indicated that the basal function of the hypothalamic–pituitary–adrenal (HPA) axis is blunted in twin fetuses, marked by lower levels of fetal plasma adrenocorticotropic hormone (ACTH) and cortisol [32, 33]. Edwards et al. demonstrated that basal levels of ACTH and cortisol plasma concentrations were lower in sheep twins than in singletons at 115–146 days and 127–146 days gestation, respectively [32]. Adrenocortical responsiveness as well as basal adrenocortical function is blunted in the twins relative to the singletons [33]. Gardner et al. reported that fetal plasma concentration of cortisol in response to acute hypoxaemia and to exogenous ACTH were blunted in twins relative to singletons [33].
It has been recognized that PGE2 activates the HPA axis [34, 35]. It was reported that PGE2 infusion into the sheep fetus caused a pronounced increase in plasma ACTH [34–37]. The fetal infusion of PGE2 also increased plasma concentration of cortisol irrespective of ACTH [36, 37]. Furthermore, increased cortisol output during pregnancy regulates expression of prostaglandin synthase type 2 (PGHS-2) in the placenta, resulting in increased PGE2 in the fetal circulation [38]. However, based on the evidence of blunted ACTH and cortisol levels in twins, PGE2 plasma concentration may be maintained at a lower level and may affect IT formation.
Another line of studies further demonstrated the difference between twins and singletons. Sheep preterm twins had a lower plasma insulin concentration than singletons did [39]. Philipps et al. showed that the inhibition of PGE2 production by the administration of indomethacin to a fetal lamb blocked the release of insulin from the fetal pancreas in response to a glucose challenge [40, 41], suggesting that PGE2 plays a role in fetal insulin production. These data also imply the possibility that plasma concentration of PGE2, which induces IT formation, may be lower in twins. To the best of our knowledge, there is currently no report showing the difference in plasma PGE2 plasma concentration and EP4 expression between preterm singletons and twins. Further study is required to clarify associations between PGE2-EP4 signaling and IT formation in multiple birth.
There are several limitations to this study. Because we utilized the DA from the experiment involving artificial placenta to make a more efficient use of resources, we were unable to exclude the effect of surgical manipulation. Additionally, we had a smaller number of twins and twins showed a trend to be lighter in body weight. Furthermore, twining is relatively common in sheep, with the majority being dizygotic. Therefore, each fetus has its own distinct placenta and placental vasculature, i.e., a diamnionic-dichorionic (DD) twin, which differs from the characteristics of human multiple pregnancies. Therefore, these results cannot simply be extrapolated to humans. However, further exploration of the differences between twin and singleton pregnancies, i.e., the contribution of PGE2, may help to explain the higher morbidity of PDA multiple births.
Acknowledgements
The authors are grateful to Yuka Sawada for her technical assistance. We thank Megumi Fukui and Takashi Nakamura for technical advice on between-species differences in pregnancy physiology.
Author contributions
The authors’ contributions are as follows: SI, UY, SW, and TM initiated the project. SI, UY, and JS were involved in the design of the experiments. SI, UY, JS, SW, TM, SS, HU, RK, YM, MS, and TH conducted the experiments. SI and UY analyzed the data. JS supported the histological experiments. SI, UY, and YI wrote the manuscript. All authors discussed the results and commented on the paper.
Compliance with ethical standards
Conflict of interest
Satoko Ito, Utako Yokoyama, Junichi Saito, Shinichi Sato, Haruo Usuda, Shimpei Watanabe, Ryuta Kitanishi, Yuichiro Miura, Masatoshi Saito, Takushi Hanita, Tadashi Matsuda, and Yoshihiro Ishikawa declare that they have no conflict of interest.
Funding
This study was funded by MEXT/JSPS KAKENHI (SI, 43008732; UY, 16H05358, 15H05761; JS, 16H07107; YI, H1605300, 16K15205), and Yokohama Foundation for Advancement of Medical Science (JS), and the Japan Agency for Medical Research and Development (AMED) (YI, 66890011, 66890023, 17ek0109240h0001, A261TS).
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Contributor Information
Utako Yokoyama, Phone: (+81) 45-787-2575, Email: utako@yokohama-cu.ac.jp.
Yoshihiro Ishikawa, Email: yishikaw@med.yokohama-cu.ac.jp.
References
- 1.Yokoyama U. Prostaglandin E-mediated molecular mechanisms driving remodeling of the ductus arteriosus. Pediatr Int. 2015;57:820–827. doi: 10.1111/ped.12769. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama U, Minamisawa S, Ishikawa Y. Regulation of vascular tone and remodeling of the ductus arteriosus. J Smooth Muscle Res. 2010;46:77–87. doi: 10.1540/jsmr.46.77. [DOI] [PubMed] [Google Scholar]
- 3.Alpan G, Scheerer R, Bland R, Clyman R. Patent ductus arteriosus increases lung fluid filtration in preterm lambs. Pediatr Res. 1991;30:616–621. doi: 10.1203/00006450-199112000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate. 2006;89:330–335. doi: 10.1159/000092870. [DOI] [PubMed] [Google Scholar]
- 5.Louwen F, Antwerpen I, Ernst T, Reichenbach L, Reitter A, Herrmann E, et al. Outcome in single and twin pregnancies at 20 to 24 weeks gestation: ten years experience in one perinatal center. Clin Exp Obstet Gynecol. 2013;40:342–344. [PubMed] [Google Scholar]
- 6.Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. 2016;16:174. doi: 10.1186/s12887-016-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkby S, Genen L, Turenne W, Dysart K. Outcomes and milestone achievement differences for very low-birth-weight multiples compared with singleton infants. Am J Perinatol. 2010;27:439–444. doi: 10.1055/s-0030-1247597. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen HC, Harvey-Wilkes K, MacKinnon B, Hung S. Neonatal outcome of very premature infants from multiple and singleton gestations. Am J Obstet Gynecol. 1997;177:653–659. doi: 10.1016/S0002-9378(97)70160-1. [DOI] [PubMed] [Google Scholar]
- 9.Qiu X, Lee SK, Tan K, Piedboeuf B, Canning R, Canadian Neonatal N. Comparison of singleton and multiple-birth outcomes of infants born at or before 32 weeks of gestation. Obstet Gynecol. 2008;111:365–371. doi: 10.1097/AOG.0b013e318162688f. [DOI] [PubMed] [Google Scholar]
- 10.Brunner B, Hoeck M, Schermer E, Streif W, Kiechl-Kohlendorfer U. Patent ductus arteriosus, low platelets, cyclooxygenase inhibitors, and intraventricular hemorrhage in very low birth weight preterm infants. J Pediatr. 2013;163:23–28. doi: 10.1016/j.jpeds.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Edstedt Bonamy AK, Gudmundsdottir A, Maier RF, Toome L, Zeitlin J, Bonet M, et al. Patent ductus arteriosus treatment in very preterm infants: a European population-based cohort study (EPICE) on variation and outcomes. Neonatology. 2017;111:367–375. doi: 10.1159/000454798. [DOI] [PubMed] [Google Scholar]
- 12.Miura Y, Matsuda T, Funakubo A, Watanabe S, Kitanishi R, Saito M, Hanita T. Novel modification of an artificial placenta: pumpless arteriovenous extracorporeal life support in a premature lamb model. Pediatr Res. 2012;72:490–494. doi: 10.1038/pr.2012.108. [DOI] [PubMed] [Google Scholar]
- 13.Miura Y, Matsuda T, Usuda H, Watanabe S, Kitanishi R, Saito M, Hanita T, Kobayashi Y. A parallelized, pumpless artificial placenta system significantly prolonged survival time in a preterm lamb model. Artif Organs. 2016;40:E61–E68. doi: 10.1111/aor.12656. [DOI] [PubMed] [Google Scholar]
- 14.Slomp J, Gittenberger-de Groot AC, Glukhova MA, Conny van Munsteren J, Kockx MM, Schwartz SM, et al. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol. 1997;17:1003–1009. doi: 10.1161/01.ATV.17.5.1003. [DOI] [PubMed] [Google Scholar]
- 15.Tada T, Wakabayashi T, Nakao Y, Ueki R, Ogawa Y, Inagawa A, et al. Human ductus arteriosus. A histological study on the relation between ductal maturation and gestational age. Acta Pathol Jpn. 1985;35:23–34. [PubMed] [Google Scholar]
- 16.Toda T, Tsuda N, Takagi T, Nishimori I, Leszczynski D, Kummerow F. Ultrastructure of developing human ductus arteriosus. J Anato. 1980;131:25–37. [PMC free article] [PubMed] [Google Scholar]
- 17.Gittenberger-de Groot AC. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. 1977;39:610–618. doi: 10.1136/hrt.39.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jager BV, Wollenman OJ. An anatomical study of the closure of the ductus arteriosus. Am J Pathol. 1942;18:595–613. [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoyama U, Ichikawa Y, Minamisawa S, Ishikawa Y. Pathology and molecular mechanisms of coarctation of the aorta and its association with the ductus arteriosus. J Physiol Sci. 2017;67:259–270. doi: 10.1007/s12576-016-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clyman RI, Chen YQ, Chemtob S, Mauray F, Kohl T, Varma DR, et al. In utero remodeling of the fetal lamb ductus arteriosus: the role of antenatal indomethacin and avascular zone thickness on vasa vasorum proliferation, neointima formation, and cell death. Circulation. 2001;103:1806–1812. doi: 10.1161/01.CIR.103.13.1806. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Dagher E, Johnson DJ, Bedell-Hogan D, Keeley FW, Kagan HM, et al. A developmentally regulated program restricting insolubilization of elastin and formation of laminae in the fetal lamb ductus arteriosus. Lab Invest. 1993;68:321–331. [PubMed] [Google Scholar]
- 22.Gittenberger-de Groot AC, Strengers JL, Mentink M, Poelmann RE, Patterson DF. Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol. 1985;6:394–404. doi: 10.1016/S0735-1097(85)80178-9. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama U, Minamisawa S, Shioda A, Ishiwata R, Jin MH, Masuda M, et al. Prostaglandin E2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation. 2014;129:487–496. doi: 10.1161/CIRCULATIONAHA.113.004726. [DOI] [PubMed] [Google Scholar]
- 25.Tada T, Kishimoto H. Ultrastructural and histological studies on closure of the mouse ductus arteriosus. Acta Anat (Basel) 1990;139:326–334. doi: 10.1159/000147020. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama U, Minamisawa S, Quan H, Akaike T, Suzuki S, Jin M, et al. Prostaglandin E2-activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J Biol Chem. 2008;283:28702–28709. doi: 10.1074/jbc.M804223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama U, Minamisawa S, Katayama A, Tang T, Suzuki S, Iwatsubo K, et al. Differential regulation of vascular tone and remodeling via stimulation of type 2 and type 6 adenylyl cyclases in the ductus arteriosus. Circ Res. 2010;106:1882–1892. doi: 10.1161/CIRCRESAHA.109.214924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deayton JM, Young IR, Thorburn GD. Early hypophysectomy of sheep fetuses: effects on growth, placental steroidogenesis and prostaglandin production. J Reprod Fertil. 1993;97:513–520. doi: 10.1530/jrf.0.0970513. [DOI] [PubMed] [Google Scholar]
- 29.Bouayad A, Kajino H, Waleh N, Fouron JC, Andelfinger G, Varma DR, et al. Characterization of PGE2 receptors in fetal and newborn lamb ductus arteriosus. Am J Physiol Heart Circ Physiol. 2001;280:H2342–H2349. doi: 10.1152/ajpheart.2001.280.5.H2342. [DOI] [PubMed] [Google Scholar]
- 30.Smith GC, Wu WX, Nijland MJ, Koenen SV, Nathanielsz PW. Effect of gestational age, corticosteroids, and birth on expression of prostanoid EP receptor genes in lamb and baboon ductus arteriosus. J Cardiovasc Pharmacol. 2001;37:697–704. doi: 10.1097/00005344-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Bouayad A, Kajino H, Waleh N, Fouron JC, Andelfinger G, Varma DR, et al. Characterization of PGE2 receptors in fetal and newborn lamb ductus arteriosus. Am J Physiol Heart Circ Physiol. 2001;280(5):2342–2349. doi: 10.1152/ajpheart.2001.280.5.H2342. [DOI] [PubMed] [Google Scholar]
- 32.Edwards LJ, McMillen IC. Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo-pituitary adrenal axis in sheep during late gestation. Biol Reprod. 2002;66:1562–1569. doi: 10.1095/biolreprod66.5.1562. [DOI] [PubMed] [Google Scholar]
- 33.Gardner DS, Jamall E, Fletcher AJ, Fowden AL, Giussani DA. Adrenocortical responsiveness is blunted in twin relative to singleton ovine fetuses. J Physiol. 2004;557:1021–1032. doi: 10.1113/jphysiol.2004.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorburn GD. The placenta, PGE2 and parturition. Early Hum Dev. 1992;29:63–73. doi: 10.1016/0378-3782(92)90059-P. [DOI] [PubMed] [Google Scholar]
- 35.Hollingworth SA, Deayton JM, Young IR, Thorburn GD. Prostaglandin E2 administered to fetal sheep increases the plasma concentration of adrenocorticotropin (ACTH) and the proportion of ACTH in low molecular weight forms. Endocrinology. 1995;136:1233–1240. doi: 10.1210/endo.136.3.7867577. [DOI] [PubMed] [Google Scholar]
- 36.Young IR, Deayton JM, Hollingworth SA, Thorburn GD. Continuous intrafetal infusion of prostaglandin E2 prematurely activates the hypothalamo-pituitary-adrenal axis and induces parturition in sheep. Endocrinology. 1996;137:2424–2431. doi: 10.1210/endo.137.6.8641195. [DOI] [PubMed] [Google Scholar]
- 37.Louis TM, Challis JR, Robinson JS, Thorburn GD. Rapid increase of foetal corticosteroids after prostaglandin E2. Nature. 1976;264:797–799. doi: 10.1038/264797a0. [DOI] [PubMed] [Google Scholar]
- 38.Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, et al. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124:1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- 39.Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW, Jr, et al. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metabol. 2011;300:E817–E823. doi: 10.1152/ajpendo.00572.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philipps AF, Matty PJ, Porte PJ, Raye JR. Inhibition of glucose-induced insulin secretion by indomethacin and sodium salicylate in the fetal lamb. Am J Obstet Gynecol. 1984;148:481–487. doi: 10.1016/0002-9378(84)90730-0. [DOI] [PubMed] [Google Scholar]
- 41.Rumball CW, Harding JE, Oliver MH, Bloomfield FH. Effects of twin pregnancy and periconceptional undernutrition on maternal metabolism, fetal growth and glucose-insulin axis function in ovine pregnancy. J Physiol. 2008;586:1399–1411. doi: 10.1113/jphysiol.2007.144071. [DOI] [PMC free article] [PubMed] [Google Scholar]


