Abstract
Animals collect sensory information through self-generated movements. Muscle movements drive active feedback of sensory information and determine large parts of the sensory inputs the animal receives; however, little is known about how this active feedback process modulates the ongoing dynamics of the brain. We made electrophysiological recordings from layer 2/3 neurons of the mouse neocortex and compared spontaneous cortical activity in local field potentials and intracellular potential fluctuations between normal and hypomyotonic conditions. We found that pancuronium-induced paralysis did not affect the electrophysiological properties of ongoing cortical activity and its perturbation evoked by visual and tactile stimuli. Thus, internal cortical dynamics are not much affected by active muscle movements, at least, in an acute phase.
Keywords: Cortex, Anesthesia, Paralysis, Pyramidal neuron, Spontaneous activity, Patch-clamp recording
Introduction
To maintain the mechanical stability of recordings, most electrophysiological studies in vivo are conducted on animals under anesthesia. The state of a neocortical neuron in an anesthetized animal is characterized by bistable membrane potentials fluctuating slowly between periodic hyperpolarization (Down state) and depolarization (Up state), which resembles the electrical activity during slow-wave-sleep (SWS). On the other hand, for a neocortical neuron of an awake animal, highly variable dynamics with persistent depolarization are observed [1, 2]. Recently, the significance of monitoring the electrophysiological properties in vivo in an awake state has been reconfirmed because of its distinct characteristics on information processing properties [2–4]. For example, active scanning of the environment via sensory signals enhances sensory resolution [5] but, in turn, affects signal representation by recruiting movement-related signals per se through the sensory pathway [6].
Meanwhile, researchers have attempted in various ways to overcome mechanical instabilities inherent in electrophysiological experiments in awake animals. One solution applied is head-restriction to suppress movement of the brain [2, 7]. Some studies have used muscle relaxants and sedative drugs to prevent movement of the animals [8, 9]. Because of the importance of active movement [2], however, muscle relaxant-induced prevention of voluntary muscle movement might have a dramatic effect on cortical dynamics. Moreover, direct application of these to the brains has effects, for example, seizure and neuronal death [10, 11], although muscle relaxants are relatively hydrophilic and thought not to cross the blood–brain barrier or act on the central nervous system. We focused this work on the effects of pancuronium bromide, a muscle relaxant, on the spontaneous activity of the neocortex in awake mice by monitoring local field potentials (LFPs) and spontaneous membrane potential fluctuations of single neurons. Here we report that peripherally applied pancuronium bromide has no apparent effects on ongoing cortical dynamics, in marked contrast with the effect of ketamine–xylazine, a general anesthetic.
Materials and methods
All experiments were performed in compliance with the Guide of the Committee for Animal Experiments (Approval No. A21-6). Our experimental procedures were further inspected and approved by an extra institutional review board, because pancuronium bromide was used in this study.
Male ICR mice (18–20 days old) were anesthetized with ketamine (50 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). The anesthesia was confirmed by a lack of paw withdrawal, whisker movement, and eye blink reflexes. A heating pad maintained the rectal temperature at 37°C. The skin was removed and the animal was implanted with a metal head-holder. After 2 days of recovery, head-fixation training on a custom-made stereotaxic fixture was repeated for 1–3 h per day until the implanted animal learned to remain quiet. This habituation procedure required 6–10 days, depending on the animal. During and after each session, the animal was rewarded with free access to sucrose-containing water. During the last 3 sessions, sham experiments were conducted to habituate the animal to experimental conditions and noise. The weight of the animal was monitored every day during the habituation procedure. On the final days, the animals could be kept virtually immobile, i.e., quiet awake, for more than 2 h. After full habituation, the animal was anesthetized with ketamine/xylazine and analgesized with 0.2% lidocaine during the entire period of experiments. Lidocaine was embrocated to surgical sites at twofold of the dose that induced complete analgesia in awake animals. The dose that produced complete analgesia was determined by observing that elimination of pain-like reaction continued for more than 2 h in awake, free-moving mice. The anesthetized mice received tracheal cannulation. A small craniotomy (approx. 1 × 1 mm2) was made over either the primary visual cortex (V1) 3.5 mm caudal to the bregma and 2 mm ventrolateral to the sagittal suture or the somatosensory barrel cortex 0.5 mm caudal to the bregma and 2.5 mm ventrolateral to the sagittal suture, unless otherwise specified. After removal of the dura, the exposed cortex surface was covered with 1.5–2% agar at a thickness of 500 μm. To compare spontaneous neuronal activity, anesthesia was induced with ketamine (50 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and paralysis was induced with pancuronium bromide (0.3 mg/kg, i.p.), while animals were ventilated with a volume cycled ventilator (10 ml/kg, 60 breaths/min; SN-480-type7; Shinano, Tokyo, Japan). The total periods of recording were restricted to less than 1 h to minimize stress of the animals. The time spent in anesthesia, waking, and paralysis varied from animal to animal and was approximately 15–20 min each. Heart rate and blood pressure were monitored by non-invasive recording using a BP-98A tail-cuff device (Softron, Tokyo, Japan). This apparatus uses an infrared sensor to measure the pulsation of the caudal artery, and the value may not reflect the exact blood pressures, although the heart rate was accurate. Thus, we compared the relative values of the mean blood pressure between groups in an arbitrary unit (Fig. 1b). Spontaneous whisker movements and rising heart rates confirmed that the mouse woke from anesthesia. Paralysis was confirmed by the disappearance of spontaneous whisker movements and reaction to limb touch, and the relaxation of limbs.
Fig. 1.
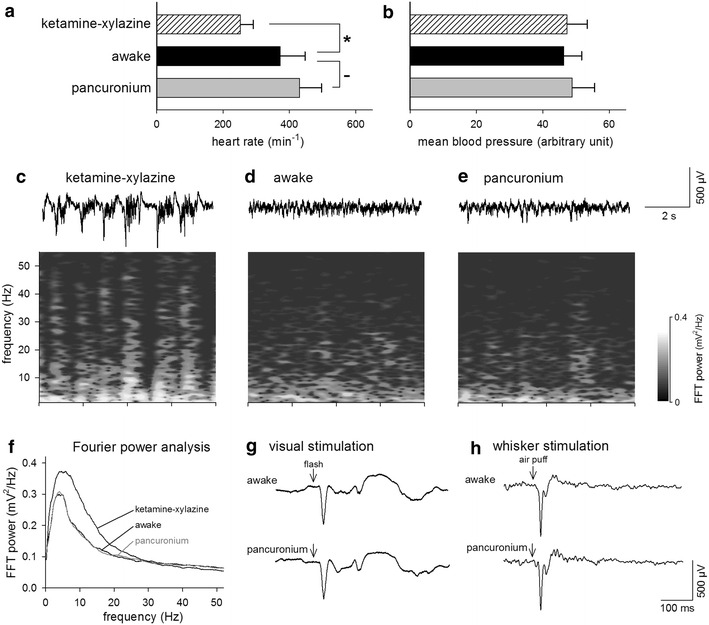
Pancuronium does not affect heart rate, mean blood pressure, or local field potentials of the cortical superficial layer in the mouse. Heart rate and blood pressure were monitored for mice under ketamine–xylazine anesthesia, awake condition, and pancuronium-induced paralysis (a, b). Data are presented as mean ± standard deviations. LFPs were recorded for a mouse under ketamine–xylazine anesthesia (c), awake conditions (d), and pancuronium-induced paralysis (e). Each panel includes an original LFP trace (top) and its Fourier power analysis (bottom). f Population average of the fast-Fourier transform of the LFP was calculated from results for 6 mice. (g, h) Representative LFP responses to brief visual (g) and whisker (h) stimulation were recorded under awake condition and paralysis. Recordings of 20 trials were averaged. Experiments were repeated on 6 mice, producing similar results
For LFP recordings, borosilicate glass capillaries with inner filaments were pulled (1 MΩ) with a P-97 puller (Sutter Instruments, Novato, USA) and filled with artificial cerebrospinal fluid (in mM): NaCl 127, KCl 1.6, KH2PO4 1.24, MgSO4 1.3, CaCl2 2.4, NaHCO3 26, and glucose 10. The tips of the electrodes were lowered to a depth of 150–200 μm below the cortex surface, which corresponds to the upper border of layer 2 in the V1, by use of a DMX-11 electric manipulator (Narishige, Tokyo, Japan). Signals were subjected to a low-pass filter, cutting the frequencies above 0.5 kHz. Whole-cell current-clamp recordings were obtained from layer 2/3 neurons at depths of 150–300 mm with borosilicate glass electrodes (5–8 MΩ) filled with intra-pipette solution consisting of (in mM): K-gluconate 135, KCl 4, CaCl2 0.1, HEPES 10, EGTA 1, Mg-ATP 4, and Na2GTP 0.4 at pH 7.2 [12]. All recordings were amplified by MultiClamp 700B and analyzed by pCLAMP 9.2 (Molecular Devices, Union City, CA, USA). Signals were digitized at 20 kHz. Off-line analysis was conducted using Matlab, and figures were produced by using SigmaPlot. For averaged data, values are represented as the mean ± standard deviation.
Results
We monitored the heart rate and blood pressure of mice throughout recordings. Spontaneous whisker movements and heart rate increased as the mouse woke from ketamine–xylazine induced anesthesia (Fig. 1a). Whisker movements ceased as pancuronium was administrated, but the heart rate did not change, indicating that the mouse was in good health. The mean blood pressure did not change throughout all three states (Fig. 1b). After confirming that, we monitored the population activity of neocortical neurons. LFPs were recorded from superficial layers of the V1 in mice anesthetized by ketamine–xylazine, followed by an awake state as control. Further recordings were then made under paralysis by pancuronium (Fig. 1c, d, e). These three states were sequentially monitored for 3 min each for the same mice. LFPs in anesthetized animals were characterized by high-amplitude fluctuations, known as slow-wave oscillations (Fig. 1c), whereas LFPs in awake and paralyzed states showed sustained, low-amplitude, high-frequency oscillations (Fig. 1d, e). Frequency analysis revealed that anesthetized states exhibited higher powers at lower frequencies (0.1–15 Hz) and that the power spectrum did not apparently differ between awake and paralyzed states (Fig. 1f). Furthermore, we investigated the LFP responses to visual or tactile stimulation under awake and paralyzed conditions. We monitored LFPs in V1, while visual stimulation was given by a 1-ms flash (Fig. 1g) or in the barrel cortex while brief stimulation of air puff (1 ml for 50 ms through a φ1-mm hole) was given to whiskers contralateral to the recorded hemisphere (Fig. 1h). Responses from 20 trials were averaged. There was no apparent difference between these two states. Similar results were obtained from LFPs of all six mice.
Next, we conducted in-vivo whole-cell patch-clamp recordings to monitor the effect of pancuronium on neuronal excitability at the single-cell level. Layer 2/3 neurons were held in the current-clamp configuration throughout anesthetized, awake, and paralyzed states. Experiments in which the series resistance reached over 70 MΩ or changed beyond 15% during the entire recording session were discarded in subsequent analyses to ensure reliable data comparison. Of more than 100 trials, six recordings met the criteria, because this was technically demanding; note that recordings often failed during a transition from anesthesia to wakefulness, probably because of the brain movement caused by abrupt changes in intracranial pressure and temperature.
During ketamine-xylazine anesthesia, slow fluctuations of the membrane potentials were apparent, composed of depolarizing (Up state) and hyperpolarizing (Down state) phases. This produces a bimodal Gaussian distribution (Fig. 2a). On the other hand, the membrane potential was persistently depolarized in awake states and conformed to a unimodal Gaussian distribution (Fig. 2b). This distribution was also demonstrated in pancuronium-induced paralysis (Fig. 2c). The mean and standard deviation of the membrane potential fluctuations were calculated. The mean value under paralyzed states was similar to that of the awake state, whereas that of anesthetized states was significantly lower than for the two other states (Fig. 2d). Likewise, there was no significant difference in the standard deviations between awake and paralyzed states, whereas for anesthetized states standard deviations were larger than for the other states (Fig. 2e).
Fig. 2.
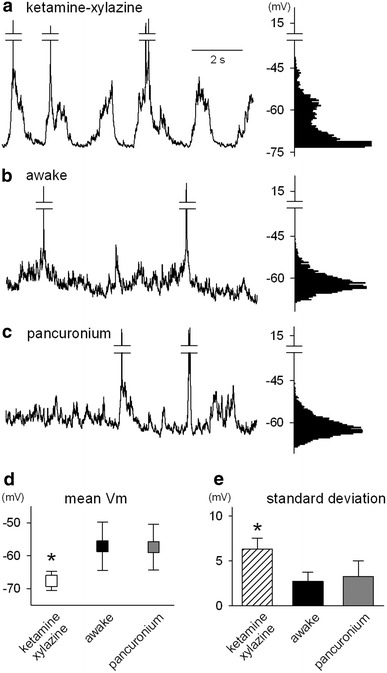
Pancuronium does not affect membrane potential properties of V1 layer 2/3 neurons. a–c Neurons were whole-cell recorded in a current-clamp configuration for a mouse under ketamine–xylazine anesthesia (a), awake conditions (b), and pancuronium-induced paralysis (c). Each panel includes an original trace of spontaneous activity in a representative neuron (left) and its histogram of the membrane potential (right). d Mean membrane potential (Vm) of the spontaneous activity of neurons recorded in each state (n = 6 mice). e Standard deviation (SD) of the spontaneous activity of neurons recorded in each state (n = 6 mice). *P < 0.05 versus awake and pancuronium; Dunnett’s test after one-way ANOVA
In the same datasets of whole-cell patch-clamp recordings, we also analyzed spike characteristics of neurons in each state (Fig. 3a–c). Layer 2/3 cortical neurons had highly variable spike frequencies, and no spontaneous action potential was observed for some neurons. Their mean spike frequencies were far less than 1 Hz, consistent with previous reports [7, 13]. There was no difference in the mean spike rate among the three states (Fig. 3d). Next, we focused on inter-spike intervals (ISIs). Because layer 2/3 neurons have low rates of spontaneous firing, data from six recordings were pooled. Each state was recorded for 3 min, meaning that the total 18-min recordings were analyzed in each state. Cumulative ISI analysis revealed that spike dynamics for paralyzed states were similar to those for awake states, whereas the cumulative ISI distribution shifted to the right in the anesthetized state (Fig. 3e). In particular, ISIs of less than 30 s were apparently fewer, indicating that massed firing was suppressed by anesthesia, consistent with a previous study [14].
Fig. 3.
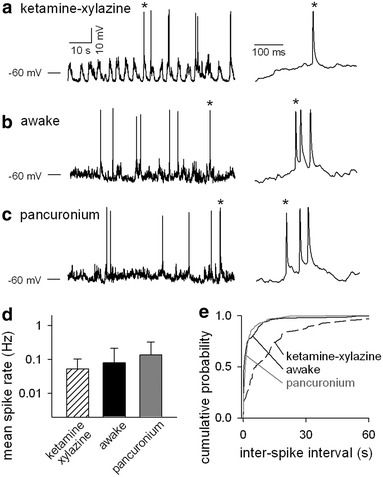
Pancuronium does not affect spike characteristics of V1 layer 2/3 neurons. a–c, Neurons were whole-cell recorded in a current-clamp configuration for a mouse under ketamine–xylazine anesthesia (a), awake conditions (b), and pancuronium-induced paralysis (c). Each panel includes an original trace of spontaneous activity in a representative neuron (left) and a time-magnified trace of spiking activity indicated by an asterisk (right). d Mean spike rate of neurons of mice in each state (n = 6 mice). e Cumulative probability of the inter-spike intervals (ISIs) including data from all of the neurons recorded for 6 mice. P < 0.01 versus awake and pancuronium; Smirnov–Kolmogorov test
Discussion
In this study, we discovered that persistent depolarization of neurons in awake mice is independent of the feedback information brought by active movements. This was shown by comparing the LFPs, the mean and standard deviation of membrane potential, and ISIs of V1 layer 2/3 neurons of pancuronium-treated or ketamine-xylazine-treated mice with those for awake mice. Statistically, the results for control awake mice were significantly different from those for mice under ketamine–xylazine anesthesia but not from those for mice under pancuronium-induced paralysis. Thus, spontaneous activity of neocortical neurons in pancuronium-paralyzed mice seems similar to that of natural brain states, whereas paralysis is a completely different state from anesthesia. The former was further confirmed in the LFP responses to sensory stimuli.
There are substantial changes in cortical dynamics in different behavioral states such as anesthesia, sleep, and wakefulness [14, 15]. Behavioral states even affect properties at the single neuronal level [3, 16]. Moreover, processing of visual information can be modulated by brain states [17, 18]. Active movements drive feedback information to the brain. For example, active whisking of mice determines the somatosensory information that they obtain through their whiskers. Indeed, some reports have demonstrated the significance of active movements in the processing of sensory information [7, 19]. Considering the importance of active movements, lack of activity forecasted possibilities that internal cortical dynamics are affected. Unexpectedly, we showed that the major properties of spontaneous cortical dynamics are not changed. This seems consistent with the report that removal of whiskers does not change internal membrane potential dynamics in the barrel cortex of mice [4], but this work provides more potent evidence, because our data were obtained from animals that were completely deprived of muscle movements. Further investigation was carried out by comparing responses of cortical neurons to external stimuli. Although such artificial sensory stimuli might be different from actively collected sensory inputs, no apparent difference was observed between awake and paralysis. More detailed analysis should be carried out on responses of cortical neurons to active sensing.
Some studies have used a muscle relaxant, for example pancuronium bromide or vecuronium bromide, to enhance mechanical stability during electrophysiological recordings under sedation [8]. These muscle relaxants contain quaternary ammonium cations, indicating that these drugs are unlikely to pass through the blood–brain barrier, although relatively small intravenous doses of d-tubocurarine can result in detectable cerebrospinal fluid concentrations in humans [20]. Nonetheless, it is surprising that there are no reports that intraperitoneal administration of muscle relaxant does not affect the central nervous system. Our work is the first to show that administration of pancuronium did not have any acute effects, at least, on internal spontaneous activity.
Acknowledgments
This work was supported in part by Grants-in-Aid for Science Research (nos. 18021008, 22650080 and 22680025) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Suzuken Memorial Foundation; the Kanae Foundation for the Promotion of Medical Science; the Daiichi-Sankyo Foundation of Life Science; and the Funding Program for Next Generation World-Leading Researchers (no. LS023).
Footnotes
G. Minamisawa and K. Funayama contributed equally to this work.
References
- 1.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crochet S, Poulet JF, Kremer Y, Petersen CC. Synaptic mechanisms underlying sparse coding of active touch. Neuron. 2011;69:1160–1175. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 4.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 5.Nelson ME, MacIver MA. Sensory acquisition in active sensing systems. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:573–586. doi: 10.1007/s00359-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 6.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 7.de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc Natl Acad Sci USA. 2009;106:16446–16450. doi: 10.1073/pnas.0904143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- 9.Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szenohradszky J, Trevor AJ, Bickler P, Caldwell JE, Sharma ML, Rampil IJ, et al. Central nervous system effects of intrathecal muscle relaxants in rats. Anesth Analg. 1993;76:1304–1309. doi: 10.1213/00000539-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Dasheiff RM. d-Tubocurarine causes neuronal death when injected directly into rat brain. Exp Neurol. 1985;89:172–188. doi: 10.1016/0014-4886(85)90274-2. [DOI] [PubMed] [Google Scholar]
- 12.Minamisawa G, Takahashi N, Matsuki N, Ikegaya Y. Laterality of neocortical slow-wave oscillations in anesthetized mice. Neurosci Res. 2009;64:240–242. doi: 10.1016/j.neures.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 15.Cotillon-Williams N, Edeline JM. Evoked oscillations in the thalamo-cortical auditory system are present in anesthetized but not in unanesthetized rats. J Neurophysiol. 2003;89:1968–1984. doi: 10.1152/jn.00728.2002. [DOI] [PubMed] [Google Scholar]
- 16.Potez S, Larkum ME. Effect of common anesthetics on dendritic properties in layer 5 neocortical pyramidal neurons. J Neurophysiol. 2008;99:1394–1407. doi: 10.1152/jn.01126.2007. [DOI] [PubMed] [Google Scholar]
- 17.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- 19.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 20.Matteo RS, Pua EK, Khambatta HJ, Spector S. Cerebrospinal fluid levels of d-tubocurarine in man. Anesthesiology. 1977;46:396–399. doi: 10.1097/00000542-197706000-00004. [DOI] [PubMed] [Google Scholar]


