Abstract
Thyroid hormones are vital for survival of mammalian species and play critical roles in growth, development, and metabolism. Both fetal hypothyroidism and sex can affect carbohydrate metabolism during adult life. This study aims to assess carbohydrate metabolism in male and female offspring born from mothers who were hypothyroid during pregnancy. Pregnant rats were divided into two groups; the controls consumed water and the hypothyroid group received water containing 0.025 % 6-propyl-2-thiouracial throughout gestation. The intravenous glucose tolerance test (0.5 g/kg glucose) was carried out in 3-month-old offspring. Findings showed that compared to controls, male fetal hypothyroid rats during adulthood had glucose intolerance (area under the curve: 446.4 ± 9.7 vs. 486.4 ± 8.8, p < 0.01 in control and fetal hypothyroid groups, respectively) whereas females had improved glucose tolerance (478.1 ± 7.0 vs. 455.9 ± 8.5, p < 0.01). In conclusion, sex could modulate the effects of fetal hypothyroidism on glucose tolerance in rats.
Keywords: Fetal hypothyroidism, Glucose tolerance, Insulin, Sex differences, 6-Propyl-2-thiouracil, Rat
Introduction
Epidemiological and laboratory studies indicate that a suboptimal environment during fetal, neonatal, and infant development is associated with the development of impaired glucose tolerance, type 2 diabetes mellitus, and insulin resistance in later adult life [1, 2]. Thyroid hormones (THs) are important for intrauterine growth and play fundamental roles in the development, growth and metabolism throughout life [3]. Data shows that maternal hormonal status significantly influences intrauterine growth and development [4–6]. THs and insulin act as metabolic and maturational signals and any change in their concentrations and bioactivity in response to environmental challenges alter fetal development, producing long-term effects on cardiovascular, reproductive, and metabolic function [7, 8]. We have previously shown that fetal hypothyroidism can alter carbohydrate metabolism in male adult euthyroid rat offspring, which may increase susceptibility to the development of glucose intolerance and occurrence of type 2 diabetes later in life [9–11]. The prevalence of diabetes and abnormalities of glucose metabolism are higher in males than females [12, 13]. In addition, a previous study demonstrated that female rats are protected against metabolic defects typically produced by fructose feeding and detrimental effects of fructose on metabolism are less severe in this group [14]. Therefore, there is a possibility that the sexual differentiation of offspring can be affected by fetal hypothyroidism [15, 16]. Since limited data are available on the relation between fetal hypothyroidism, sex differences, and carbohydrate metabolism during adult life, in this study, we hypothesized that thyroid hormone deficiency during fetal life could impair glucose tolerance in female rat offspring.
Materials and methods
Animals and induction of hypothyroidism
Female Wistar rats were bred locally in the animal facility of the Research Institute for Endocrine Sciences (RIES) of Shahid Beheshti University of Medical Sciences. Female rats (180–220 g), being in the pro-estrus phase of estrus cycle, were housed with males in polypropylene cages overnight in an environmentally controlled room (temperature 22 ± 3 °C) with 12 h light/dark cycles. All experiments were carried out in accordance with standards approved by the local ethics committee of the RIES. The presence of spermatozoa in the vaginal smears on the morning after caging was considered as an index of pregnancy [17] and this day was considered as day 0 of pregnancy. Pregnant females were randomly divided into fetal hypothyroid (FH) and control (C) groups and then transferred to separate cages. The FH group received 0.025 % 6-Propyl-2-thiouracil (PTU) (Sigma-Aldrich, Germany) in drinking water throughout pregnancy while the C group consumed tap water; treatment was initiated on day 1 of the pregnancy and discontinued after delivery [18, 19]. After weaning, the male and female offspring of the C and FH rats were housed in groups of four per cage, until 3 months of age. After birth, the body weights of the pups were recorded weekly (A&D scale EK-300i, Japan; sensitivity 0.1 g) from the first day of the birth till the end of the third month. Food intakes of offspring were measured weekly after weaning until the end of the third month.
Glucose and hormones measurements
Blood samples were obtained from the mothers after delivery and offspring during adulthood by means of a small incision at the end of their tails [20] and from neonates by cutting the head [21]. Blood was centrifuged at 3000×g for 10 min at 4 °C and sera were kept at −20 °C. Serum glucose was measured by the glucose oxidase method (Pars Azmoon Co., Tehran, Iran). Serum insulin was measured using the enzyme-linked immunosorbent assay (ELISA) method (Mercodia, Uppsala, Sweden), 17 β-estradiol (E2) was measured by the ELISA method (Diagnostics Biochem Canada Inc.), total triiodothyronine (TT3) and total thyroxine (TT4) levels were measured by the ELISA method (Pishtazteb Zaman Co., Tehran, Iran). Intra- and inter assay coefficients of variation for insulin and glucose measurements were 5.8, 9.3%, and 2.4, 8.7 %, and for estrogen, TT3 and TT4 were 3.5, 6.6, 3.2, 4.8 %, and 4.5, 5.7 %, respectively.
Intravenous glucose tolerance test (IVGTT)
To perform IVGTT, female rats at the estrous phase (determined by vaginal smears) and age-matched males were fasted overnight (12–14 h), anesthetized with an intraperitoneal (i.p) injection of pentobarbital sodium (60 mg/kg) and the femoral vein was exposed for glucose infusion. The femoral vein was cannulated with a PE-50 polyethylene tube filled with heparinized saline (20 IU/ml). Initially, the first blood sample from the tail cut at time zero was obtained, then a 20 % glucose solution (0.5 g/kg) was injected through the vein and blood samples (0.3 ml each) were collected at 5, 10, 15, 20, 30, and 60 min for glucose and insulin measurement. An equal volume of heparinized saline was infused through the catheter for replacing blood removed (10). Computation of the homeostasis model assessment of insulin resistance (HOMA-IR) index was performed by the formula: HOMA-IR = fasting insulin (μU/ml) × fasting glucose (mmol/L)/22.5 [22].
Statistical analysis
GraphPad Prism software (version 5) was used for statistical analyses. Two-way analysis of variance (ANOVA), followed by a Bonferroni post hoc test were used for comparing animal weights, food intake, and serum glucose and insulin levels during glucose tolerance test between groups. Overall changes in glucose and insulin during IVGTT were calculated as area under the curve (AUC) above the basal level. Student t test was used for comparing hormone values and AUC between groups. All data were expressed as mean ± SEM and P values below 0.05 were considered significant.
Results
Hormone determinations, weight gain, and food intake of the animals
Administration of PTU in drinking water decreased circulating TT3 and TT4 in hypothyroid mothers and their neonates. In adult offspring of the PTU-treated group, hormone levels were not different from those of adult offspring of controls (Table 1). In female adult offspring of the fetal hypothyroid group, E2 hormone level was not different from the control group (Fig. 1). Although body weight of FH rats in both sexes was significantly lower compared to controls from the first day until the end of 12 weeks, there was no difference in body weight of offspring at the age of 13 weeks in the C and FH groups (Fig. 2). Food intake was not significantly different between FH and control rats in both sexes (Fig. 3).
Table 1.
Serum T3 and T4 concentrations in offspring of the fetal hypothyroid (FH) and control groups at birth and adulthood, and their mothers, at the time of delivery
| Mothers | Offspring | |||||||
|---|---|---|---|---|---|---|---|---|
| At the time of delivery | At the time of birth | Adulthood | ||||||
| Male | Female | |||||||
| Control | Hypothyroid | Control | FH | Control | FH | Control | FH | |
| Triiodothyronine (ng/dL) | 93.4 ± 3.8 | 51.7 ± 4.9* | 83.0 ± 7.8 | 39.5 ± 4.3* | 95.7 ± 4.3 | 87.7 ± 5.3 | 86.8 ± 4.2 | 84.8 ± 5.6 |
| Thyroxine (µg/dL) | 2.4 ± 0.2 | 0.52 ± 0.04* | 0.73 ± 0.06 | 0.37 ± 0.04* | 3.8 ± 0.10 | 3.4 ± 0.20 | 3.5 ± 0.10 | 3.2 ± 0.10 |
Value sera mean ± SEM
n = 12 in each group
FH fetal hypothyroid
* p < 0.001 compared to control group
Fig. 1.
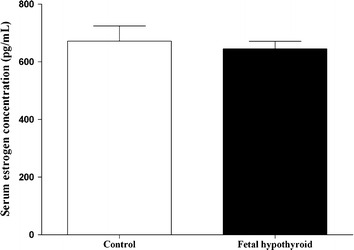
Serum estrogen concentrations in female (n = 8) fetal hypothyroid and control rats
Fig. 2.
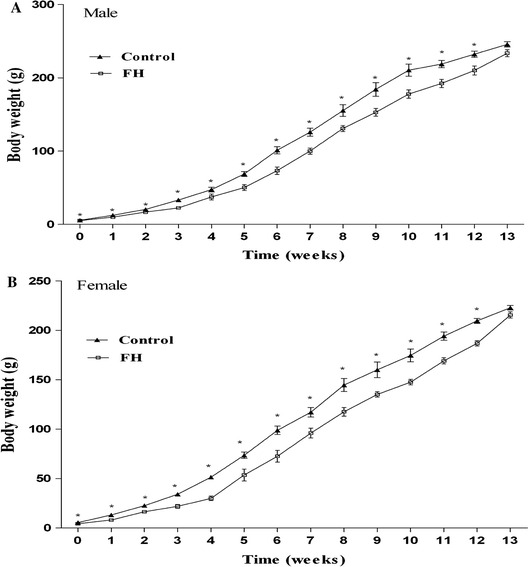
Body weight of the animals during study period in the male (n = 14) and female (n = 14) fetal hypothyroid (FH) and control rats. *p < 0.01, statistically significant differences between treatment (FH and controls)
Fig. 3.
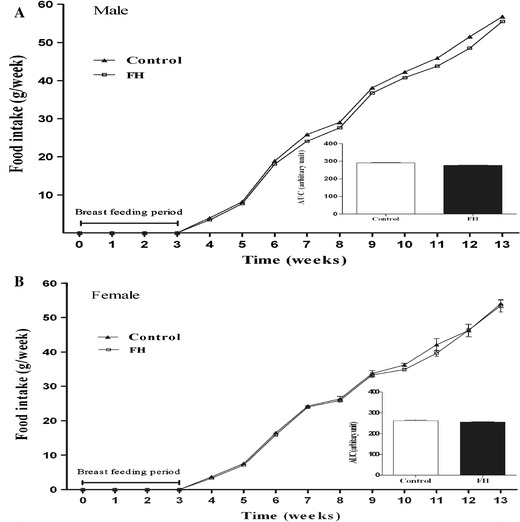
Food intake of the animals during the study period in male (n = 9) and female (n = 9) fetal hypothyroid (FH) and control rats. Inset shows area under the curve
Serum glucose and insulin concentration during IVGTT
Glucose tolerance curves for the four groups are shown in Fig. 4. Fasted serum glucose levels appeared to be identical in the control and FH groups (Table 2). In male animals, mean serum glucose of the FH group was significantly higher at 5 and 10 min after glucose injection, as compared to the C group, whereas in female animals, corresponding values were significantly lower compared with the C group (Fig. 4a). In female but not in male animals, the AUC of the serum glucose concentration in FH group during IVGTT was significantly lower compared to its respective C group (Table 2). Results of the IVGTT in male and female rats from the control and FH groups were also compared; while no significant differences were observed between male and female rats in the C group, mean serum glucose concentrations at 5 and 10 min during IVGTT (Fig. 4a), and the AUC (Table 2) in male animals of the FH group were significantly higher, compared with the females of the same group. As shown in Fig. 4b, no significant differences were observed between male and female animals of the control group, but the mean serum insulin concentrations of the FH group in male animals were lower during the IVGTT, compared to females. The AUC of the serum insulin concentration of male animals were also significantly lower compared to the female animals in the FH group (Table 2). In male animals, although means for serum insulin concentrations of FH group during IVGTT were significantly lower (Fig. 4b), the AUC of the serum insulin concentration of this group was not, as compared to the C group (Table 2). In female animals, means of serum insulin concentrations of the FH group and AUC of the serum insulin were both significantly higher during the IVGTT, as compared to the C group (Fig. 4b; Table 2). Only the HOMA-IR index of the male offspring of the FH group was significantly different from that of the C group. There were no differences in HOMA-IR index between male and female animals of either group (Table 2).
Fig. 4.
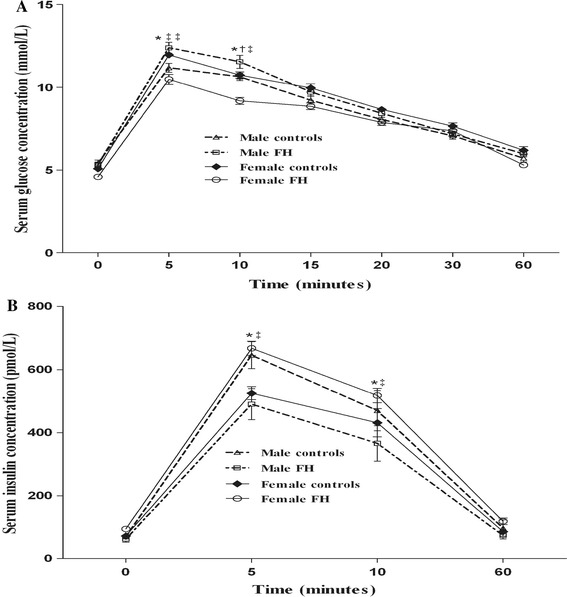
Evaluation of intravenous glucose tolerance test (IVGTT). Comparison of changes in serum glucose (a) and insulin (b) concentrations following IVGTT at different time points in the male (n = 12) and female (n = 12) fetal hypothyroid (FH) and control rats. *p < 0.01, statistically significant differences between different sexes, † p < 0.05, ‡ p < 0.01, statistically significant differences between different treatments (FH and controls)
Table 2.
Variations of serum glucose and insulin concentrations during intravenous glucose tolerance test in the fetal hypothyroid (FH) and control groups
| Control | FH | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| AUC for glucose (mmol min−1 L−1) | 446.4 ± 9.7‡ | 478.1 ± 7.0 | 486.4 ± 8.8 | 455.9 ± 8.5‡ |
| AUC for insulin (pmol min−1 L−1) | 18740 ± 2063 | 16818 ± 12 | 14513 ± 1821*,† | 20775 ± 751 |
| HOMA-IR | 2.3 ± 0.2† | 2.4 ± 0.3 | 2.8 ± 0.1 | 2.1 ± 0.3 |
Values are mean ± SEM
AUC area under the curve, HOMA-IR homeostatic model assessment of insulin resistant, FH fetal hypothyroid
* p < 0.05, Statistically significant differences between different sex
† p < 0.05, ‡ p < 0.01 statistically significant differences between different treatments (FH and controls)
Discussion
The main finding of this study is that fetal hypothyroidism had different effects on glucose tolerance in male and female rats. During IVGTT, higher serum glucose and lower serum insulin concentrations were observed in the FH group of male rats, while adult female offspring born from hypothyroid mothers had lower serum glucose and higher serum insulin concentrations compared to the C group.
In our study, PTU administration decreased TT3 and TT4 in both mothers and neonates, confirming the induction of hypothyroidism [23]. Adult offspring born from hypothyroid mothers had normal serum TT3 and TT4 levels. In line with our findings, previous studies [24] have also reported normal TT3 and TT4 levels of adult offspring rats exposed to maternal hypothyroidism. In the present study, neonate rats born from mothers with hypothyroidism had significantly lower birth weight, a finding similar to those of other studies [9–11, 24–26]. Although, from the results of this study, we could not explain the lower body weight in presence of equal food intake, it has been shown that thyroid hormones are strongly involved in the regulation of body growth during fetal and neonatal periods through stimulation of growth factors production [26]; in addition, fetal hypothyroidism leads to an asymmetrical type of intrauterine growth restriction, with increased reduction in muscle mass [27].
The results of the present study indicate that adult male offspring of fetal hypothyroid mothers showed glucose intolerance, findings in agreement with our previous reports [9–11]. Rodriguez-Castelan et al. [28] have recently investigated the effects of hypothyroidism on isolated islets of female rabbits, and they found no difference in the density, number, or the area of islets, and the number of cells per islet between the control and hypothyroid groups; in line with these findings, in our study, adult female offspring rats, unlike their male counterparts not only did not show glucose intolerance but also had improved glucose tolerance according to IVGTT. We previously reported that serum glucose concentration during IVGTT was higher at 5 min in 3-month old FH offspring compared to their controls, a result is in line with our current finding; however in previous study, the AUC of serum glucose concentration did not differ significantly between the FH and C groups, while in current study, AUC was significantly higher in males; one possible explanation for this discrepancy may be related to the different doses of the PTU; as in the previous study we used 200 ppm but in the current study, higher dose (250 ppm) was used [24].
In the current study, female rats in the FH group showed improved glucose tolerance, a finding in line with that of, Berlezet et al. [29] who showed that in female rats, a nutrition deficiency during gestational and postnatal life of female rat offspring increases both glucose tolerance and insulin sensitivity, possibly due to increased hepatic glycogen concentration, increased hepatic glycogen synthesis, and the higher glucose uptake in skeletal muscle. Estrogen may contribute to the gender-specific difference observed in glucose tolerance [30]; it has been suggested that estrogen improves β-cell function, through binding to its receptor in rat islets [31], and stimulates insulin release [32]. Studies have demonstrated that the development of glucose intolerance after menopause is related to an imbalance of ovarian hormones, in particular to the decrease in estrogen [33, 34]. In addition, the prevalence of insulin resistance and type 2 diabetes is higher in post-menopausal women [35], where estrogen replacement therapy could improve glucose tolerance [36, 37]. We could not explain the exact role of estrogen, as a protective factor against insulin resistance [38], in the improvement of glucose tolerance in female rats, however, serum E2 concentration was comparable between control and fetal hypothyroid groups in female rats.
Conclusion
Sex-specific differences were found in the effects of fetal hypothyroidism on carbohydrate metabolism, with female offspring exhibiting better glucose tolerance.
Acknowledgments
This study was supported by a grant (NO. 499) funding from the Endocrine Physiology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences. We would like to thank Ms N. Shiva for critical editing of English grammar and syntax of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Poulsen P, Vaag AA, Kyvik KO, Jensen DM, Beck-Nielsen H. Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia. 1997;40:439–446. doi: 10.1007/s001250050698. [DOI] [PubMed] [Google Scholar]
- 2.Zambrano E, Bautista CJ, Deas M, Morales J, Gonzalez-Zamorano M, Ledesmal H. A low maternal protein diet during pregnancy and lactation has sex-and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krassas GE. Thyroid disease and female reproduction. Fertil Steril. 2000;74:1063–1070. doi: 10.1016/S0015-0282(00)01589-2. [DOI] [PubMed] [Google Scholar]
- 4.Lesage J, Hahn D, Leonhardt M, Blondeau B, Breant B, Dupouy JP. Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. J Endocrinol. 2002;174:37–43. doi: 10.1677/joe.0.1740037. [DOI] [PubMed] [Google Scholar]
- 5.Holness M, Langdown M, Sugden M. Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of type 2 diabetes mellitus. Biochem J. 2000;349:657–665. doi: 10.1042/bj3490657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moraes C, Rebelato HJ, Amaral MEC, Resende TM, Silva EV, Esquisatto MA. Effect of maternal protein restriction on liver metabolism in rat offspring. J Physiol Sci. 2014;64:347–355. doi: 10.1007/s12576-014-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 8.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology. 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 9.Karbalaei N, Ghasemi A, Hedayati M, Godini A, Zahediasl S. The possible mechanisms by which maternal hypothyroidism impairs insulin secretion in adult male offspring in rats. Exp Physiol. 2014;99:701–714. doi: 10.1113/expphysiol.2013.073825. [DOI] [PubMed] [Google Scholar]
- 10.Farahani H, Ghasemi A, Roghani M, Zahediasl S. The effect of maternal hypothyroidism on the carbohydrate metabolism and insulin secretion of isolated islets in adult male offspring of rats. Horm Metab Res. 2010;42:792–797. doi: 10.1055/s-0030-1262826. [DOI] [PubMed] [Google Scholar]
- 11.Farahani H, Ghasemi A, Roghani M, Zahediasl S. Effect of neonatal hypothyroidism on carbohydrate metabolism, insulin secretion, and pancreatic islets morphology of adult male offspring in rats. J Endocrinol Invest. 2013;36:44–49. doi: 10.3275/8468. [DOI] [PubMed] [Google Scholar]
- 12.Nuutila P, Knuuti MJ, Maki M, Laine H, Ruotsalainen U, Teras M. Gender and insulin sensitivity in the heart and in skeletal muscles: studies using positron emission tomography. Diabetes. 1995;44:31–36. doi: 10.2337/diab.44.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl J, Hilding A, Ostenson CG, Grill V, Efendic S, Bavenholm P. Characterisation of subjects with early abnormalities of glucose tolerance in the Stockholm Diabetes Prevention Programme: the impact of sex and type 2 diabetes heredity. Diabetologia. 2005;48:35–40. doi: 10.1007/s00125-004-1614-1. [DOI] [PubMed] [Google Scholar]
- 14.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. 2002;283:2478–2484. doi: 10.1152/ajpheart.00243.2002. [DOI] [PubMed] [Google Scholar]
- 15.Capuco AV, Wood DL, Elsasser TH, Kahl S, Erdman RA, Van Tassell CP. Effect of somatotropin on thyroid hormones and cytokines in lactating dairy cows during ad libitum and restricted feed intake. J Dairy Sci. 2001;84:2430–2439. doi: 10.3168/jds.S0022-0302(01)74693-0. [DOI] [PubMed] [Google Scholar]
- 16.Lookin O, Kuznetsov D, Protsenko Y (2015) Sex differences in stretch-dependent effects on tension and Ca2+ transient of rat trabeculae in monocrotaline pulmonary hypertension. J Physiol Sci 65:89–94 [DOI] [PMC free article] [PubMed]
- 17.Hapon M, Simoncini M, Via G, Jahn G. Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction. 2003;126:371–382. doi: 10.1530/rep.0.1260371. [DOI] [PubMed] [Google Scholar]
- 18.Savage DD, Montano CY, Otero MA, Paxton LL. Perinatal hypothyroidism decreases hippocampal 3H-vinylidene kainic acid binding in rats. Neuroendocrinology. 1990;51:38–44. doi: 10.1159/000125313. [DOI] [PubMed] [Google Scholar]
- 19.Khaksari M, Shafiee M, Ghasemi A, Asl SZ. Effect of orally administered propylthiouracil in pregnant and lactating rats on isolated aorta contractility of their adult male offspring. Med Sci Monit: Int Med J Exp Clin Res. 2009;15:123–127. [PubMed] [Google Scholar]
- 20.Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34:372–378. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- 21.Daikoku S, Adachi T, Kawano H, Wakabayashi K. Development of the hypothalamic–hypophysial–gonadotrophic activities in fetal rats. Experientia. 1981;37:1346–1347. doi: 10.1007/BF01948403. [DOI] [PubMed] [Google Scholar]
- 22.Song HK, Han DH, Song JH, Ghee JY, Piao SG, Kim SH. Influence of sirolimus on cyclosporine-induced pancreas islet dysfunction in rats. Am J Transplant. 2009;9:2024–2033. doi: 10.1111/j.1600-6143.2009.02751.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed OM, Abd El-Tawab SM, Ahmed RG. Effects of experimentally induced maternal hypothyroidism and hyperthyroidism on the development of rat offspring: I. The development of the thyroid hormones-neurotransmitters and adenosinergic system interactions. Int J Dev Neurosci. 2010;28:437–454. doi: 10.1016/j.ijdevneu.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Karbalaei N, Ghasemi A, Faraji F, Zahediasl S. Comparison of the effect of maternal hypothyroidism on carbohydrate metabolism in young and aged male offspring in rats. Scand J Clin Lab Invest. 2013;73:87–94. doi: 10.3109/00365513.2012.743164. [DOI] [PubMed] [Google Scholar]
- 25.Ghasemi A, Mehrazin F, Zahediasl S. Effect of nitrate and l-arginine therapy on nitric oxide levels in serum, heart, and aorta of fetal hypothyroid rats. J Physiol Biochem. 2013;69:751–759. doi: 10.1007/s13105-013-0251-x. [DOI] [PubMed] [Google Scholar]
- 26.Hamouli-Said Z, Tahari F, Hamoudi F, Hadj-Bekkouche F. Comparative study of the effects of pre and post natal administration of a thyroid drug on testicular activity in adult rat. Folia Histochem Cytobiol. 2007;45:51–57. [PubMed] [Google Scholar]
- 27.Fowden AL. Endocrine regulation of fetal growth. Reprod Fertil Dev. 1995;7:351–363. doi: 10.1071/RD9950351. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Castelán J, Nicolás L, Morimoto S, Cuevas E (2014) The Langerhans islet cells of female rabbits are differentially affected by hypothyroidism depending on the islet size. Endocrine (in press) [DOI] [PubMed]
- 29.Berleze KJ, Muller AP, Schweigert ID, Longoni A, Sordi F, de Assis AM. Gestational and postnatal low protein diet alters insulin sensitivity in female rats. Exp Biol Med. 2009;234:1437–1444. doi: 10.3181/0903-RM-111. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesone M, Chazenbalk GD, Ballejos G, Charreau EH. Estrogen receptor in rat pancreatic islets. J Steroid Biochem. 1979;11:1309–1314. doi: 10.1016/0022-4731(79)90201-2. [DOI] [PubMed] [Google Scholar]
- 32.SutterDub MT. Preliminary report: effects of female sex hormones on insulin secretion by the perfused rat pancreas. J De Physiologie. 1976;72:795–800. [PubMed] [Google Scholar]
- 33.El Seifi S, Green I, Perrin D. Insulin release and steroid-hormone binding in isolated islets of Langerhans in the rat: effects of ovariectomy. J Endocrinol. 1981;90:59–67. doi: 10.1677/joe.0.0900059. [DOI] [PubMed] [Google Scholar]
- 34.Bailey C, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Diabetologia. 1980;19:475–481. doi: 10.1007/BF00281829. [DOI] [PubMed] [Google Scholar]
- 35.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. Diabetes Care. 1998;21:1589–1595. doi: 10.2337/diacare.21.10.1589. [DOI] [PubMed] [Google Scholar]
- 37.Rijpkema A, Van der Sanden A, Ruijs A. Effects of post-menopausal oestrogen-progestogen replacement therapy on serum lipids and lipoproteins: a review. Maturitas. 1990;12:259–285. doi: 10.1016/0378-5122(90)90007-S. [DOI] [PubMed] [Google Scholar]
- 38.Louet J-F, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6:180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]


