Abstract
Gender-related differences in various gastric functions and diseases have been reported, with women having a higher prevalence of gastrointestinal disturbances than men. The aim of this study was to investigate sex-dependent differences in activation of the Rho-associated protein kinase (ROCK; RhoA/Rho kinase) pathway and muscle contraction in the stomach using single gastric smooth muscle cells (GSMC) from male and female Sprague–Dawley rats. Expression of ROCK1 and ROCK2 protein and acetylcholine (ACh)-induced activation of RhoA and ROCK were measured using a specifically designed enzyme-linked immunosorbent assay and activity assay kits, respectively. Contraction of a single GSMC was measured by scanning micrometry in the presence or absence of the ROCK inhibitor Y27632 dihydrochloride. ACh-induced activation of RhoA and ROCK and subsequent contraction were greater in male rats than in female rats but neither was related to differences in the expression of ROCK1 or ROCK2 or total RhoA amount. Most important, Y27632 inhibited and abolished differences in ACh-induced contraction in both sexes. In conclusion, increased ACh-induced contraction in the GSMC of male rats is attributable to greater RhoA/ROCK activation independent of differences in the expression of ROCK isoforms or total RhoA.
Keywords: Gastrointestinal system, Smooth muscle, Contraction, Rho kinase
Introduction
An accumulating body of evidence suggests that sex can affect gastrointestinal (GI) motility. It has been shown that women have a longer gastric half-emptying time for solids as compared to men [1] and that accordingly women are more likely to be affected by gastroparesis––a chronic motility disorder which is defined as delayed gastric emptying of solids and liquids in the absence of obstruction [2, 3]. Women have also been shown to be more likely to complain of GI disturbances, such as nausea, vomiting, bloating, and constipation, than men [4, 5].
Studies on colonic motility have demonstrated that healthy men have shorter GI transit times than healthy women [6–8]. Rao et al. performed 24-h ambulatory colonic manometry in healthy humans and demonstrated that the female subjects had significantly less pressure activity in the colon during daytime hours than the male subjects [9]. Moreover, the healthy men were found to have higher minimum and maximum basal anal sphincter pressures, higher anal pressures during maximum conscious sphincter contraction, lower rectal volumes required to cause an anal relaxation, and higher volumes to induce a desire to defecate. These results suggest that healthy men have stronger anal sphincter pressures than women [10].
Epidemiological data on the sex ratio in inflammatory bowel syndrome suggest that this syndrome is predominantly diagnosed in women, with a female to male sex ratio ranging from 2:1 to 4:1 [11]. Interestingly, most of the extraintestinal diseases associated with this syndrome also share this female predominance, such as fibromyalgia [12], migraine [13], other functional GI disorders (e.g., functional dyspepsia [14]), chronic pelvic pain [15], chronic fatigue syndrome [16], and depression [17].
In the physiological context, smooth muscle cells (SMC) are considered the final effectors responsible for producing fine and delicate GI tract movements. Contraction of smooth muscle is regulated by both Ca2+-dependent and Ca2+-independent (Ca2+ sensitization) mechanisms [18]. An increase in intracellular Ca2+ levels leads to myosin light chain (MLC) kinase activation, resulting in an increase in MLC20 phosphorylation and thus smooth muscle contraction. Importantly, MLC20 phosphorylation can also be increased through inhibition of MLC phosphatase, which enhances smooth muscle force generation without a change in intracellular Ca2+ level [19]. Rho-associated protein kinase (ROCK), a serine/threonine kinase and an important downstream effector of the small G protein RhoA, has been found to be an important factor in developing smooth muscle tone. Activation of the RhoA/ROCK pathway maintains the level of MLC20 phosphorylation, the essential step in smooth muscle contraction, via the inhibition of MLC phosphatase activity by phosphorylation of the MLC phosphatase target subunit (MYPT1). The importance of the RhoA/ROCK pathway has been demonstrated in the pathogenesis of cardiovascular disorders and as a target in the development of new drugs, such as fasudil, a Rho-kinase inhibitor [20–22].
Sex differences in signaling pathways within vascular muscle contribute to differences in contractile function, as exemplified in male mice, whose arteries contract to a greater magnitude in response to selected agonists than those of female mice [23]. This difference in contractile response is due to agonist-specific activation of the signaling pathways that regulate the Ca2+sensitivity of contractile proteins. Most importantly, the RhoA/ROCK pathway has been found to be upregulated in male vascular smooth muscle relative to that of females [23]. Whether gender-related differences in GI motility are due to differential activation of the RhoA/ROCK pathway—and thus smooth muscle contraction—in the sexes is not known. The aim of this study was to test the hypothesis that there is a sex-related difference in gastric muscle cell contraction which may result from differences in the activation of the RhoA/ROCK pathway. Since motility disorders are the major characteristics of many GI disturbances, the findings of this study may be of considerable importance in improving our understanding of the cause of their disproportionate prevalence among women. They may also pave the way for a better understanding of the role of the RhoA/ROCK pathway in smooth muscle contraction and thereby contribute to the development of novel therapeutic approaches for treatment of GI disorders.
Materials and methods
Animals
Young mature male and female Sprague–Dawley rats (approx. 12 weeks of age, 250–300 g) were provided by the animal house of the Jordan University of Science and Technology. Mature female rats were randomly selected regardless of the stage of the ovarian cycle. The average data from all mature female rats should roughly represent the average changes in gastric smooth muscle contraction during different stages of the ovarian cycle. Considering the short ovarian cycle (every 4–5 days) and estrous stage (12 h) on the whole, any possible fluctuations in estrogen levels at the specific stages of the ovarian cycle should cancel out. Rats were euthanized by CO2 inhalation and the stomach rapidly excised. All procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of Jordan University of Science and Technology.
Preparation of dispersed gastric SMC
Smooth muscle cells were isolated from the circular muscle layer of the rat stomach by sequential enzymatic digestion, filtration, and centrifugation, as described previously [24]. Briefly, strips of circular muscle free of mucosa were dissected from all regions of the stomach and incubated at 31 °C for 30 min in HEPES medium (pH was adjusted to 7.4) containing 120 mM NaCl, 4 mM KCl, 2.0 mM CaCl2, 2.6 mM KH2PO4, 0.6 mM MgCl2, 25 mM HEPES, 14 mM glucose, 2.1 % Eagle’s essential amino acid mixture, 0.1 % collagenase, and 0.01 % soybean trypsin inhibitor. The tissue was continuously gassed with 100 % oxygen during the entire isolation procedure. The partly digested strips were then washed twice with 50 ml of enzyme-free medium and the muscle cells allowed to disperse spontaneously for 30 min. The cells were harvested by filtration through a 500-μm Nitex mesh (precision woven open-mesh monofilament fabric with 500-µm pores; Amazon) and centrifuged twice at 350 g for 10 min to remove broken cells and organelles. The harvested cells were counted in a hemocytometer; it was estimated that 95 % of the cells excluded trypan blue. The cell isolation procedure consistently yielded spindle-shaped and viable GSMC that showed significant contraction in response to contractile stimuli. All of the experiments were performed within 2–3 h of cell dispersion.
Identification of SMC
The identity of the gastric cells isolated was confirmed as SMC by immunohistochemical staining of paraffin-embedded rat gastric muscle using anti-calponin antibody ab46794 (Abcam, Cambridge, MA) at a 1:100 dilution.
Expression of ROCK1 and ROCK2 protein by enzyme-linked immunosorbent assay
The membranes of the collected GSMC were broken down by being subjected to repeated freeze–thaw cycles. After centrifugation of the lysates (20,000 g, 10 min, 4 °C), the protein concentrations of the supernatant were determined using a DC Protein Assay kit from Bio-Rad Laboratories (Hercules, CA). Samples of equal amounts of proteins were quantitated for ROCK1 and ROCK2 protein using the respective enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s instructions [ROCK1 ELISA kit (MBS909842); MyBioSource, San Diego, CA; ROCK2 ELISA kit (CSB-EL020059RA); Cusabio Biotech, Newark, DE]. Briefly, the protein concentration of the supernatants of the lysed cells was measured and then adjusted where needed so that all samples had the same protein concentration; they were then added to 96-well plates precoated with ROCK1 (ROCK1 ELISA) or ROCK2 (ROCK2 ELISA) and incubated for 2 h at 37 °C, following which 100 μL of Biotin antibody specific for ROCK1 (ROCK1 ELISA) or for ROCK2 (ROCK2 ELISA) was added to each well and the plates incubated for 1 h at 37 °C. After washing, 100 μL of horseradish peroxidase–avidin was added to each well and the plates incubated for 1 h at 37 °C. Optical density was determined using the BioTek a microplate reader (BioTek Instruments, Winooski, VT) at 450 nm.
Measurement of ROCK activity
ROCK activity was analyzed by enzyme immunoassay with Cell Biolabs’ 96-well ROCK activity assay kit (STA-416; Cell Biolabs Inc., San Diego, CA). All experiments were carried out according to the manufacture’s protocol using 10 µL of protein lysate. The total starting protein concentration in each sample was 1 mg/mL. In brief, the protein concentration of the supernatants of the lysed cells was measured and then adjusted where needed so that all samples had the same protein concentration. The samples were then added (90 μL/well) to 96-well plates precoated with substrates corresponding to the C-terminus of MYPT1, which contains a threonine residue that may be phosphorylated by ROCK, and 10 μL of kinase reaction buffer was added to initiate the kinase reaction. The mixture was allowed to incubate for 60 min at 30 °C. After the plates were washed with the washing buffer provided in the kit, 100 μL of diluted anti-phospho-MYPT1 (Thr696) antibody was added to each well and the plates incubated at room temperature for 1 h. The specific detector antibody was horseradish peroxidase-conjugated anti-phospho-MYPT1 (incubated at room temperature for 1 h). The amount of phosphorylated substrates was measured by binding with horseradish peroxidase-conjugated anti-phospho-MYPT1, which catalyzes the conversion of tetramethylbenzidine to a yellow solution. The results were quantified using a BioTek microplate reader at 450 nm.
Measurement of active and total RhoA
Levels of activated and total RhoA were determined using the RhoA G-LISA™ Activation Assay kit (BK124) and the Total RhoA ELISA kit (BK150) (Cytoskeleton Inc., Denver, CO), respectively, according to the manufacturer’s instructions, using 10 µL of protein lysate. The total starting protein concentration for all samples was 1 mg/mL. Briefly, GSMC were lysed using the cell lysis buffer provided and the protease inhibitor cocktail provided in the kits. Lysates were quantified using the DC Protein Assay kit from Bio-Rad by measuring the absorbance at 750 nm on the BioTek microplate reader. Lysates were diluted to 0.5 mg/mL using lysis buffer and then loaded onto to the G-LISA (activated RhoA) or ELISA (total RhoA) plate for protein analysis. The results were obtained as absorbance values at 490 nm. For total RhoA, the results were converted to total protein levels using a standard curve obtained with each test.
Measurement of contraction in dispersed SMC
Contraction in freshly dispersed GSMC was determined by scanning micrometry [25]. Aliquots (0.4 ml) of cells containing approximately 104 cells/ml were prepared and categorized into aliquots of cells from either male or female rats. Cells were stimulated with acetylcholine (ACh; 0.1 µM) for 10 min at room temperature in the presence or absence of the ROCK inhibitor Y27632 (Santa Cruz Biotechnology, Dallas, TX), and the reaction was terminated with the addition of 1 % acrolein at a final concentration of 0.1 %. Acrolein kills and fixes cells without affecting cell length. The cells were viewed using a 10× or 20× objective on an inverted Nikon TMS-f microscope (Nikon Corp., Tokyo, Japan), and cell images were acquired using a Canon digital camera (Canon Inc., Tokyo, Japan) and Image J acquisition software. The resting cell length was determined in control experiments in which muscle cells were not treated with ACh. The mean length of at least 50 muscle cells from each group was measured by image J software. The contractile response to ACh was defined as the decrease in the average length of at least 50 cells and expressed as the percentage of change in length relative to the average resting length.
Materials
All chemicals, other than those whose suppliers are specifically mentioned above, were obtained from Sigma (St. Louis, MO) USA. The stock solution of Y27632 was prepared in dimethylsulfoxide.
Statistical analysis
Each experiment was performed on GSMC that were harvested from at least five to ten different rats of each sex. Statistical analysis of all experiments was performed using Prism 5.0 software (GraphPad Software, San Diego, CA). The unpaired Student’s t test was used to reveal significant differences between two means, and one-way analysis of variance followed by Fisher’s post hoc analysis was performed for the comparison of more than two means. A P < 0.05 was required for statistical significance in all the experiments. All data are shown as the mean ± standard error of the mean.
Results
Smooth muscle identification
Freshly isolated GSMC observed under a phase-contrast microscope were of variable length and had a spindle-shaped morphology. Some cells were at rest while others were at different phases of contraction (Fig. 1a). The length of resting cells from male and female rats was 113.53 ± 4 and 114.39 ± 3 µm, respectively. The smooth muscle identity of the rat gastric cells was verified by immunohistostaining with anti–calponin antibody (Fig. 1b), which showed that more than 95 % of cells stained positive for calponin (h1-calponin is specific to differentiated SMC [26]).
Fig. 1.
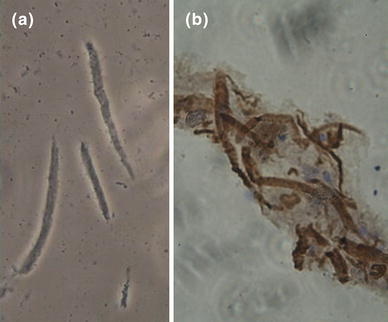
Identification of rat gastric smooth muscle cells (GSMC). a Freshly isolated GSMC were of variable length and had a spindle-shaped form by phase contrast microscopy. Cells were viewed under a 20× objective of an inverted Nikon TMS-f microscope, and the image was captured with a Canon digital camera. Average length of resting SMC isolated from male and female rats was 113.53 ± 4 and 114.39 ± 3 µm, respectively. b Immunohistochemical staining of paraffin-embedded rat smooth muscle using anti-h1-calponin antibody at 1/100 dilution
ROCK activity
Treatment of freshly dispersed male or female GSMC with 0.1 µM ACh, a Gαq/13-coupled receptor agonist, for 10 min significantly increased ROCK activity above the basal level. Importantly, ACh-induced ROCK activity was greater in male versus female GSMC (P < 0.01) (Fig. 2). Basal ROCK activity was similar in both male and female groups of cells (data not shown).
Fig. 2.
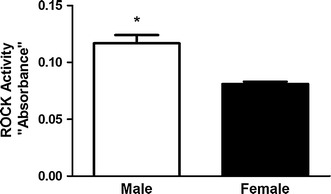
Acetylcholine (ACh)-induced activity of Rho-associated protein kinase (ROCK) in GSMC isolated from male and female rats. ROCK activity is expressed as absorbance at 450 nm. Treatment of GSMC with ACh (0.1 µM) significantly increased ROCK activity in the cells of both sexes. Note that the ACh-induced activation of ROCK was significantly higher in cells isolated from male rats than in those isolated from female rats (*P < 0.01 vs. female; n = 10 males, n = 7 females)
ROCK expression
To determine whether the sex differences in ROCK activity correlates with its expression profile we compared the protein levels of ROCK1 and ROCK2, the predominant smooth muscle isoforms [27], in GSMC isolated from male and female rats, respectively, by ELISA. Despite the higher agonist-stimulated ROCK activity in male gastric muscle cells compared to female ones, the expression of both ROCK1 and ROCK2 protein was not different in the two groups of cells (P > 0.05) (Fig. 3a, b). This observation raises the possibility that gender differences in ROCK activity might be due to an effect on other upstream regulators of the enzyme, such as RhoA.
Fig. 3.
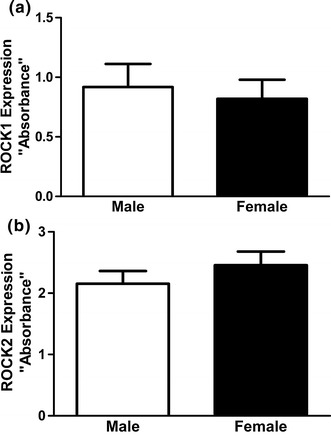
Expression of ROCK1 and ROCK2 proteins in GSMC of male and female rats. ROCK1 (a) and ROCK2 (b) protein expression level is expressed as absorbance at 450 nm. The values shown are representative of at least four independent experiments performed in triplicate. Note that there was no significant difference in both ROCK1 and ROCK2 protein levels in the GSMC of male and female rats (P > 0.05; n = 11 males, n = 7 females)
RhoA activation
Treatment of freshly dispersed male or female GSMC with 0.1 µM ACh for 10 min significantly augmented RhoA activation above the basal level. Most importantly, ACh-induced RhoA activation was greater in male GSMC than in female ones (P < 0.01) (Fig. 4a). Basal RhoA activity was similar in the two groups of isolated from male and female rats (data not shown). Interestingly, despite the higher RhoA activation in male GSMC, total RhoA protein level (active and inactive forms) was similar in both male and female cells (Fig. 4b).
Fig. 4.
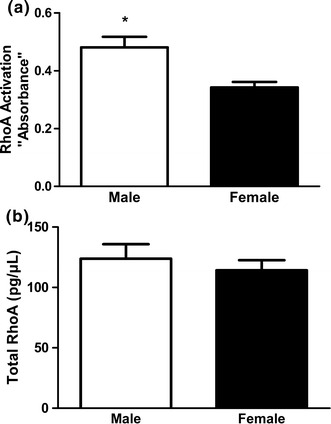
Acetylcholine-induced RhoA activation in GSMC of male and female rats. a Activated RhoA is expressed as absorbance at 450 nm. Analysis using the RhoA G-LISA™ Activation Assay kit revealed increased levels of activated RhoA in response to ACh (0.1 µM) in both male and female GSMC. Note that ACh-induced activation of RhoA was significantly greater in male cells versus female. b Total RhoA protein was similar in both male and female GSMC (*P < 0.01 vs. female, n = 10 males, n = 7 females)
Smooth muscle contraction
Freshly dispersed GSMC from male and female rats were treated with 0.1 µM ACh, and the decrease in muscle cell length was measured by scanning micrometry. The lengths of resting muscle cells from male and female rats were similar (data not shown). ACh caused both male and female muscle cells to contract, but male muscle cells showed a significantly greater contraction than female cells (P < 0.01) (Fig. 5). Preincubation of GSMC with the ROCK inhibitor Y27632, (1 µM) significantly reduced ACh-induced contraction in the muscles cells of both male and female rats (P < 0.01) (Fig. 6). Most importantly, Y27632 abolished the sex differences in ACh-induced contractions (Fig. 6).
Fig. 5.
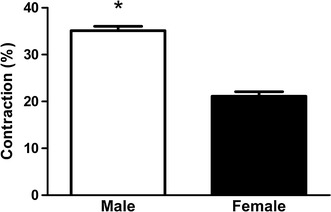
Acetylcholine-induced contraction of GSMC isolated from male and female rats. GSMC of male and female rats were stimulated with ACh (0.1 µM) and viewed under a Nikon microscope. Images of treated and non-treated single cells were acquired and cell contraction measured. ACh caused muscle cell contraction in GSMC from both male and female rats. Note that contraction in response to ACh was significantly greater in male cells than in female ones (*P < 0.01 vs. female; n = at least 50 cells from 10 different rats of each sex)
Fig. 6.
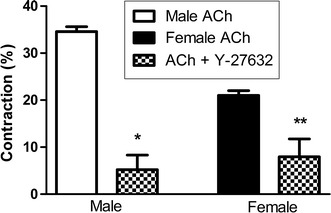
Effect of the ROCK inhibitor Y27632 on ACh-induced contraction in GSMC of male and female rats. GSMC of male and female rats were stimulated with ACh (0.1 µM) in the presence or absence of 1 µM Y27632 and then viewed under a Nikon microscope. Images of treated and non-treated single cells were acquired and cell contraction measured. The ROCK inhibitor significantly inhibited ACh-induced contraction in GSMC of both male and female rats. Importantly, Y27632-induced inhibition of gastric muscle contraction abolished the sex differences in the strength of ACh-induced contractions (*P < 0.01 vs. male ACh, **P < 0.01 vs. female ACh; n = at least 50 cells from 10 different rats of each sex)
Discussion
The main findings of this study are:
The contraction of GSMC was sex-dependent
Contraction to ACh was greater in gastric muscle cells isolated from male rats than in those from female rats.
In our study of GSMC isolated from male and female rats, ACh-induced activation of ROCK was higher in GSMC from male rats than in those from female rats. This increased activation of ROCK was associated with a greater activation of RhoA even though the total RhoA protein level was similar (active and inactive forms) in cells from both sexes. Despite the higher agonist-induced ROCK activation in male cells, the protein levels of both ROCK1 and ROCK2 were similar in male and female gastric muscle cells. Inhibition of ROCK abolished the sex-dependent differences in contraction induced by ACh. These results suggest that activation of RhoA and ROCK are regulated differently in GSMC from male and female rats.
Sex differences in smooth muscle function have been reported in several different tissue organs and in different species; for example, the authors of two separate vascular studies reported that the arteries of males have a greater myogenic tone than those of females [28, 29]. Further, it has also been suggested that sex differences in vascular reactivity may be related to differential regulation of the Rho/Rho kinase pathway [30, 31]. Chrissobolis et al. reported greater Rho kinase function and stronger vasodilator response to Y27632 in the male cerebral circulation of rats than in that of female rats, but the measured protein levels of RhoA and Rho kinase were not different [30]. To the best of my knowledge, this is the first study to show differential activation of the RhoA/ROCK pathway and different degrees of contraction of single GSMC isolated from male and female rats. Because gender-related differences in gastric contraction may be due to differences in the various types of gastric cells, studying muscle contraction and regulation of the RhoA/ROCK pathway in multicellular preparations, as commonly attempted in previous studies, may be difficult and non-specific. To avoid the contribution of other non-muscle cell types, in the current study all experiments were performed using single SMC freshly isolated from the stomach of rats.
Various receptor agonists generate both initial/transient (<1 min) Ca2+-dependent and sustained (>5 min) Ca2+-independent contraction in GI SMC [32–34]. Recent studies have revealed that ROCK, a serine/threonine kinase downstream of the small G protein RhoA, is important in developing smooth muscle tone. It inhibits MLC phosphatase activity by phosphorylation of MYPT1. A decrease in MLC phosphatase activity maintains phosphorylation of myosin and therefore contributes to smooth muscle contraction at low concentrations of intracellular Ca2+ [35].
In the experimental design of the present study, single GSMC were treated with the agonist for 10 min; consequently, the results of the kinase and contraction assays mostly represent differences in the sustained phase of contraction. Gender differences in the initial phase of contraction, such as changes in Ca2+ level and/or in the activity of various kinases (e.g., MLC kinase), should be tested in future studies.
Consistent with the results of many previous studies, the treatment of muscle cells with ACh significantly enhanced ROCK activity in the cells from both males and females [36, 37]. Moreover, the results presented here support previously reported findings on the vasculature and confirm that ACh-induced activation of ROCK is higher in males [30]. Two ROCK isoforms have been identified; ROCK1 (or β isoform) and ROCK2 (or α isoform). The ROCK assay kit used in this study uses MYPT1 Thr696 as a probe for the kinase which can be phosphorylated by both ROCK isoforms, so it does not distinguish betweeen the activities of the ROCK1 and ROCK2 isoforms. The expression levels of both kinase isoforms were measured in order to determine whether they correlated with the respective activation pattern or not. Despite the differences in ROCK activation in GSMC from both genders, expression of the two isoforms of the enzyme was similar in single GSMC from males and females, indicating that the differences in the expression of either ROCK1 or ROCK2 did not account for the greater activity of ROCK in GSMC isolated from males. The results reported here raise the possibility that sex-linked increased ROCK activity in males might be due to differences in the activation of other upstream regulators of the enzyme, such as RhoA. The inactive form of RhoA (RhoA.GDP) is present in the cytosol bound to a guanine dissociation inhibitor (GDI). Activation of RhoA, such as via ACh-stimulated muscarinic receptors (M3), is mediated by various Rho-specific guanine nucleotide exchange factors (RhoGEFs) which in turn promote the exchange of GDP for GTP. Upon GTP binding and activation, RhoA interacts with and stimulates the activity of downstream effectors, including ROCK. Active RhoA (GTP-bound) is in turn inactivated by GTPase-activating proteins (GAPs) which hydrolyze GTP to GDP [33]. Parallel to these ROCK findings are those showing that ACh-induced activation of RhoA was greater in male GSMC than in female ones. However, despite this greater ACh-induced RhoA activation in male GSMC, total RhoA levels, including both active (GTP-bound) and inactive (GDP-bound) forms, were similar in both the male and female cells. These findings suggest that the observed sex differences in RhoA activation may be related to other regulators of Rho, such as GDI inhibitors, RhoGEFs, and GAPs, which control the balance between active and inactive RhoA. The role of these regulatory proteins in this reported gender-linked RhoA activation needs to be tested in future studies.
Noteworthy, ACh-induced muscle cell contraction was greater in male gastric cells than in female ones. Most importantly, ROCK inhibitor Y27632 abolished the sex difference in agonist-induced contractions. Inhibition of ROCK normalized contractions to ACh in GSMC from male and female rats, demonstrating that the enhanced ROCK activity in the male stomach mediates the sex difference. Although higher concentrations of Y27632 also inhibited PKC, previous studies confirmed that Y27632 at 1 µM is highly selective for Rho kinase [38, 39].
In conclusion, the results of this study demonstrate that activation of RhoA and ROCK in response to ACh is greater in the male stomach than in the female one, with the result that male stomachs undergo greater contractions. The exact mechanisms by which the RhoA/ROCK pathway is differentially regulated in the sexes require further investigations. Sex differences are clearly present in the GI system, with the RhoA/ROCK pathway playing a crucial role in GI functionality. Further understanding of the role of the RhoA/ROCK pathway in modulating normal physiological and pathophysiological functions of the GI tract will enable the development of more effective and sex-tailored treatments for GI disturbances.
Acknowledgments
This work was supported by Jordan University of Science & Technology, Irbid, Jordan (Grant No. 20140071).
Compliance with ethical standards
Conflict of interest
The author declares that there are no conflicts of interest.
References
- 1.Bennink R, Peeters M, Van den Maegdenbergh V, Geypens B, Rutgeerts P, De Roo M, Mortelmans L. Comparison of total and compartmental gastric emptying and antral motility between healthy men and women. Eur J Nucl Med. 1998;25(9):1293–1299. doi: 10.1007/s002590050298. [DOI] [PubMed] [Google Scholar]
- 2.Rao JN. Estrogens and gastroparesis: a clinical relevance. Dig Dis Sci. 2013;58(6):1449–1451. doi: 10.1007/s10620-013-2683-0. [DOI] [PubMed] [Google Scholar]
- 3.Oh JH, Pasricha PJ. Recent advances in the pathophysiology and treatment of gastroparesis. J Neurogastroenterol Motil. 2013;19(1):18–24. doi: 10.5056/jnm.2013.19.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matchock RL, Levine ME, Gianaros PJ, Stern RM. Susceptibility to nausea and motion sickness as a function of the menstrual cycle. Women’s Health Issues: Off Publ Jacobs Inst Women’s Health. 2008;18(4):328–335. doi: 10.1016/j.whi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonenne J, Esfandyari T, Camilleri M, Burton DD, Stephens DA, Baxter KL, Zinsmeister AR, Bharucha AE. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil. 2006;18(10):911–918. doi: 10.1111/j.1365-2982.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 6.Teff KL, Alavi A, Chen J, Pourdehnad M, Townsend RR. Muscarinic blockade inhibits gastric emptying of mixed–nutrient meal: effects of weight and gender. Am J Physiol. 1999;276(3 Pt 2):R707–R714. doi: 10.1152/ajpregu.1999.276.3.R707. [DOI] [PubMed] [Google Scholar]
- 7.Meier R, Beglinger C, Dederding JP, Meyer-Wyss B, Fumagalli M, Rowedder A, Turberg Y, Brignoli R. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol Motil. 1995;7(4):235–238. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 8.Lampe JW, Fredstrom SB, Slavin JL, Potter JD. Sex differences in colonic function: a randomised trial. Gut. 1993;34(4):531–536. doi: 10.1136/gut.34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280(4):G629–G639. doi: 10.1152/ajpgi.2001.280.4.G629. [DOI] [PubMed] [Google Scholar]
- 10.Sun WM, Read NW. Anorectal function in normal human subjects: effect of gender. Int J Colorectal Dis. 1989;4(3):188–196. doi: 10.1007/BF01649702. [DOI] [PubMed] [Google Scholar]
- 11.Chial HJ, Camilleri M. Gender differences in irritable bowel syndrome. J Gend-Specif Med. 2002;5(3):37–45. [PubMed] [Google Scholar]
- 12.McCarberg BH. Clinical overview of fibromyalgia. Am J Ther. 2012;19(5):357–368. doi: 10.1097/MJT.0b013e3181ff7bee. [DOI] [PubMed] [Google Scholar]
- 13.Lipton RB, Stewart WF, Scher AI. Epidemiology and economic impact of migraine. Curr Med Res Opin. 2001;17(Suppl 1):s4–s12. doi: 10.1185/0300799039117005. [DOI] [PubMed] [Google Scholar]
- 14.Flier SN, Rose S. Is functional dyspepsia of particular concern in women? A review of gender differences in epidemiology, pathophysiologic mechanisms, clinical presentation, and management. Am J Gastroenterol. 2006;101(12 Suppl):S644–S653. doi: 10.1111/j.1572-0241.2006.01015.x. [DOI] [PubMed] [Google Scholar]
- 15.Walker EA, Gelfand AN, Gelfand MD, Green C, Katon WJ. Chronic pelvic pain and gynecological symptoms in women with irritable bowel syndrome. J Psychosom Obstet Gynaecol. 1996;17(1):39–46. doi: 10.3109/01674829609025662. [DOI] [PubMed] [Google Scholar]
- 16.Dinos S, Khoshaba B, Ashby D, White PD, Nazroo J, Wessely S, Bhui KS. A systematic review of chronic fatigue, its syndromes and ethnicity: prevalence, severity, co-morbidity and coping. Int J Epidemiol. 2009;38(6):1554–1570. doi: 10.1093/ije/dyp147. [DOI] [PubMed] [Google Scholar]
- 17.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65(2–3):123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 18.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 19.Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991;266(3):1708–1715. [PubMed] [Google Scholar]
- 20.Hahmann C, Schroeter T. Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell Mol Life Sci. 2010;67(2):171–177. doi: 10.1007/s00018-009-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills TM, Chitaley K, Wingard CJ, Lewis RW, Webb RC. Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol. 2001;91(3):1269–1273. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- 22.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 23.Nuno DW, Korovkina VP, England SK, Lamping KG. RhoA activation contributes to sex differences in vascular contractions. Arterioscler Thromb Vasc Biol. 2007;27(9):1934–1940. doi: 10.1161/ATVBAHA.107.144675. [DOI] [PubMed] [Google Scholar]
- 24.Murthy KS, Makhlouf GM. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am J Physiol. 1995;268(1 Pt 1):C171–C180. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Mahavadi S, Sriwai W, Grider JR, Murthy KS. Cross-regulation of VPAC(2) receptor desensitization by M(3) receptors via PKC-mediated phosphorylation of RKIP and inhibition of GRK2. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G867–G874. doi: 10.1152/ajpgi.00326.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol. 2003;284(1):C156–C167. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 27.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Sequential activation of heterotrimeric and monomeric G proteins mediates PLD activity in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G381–G388. doi: 10.1152/ajpgi.2001.280.3.G381. [DOI] [PubMed] [Google Scholar]
- 28.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca2+ -dependent K+ channels. Circ Res. 1996;79(5):1024–1030. doi: 10.1161/01.RES.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 29.Huang A, Sun D, Koller A, Kaley G. Gender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. Am J Physiol. 1997;272(4 Pt 2):H1804–H1809. doi: 10.1152/ajpheart.1997.272.4.H1804. [DOI] [PubMed] [Google Scholar]
- 30.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35(9):2200–2205. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 31.Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326(1):154–159. doi: 10.1016/j.bbrc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288((5):G849–853. doi: 10.1152/ajpgi.00530.2004. [DOI] [PubMed] [Google Scholar]
- 33.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta. Am J Physiol Cell Physiol. 2007;293(6):C1991–C2000. doi: 10.1152/ajpcell.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22(1):32–39. doi: 10.1016/S0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 37.Al-Shboul O, Mustafa A. Effect of oxidative stress on Rho kinase II and smooth muscle contraction in rat stomach. Can J Physiol Pharmacol. 2015;2015:1–7. doi: 10.1139/cjpp-2014-0505. [DOI] [PubMed] [Google Scholar]
- 38.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J. 2003;374(Pt 1):145–155. doi: 10.1042/bj20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287(4):H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]


