Abstract
It has been suggested that glycogen functions not only in carbohydrate energy storage, but also as molecular sensors capable of activating lipolysis. This study aimed to compare the variation in liver and muscle glycogen during the day due to different timing of exercise. Nine healthy young men participated in two trials in which they performed a single bout of exercise at 70% of their individual maximal oxygen uptake for 60 min in the post-absorptive (morning) or post-prandial (afternoon) state. Liver and muscles glycogen levels were measured using carbon magnetic resonance spectroscopy (13C MRS). Diurnal variations in liver and muscle glycogen compared to baseline levels were significantly different depending on the timing of exercise. The effect of the timing of exercise on glycogen fluctuation is known to be related to a variety of metabolic signals, and the results of this study will be useful for future research on energy metabolism.
Keywords: Liver, Muscle, Glycogen, Post-absorptive exercise, Post-prandial exercise
Background
The evidence regarding the health benefits of regular physical activity is well established, and previous research demonstrates that virtually everyone benefits: men and women, young children to older adults, women who are pregnant or postpartum, people living with a chronic disease, or people attempting to reduce their risk of disease [27]. Regular exercise can also be expected to achieve and maintain desired body composition, such as weight loss and weight gain inhibition, due to increased energy expenditure [27]. According to the World Health Organization physical activity guidelines, adults are recommended to undergo 150–300 min of moderate-intensity or 75–150 min of vigorous-intensity physical activity, or an equivalent combination of moderate-intensity and vigorous-intensity aerobic physical activities per week to maintain a healthy body [4]. However, while these guidelines indicate the time, frequency, and intensity of exercise, they do not describe the timing of exercise, such as post-absorptive or post-prandial states.
Recently, the timing of exercise has received much attention due to its influence on various factors, such as food intake [2], circadian rhythm [36, 41], energy substrates [39], body composition [3, 10, 24, 33], the adaptation of training [6, 38], and glucose metabolism [23, 37]. While there is still disagreement regarding some of the above, there is general consensus on the energy substrates, i.e., exercise performed in the post-absorptive state induces higher fat oxidation than exercise performed in the post-prandial state [39]. It has long been known that pre-exercise nutritional status affects energy substrates during exercise [7, 14]. Additionally, post-absorptive exercise increases 24-h fat oxidation, even in energy-balanced conditions [15, 31]. The body condition after an overnight fast is characterized by no energy intake since the supper of the previous night, moreover, overnight fasting is the only way to induce a post-absorptive state in people with traditional eating habits of three meals daily. During overnight fasting, substrate oxidation predominantly shifts from glycogenolysis to fatty acid oxidation [30], and hormonal changes activate several transcription factors and regulate gene expression [11]. A possible physiological background for these aspects is that glycogen variability is involved in whole-body energy metabolism. Glycogen in the liver and muscles is a storage source of energy and its quantity affects whole-body energy metabolism. Specifically, decreased liver glycogen levels stimulate lipolysis in adipose tissue through a central nervous system-mediated mechanism [20], and decreased muscle glycogen levels trigger sequential events, including the dissociation of the AMP-activated protein kinase (AMPK–glycogen interaction, enhanced activity and altered intracellular localization of AMPK; and the upregulated expression of genes associated with fat oxidation, such as carnitine palmitoyltransferase, fatty acid translocase, and hormone-sensitive lipase [26]. In this way, in the liver and muscle glycogen is not only a source of energy, but also a regulator of whole-body energy metabolism. Nevertheless, none of the previous studies has reported fluctuations in liver and muscle glycogen levels due to post-absorptive or post-prandial exercise.
This study aimed to clarify the effect of exercise in the morning or afternoon, i.e., post-absorptive or post-prandial, on liver and muscle glycogen fluctuations during the day. Liver and muscle glycogen levels were measured over two trials, including a 60-min exercise session in the morning before breakfast or in the afternoon using carbon magnetic resonance spectroscopy (13C MRS), which is non-invasive.
Methods
This study entailed a randomized repeated measures design including two trials with exercise sessions performed in the morning or afternoon; the two trials were separated for at least 1 week.
Each trial consisted of two consecutive days. On the first day (day 1) of each experiment, participants were permitted to engage in normal activities, such as walking up and down stairs, until arriving at the laboratory. Thereafter, they were instructed to refrain from performing strenuous exercise and consuming beverages, such as energy, caffeinated, or alcoholic drinks, except for experimental meals. Participants arrived at the laboratory before 17:30. Their body composition was subsequently measured using the bioimpedance method (InBody 770; InBody, Tokyo, Japan). They consumed supper at 18:30 and went to bed at 23:00. On the second day (day 2), two meals (breakfast at 09:00 and lunch at 13:00) were provided, and participants ran at 70% of their VO2max for 60 min, beginning at 07:00 (morning) or 16:00 (afternoon) on a treadmill. Participants were instructed to remain awake and to maintain a sedentary position, except when performing prescribed exercise sessions.
Participants
Nine healthy young men were recruited for the present study after providing written informed consent. Participants’ mean physical characteristics were as follows: age, 23.4 ± 1.3 years; height, 170.1 ± 1.5 cm; body weight, 59.3 ± 1.0 kg; and body fat, 10.1 ± 0.7%, maximal oxygen uptake (VO2max) was 60.7 ± 1.4 ml/kg/min. At the time of the study, no participant had any medical condition or was taking medications or smoking. This study was approved by the Ethics Committee of the Japan Institute of Sports Sciences (IRS-2017-048).
Pre-study evaluation
To determine the workload corresponding to 70% of the individual VO2max, all participants performed a graded exercise test including submaximal and maximal tests using a treadmill (Ohtake Root Kogyo, Japan) at least 1 week before the main experiment. The initial running speed was set at 9.0 km/h and was then increased by 1.2 km/h every 3 min at each subsequent stage up to a lactate concentration exceeding 4.0 mmol/L. Each stage was separated by a 1-min rest, and the rest was extended to 3 min when the lactate concentration exceeded 4.0 mmol/L. Then, the following running speed was set to the stage before the lactate concentration exceeded 4.0 mmol/L and was increased by 0.6 km/h every 1 min until the participants reached voluntary exhaustion. A capillary blood sample was taken from fingertips to analyze the lactate concentration (Lactate Pro 2, Arkray Co., Ltd., Kyoto, Japan) immediately before the test, after each running stage, and after 1, 3, and 5 min of exercise to exhaustion.
Oxygen uptake was considered to be maximal when at least two of the following four criteria were met: (1) VO2 reached a plateau; (2) heart rate exceeded 90% of the age-predicted maximal value; (3) respiratory exchange ratio increased above 1.10, and (4) ratings of perceived exertion at the end of the test ≥ 19. The highest VO2 for two consecutive 15 s during the test was taken as the VO2max. Respiratory gas was continuously collected using the breath-by-breath method (AE310S, Minato Medical Science Co., Osaka, Japan). Regression analysis revealed that the relative oxygen uptake and treadmill running velocity corresponded to 70% of the individual VO2max.
Experimental meals
Experimental meals were designed to achieve energy-balanced conditions, assuming a resting metabolic rate of 24.0 kcal/kg/day according to estimated energy requirements for Japanese individuals [1]. The physical activity factor was assumed to be 1.75 (2471 ± 39 kcal/day) on day 1 and 1.85 on day 2 (1732 ± 33 kcal for breakfast and lunch) based on a previous study using a room-size metabolic chamber [17]. Participants consumed 6.2 ± 0.1 g/kg of body weight of carbohydrates (370 ± 7 g/day on day 1 and 4.4 ± 0.0 g/kg of body weight (260 ± 5 g for breakfast and lunch on day 2. Experimental meals comprised 15% protein, 25% fat, and 60% carbohydrate; these values were expressed as percentages of the total energy intake.
Glycogen measurements
Muscle and liver glycogen contents were measured using 13C MRS at 20:00 on day 1, and at 06:00, 08:00, 12:00, 15:00, and 17:00 on day 2. Muscle glycogen was assessed using the calf, as it is one of the major muscles that contract during running [5]. 13C MRS was performed using a clinical 3-Tesla superconducting magnetic resonance system (Magnetom Verio and Magnetom Skyra, Siemens, Germany) as previously described [18, 35]. Briefly, the 13C MRS muscle spectra were collected in 15-min blocks at 200-ms intervals, resulting in 4500 acquisitions using a 13C–1H double-tuned surface coil, 10 cm in diameter (Takashima Seisakusho, Tokyo, Japan) that was set on the calf muscle. Muscle glycogen levels were quantified by comparing them with an external standard solution (120 mM glycogen from oysters and 50 mM KCl). Similarly, the 13C MRS liver spectra were collected in 16-min blocks at 160-ms intervals, resulting in 6000 acquisitions using the same coil as that for muscle measurement; the coil was set on the right side of the trunk by the liver. Liver glycogen levels were quantified by comparing them with an external standard solution (200 mM glycogen from oysters and 150 mM KCl). When the first measurement was taken in both the muscle and liver, the coil position was marked on the skin with a felt-tipped pen to ensure that 13C MRS data were collected at the same position during subsequent measurements.
Blood sampling and analyses
Blood samples were collected from the antecubital vein in commercially available vacuum-sealed serum collection tubes (Nipro, Osaka, Japan) at the same time as glycogen measurements. Serum samples were obtained by centrifugation at 3000 rpm for 10 min at 4 °C and were stored at − 80 °C until analysis. From the obtained samples, insulin, and triglyceride levels were measured in an independent laboratory (LSI Medience Corporation, Tokyo, Japan). Blood glucose levels were determined by blood samples taken from participants’ fingertips (Medisafe FIT, Terumo, Tokyo, Japan) [13].
Experimental exercise and physical activity
Participants ran for 60 min at 70% of their VO2max on a treadmill (Ohtake Root Kogyo, Japan).
During the experimental 60-min running session, respiratory gas was continuously collected using the breath-by-breath method (AE310S, Minato Medical Science Co., Osaka, Japan). Immediately after completing the 60-min exercise, lactate concentration was measured by blood samples taken from participants’ fingertips.
Non-exercise-related activity was estimated using a hip-worn accelerometer device (ActiGraph GT9X Link, ActiGraph, Pensacola FL), which was validated using a three-axial accelerometer and data filtering technology [28]. Participants wore accelerometers upon wakening on day 1 until the final glycogen measurement on day 2, except during experimental exercise.
Statistical analyses
Data in the main text and figures are presented as means ± standard errors. To determine the sample size, the effect size (d) from the present data was expected to be 0.7 for the comparison of changes in muscle and liver glycogen fluctuation during the day. The results of the power analysis using G*Power version 3.1.9.2 (University of Dusseldorf, Germany) indicated a minimum sample size of eight to ensure a power of 0.8 and an α level of P < 0.05. Considering the possibility of dropouts, we recruited nine participants. Mean condition pair values were compared using a paired t test. Muscle and liver glycogen levels, respiratory gas analysis during exercise sessions, and blood parameters between both trials were compared using a two-way repeated-measures analysis of variance to identify the main effect of trial and time. The interaction between trial and time was determined using Bonferroni’s correction with post hoc pairwise comparisons. Changes from baseline in muscle and liver glycogen between both trials were compared using a two-way repeated-measures analysis of variance to identify the main effect of trial and time. The interaction between trial and time was determined using Bonferroni’s (trial) and Dunnett’s (time) correction with post hoc pairwise comparisons. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS statistical software version 24 (IBM Japan, Tokyo, Japan).
Results
All participants completed both trials and there were no significant differences in body mass, body fat, or fat-free mass between trials.
During the experimental exercises in the two trials, participants ran on a treadmill at a speed equivalent to 70% of their VO2max for 60 min (12.3 ± 0.4 km/h). This intensity was lower than the lactate threshold of each individual calculated in the pre-study evaluation (14.8 ± 0.6 km/h). No significant differences were observed in the average heart rate (morning, 153 ± 4 bpm; and afternoon, 150 ± 3 bpm; P = 0.12), post-exercise blood lactate (morning, 1.7 ± 0.4 mM; and afternoon, 1.5 ± 0.3 mM; P = 0.16), and the rating of perceived exertion (morning, 11.3 ± 0.9; and afternoon, 11.1 ± 0.7; P = 0.65) between trials. The results of respiratory analysis during exercise are shown Table 1.
Table 1.
Results of respiratory analyses
| 10 min | 20 min | 30 min | 40 min | 50 min | 60 min | Average | |
|---|---|---|---|---|---|---|---|
| O2 uptake (ml/min) | |||||||
| Morning | 2494 ± 51 | 2623 ± 52 | 2618 ± 51 | 2620 ± 54 | 2614 ± 56 | 2624 ± 55 | 2599 ± 51* |
| Afternoon | 2470 ± 45 | 2577 ± 46 | 2569 ± 50 | 2566 ± 54 | 2564 ± 55 | 2561 ± 54 | 2551 ± 49 |
| CO2 production (ml/min) | |||||||
| Morning | 2315 ± 70 | 2439 ± 65 | 2423 ± 65 | 2414 ± 72 | 2414 ± 73 | 2419 ± 71 | 2404 ± 68** |
| Afternoon | 2408 ± 66 | 2541 ± 67 | 2531 ± 67 | 2521 ± 73 | 2508 ± 75 | 2506 ± 77 | 2502 ± 69 |
| Ventilation (L/min) | |||||||
| Morning | 66.8 ± 3.2 | 72.0 ± 3.2 | 73.6 ± 3.5 | 74.5 ± 4.0 | 75.1 ± 4.2 | 75.4 ± 4.2 | 72.9 ± 3.6 |
| Afternoon | 68.6 ± 3.1 | 74.6 ± 3.2 | 75.3 ± 3.2 | 75.1 ± 3.2 | 75.3 ± 3.5 | 75.8 ± 3.5 | 74.1 ± 3.2 |
| Respiratory exchange ratio | |||||||
| Morning | 0.93 ± 0.02 | 0.93 ± 0.01 | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.92 ± 0.01** |
| Afternoon | 0.97 ± 0.01 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.98 ± 0.01 | 0.98 ± 0.01 | 0.98 ± 0.01 | 0.98 ± 0.01 |
Values are shown as mean ± standard errors. *P < 0.05, **P < 0.01 vs afternoon trial
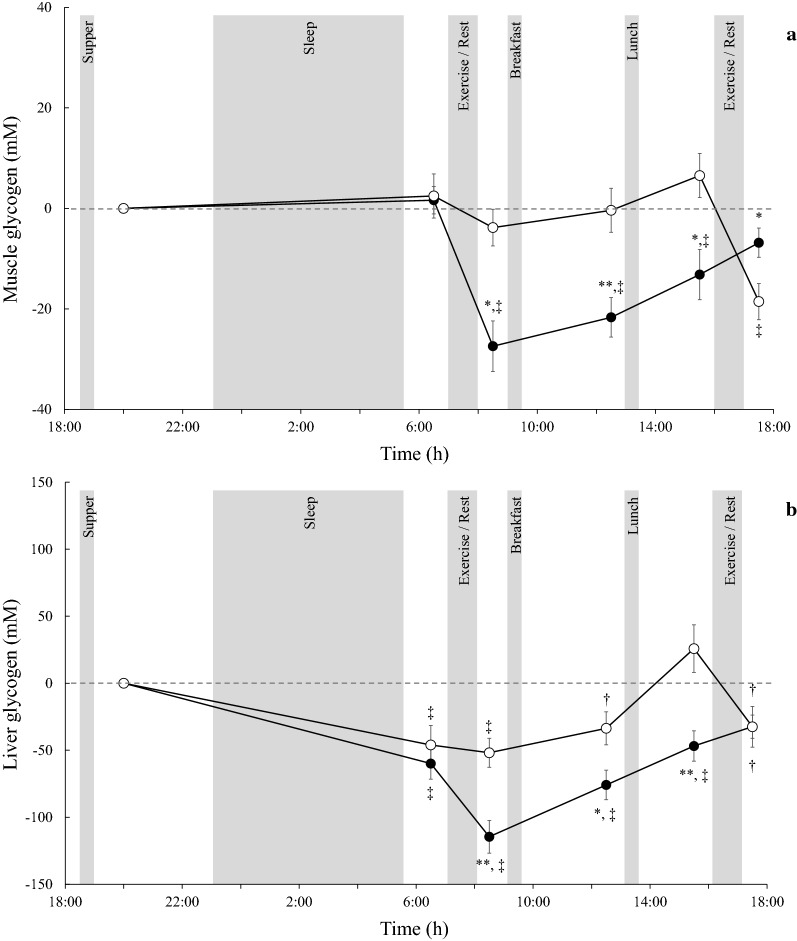
Table 2 shows the time course of glycogen levels in the muscle and liver. Significant main effects of trial (P < 0.05) and time (P < 0.01) and time and trial interactions (P < 0.01) were observed for muscle glycogen levels. Significant main effects of time (P < 0.01) and trial and time interactions (P < 0.01) and a significant trial tendency (P = 0.06) were observed when analyzing liver glycogen levels. Figure 1 shows the change from baseline in muscle and liver glycogen. Significant main effects of time (P < 0.01) and time and trial interactions (P < 0.01) were observed for change from baseline in muscle glycogen, although the main effect of trial (P = 0.11) was not statistically significant. Similarly, significant main effects of trial (P < 0.05), time (P < 0.01) and time and trial interactions (P < 0.01) were observed for change from baseline in liver glycogen.
Table 2.
Results of muscle and liver glycogen measurements
| Day 1 | Day 2 | |||||
|---|---|---|---|---|---|---|
| 20:00 | 6:00 | 8:00 | 12:00 | 15:00 | 17:00 | |
| Muscle glycogen (mM) | ||||||
| Morning | 86 ± 7 | 88 ± 7 | 59 ± 7** | 65 ± 6** | 73 ± 5** | 80 ± 6 |
| Afternoon | 90 ± 6 | 93 ± 6 | 87 ± 6 | 90 ± 7 | 97 ± 7 | 72 ± 7 |
| Liver glycogen (mM) | ||||||
| Morning | 245 ± 20 | 185 ± 14 | 130 ± 16** | 169 ± 18* | 202 ± 18** | 213 ± 20 |
| Afternoon | 239 ± 15 | 193 ± 17 | 187 ± 14 | 206 ± 14 | 266 ± 21 | 207 ± 20 |
Values are shown as mean ± standard errors. *P < 0.05, **P < 0.01 vs afternoon trial
Fig. 1.
Change from baseline (at 20:00 on day 1) in muscle (a) and liver (b) glycogen in the morning (closed circles) and afternoon trials (open circles). Values are shown as means ± SEs. *P < 0.05 vs. afternoon trial. **P < 0.01 vs. afternoon trial. †P < 0.05 vs. baseline. ‡P < 0.05 vs. baseline
Significant main effects of trial (P < 0.05) and time (P < 0.01) were observed for blood glucose levels, although the main effect of trial and time interaction was not statistically significant (Fig. 2a). Significant main effects of time were observed on serum insulin levels (P < 0.01), although the main effect of trial and time interaction was not statistically significant (Fig. 2b). Significant main effects of time (P < 0.01) and trial and time interactions (P < 0.05) were observed on serum triglyceride levels. At 15:30, serum triglyceride levels were significantly higher in the afternoon than in the morning trial (P < 0.05, Fig. 2c).
Fig. 2.
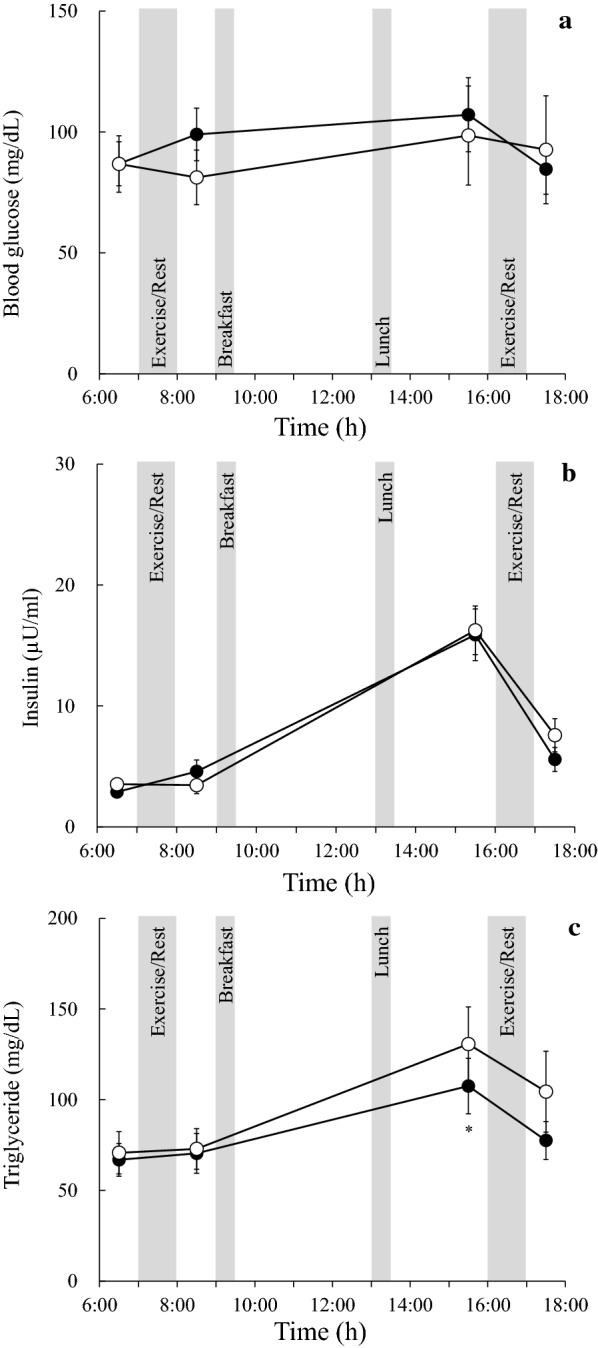
Changes in blood glucose (a), insulin (b), and triglyceride (c) levels in the morning (closed circles) and afternoon trials (open circles). Values are shown as means ± SEs. *P < 0.05 vs. afternoon trial
Participants maintained an inactive and sedentary lifestyle during both experimental days, except when they conducted exercises. No significant differences were observed between the morning and afternoon trials in non-exercise physical activity (day 1, 325 ± 27, 317 ± 24 counts/min, P = 0.81; and day 2; 139 ± 9, 138 ± 5 counts/min, P = 0.86) and step counts (day 1, 6746 ± 735, 6687 ± 651, P = 0.94; day 2, 1733 ± 239, 1639 ± 176, P = 0.28).
Discussion
The purpose of this study was to determine the effect of the timing of exercise on fluctuations in liver and muscle glycogen levels. Our main findings were that exercise in the morning was associated with relatively lower liver and muscle glycogen during the day compared to exercise in the afternoon.
Compared with baseline values (i.e., at 20:00 on day 1), liver glycogen levels decreased after overnight fasting in the morning (-23%) and afternoon (-21%) trials. A key function of liver glycogen is to maintain blood glucose levels by releasing glucose into the bloodstream [12]; therefore, its degradation after overnight fasting is reasonable [18]. The morning trial induced further low liver glycogen levels (-46%, at 08:00 on Day 2) by experimental exercise, and liver glycogen remained significantly decreased relative to baseline, even at the last measurement. However, there was no significant difference in liver glycogen levels before experimental exercise compared to baseline in the afternoon trial. This was due to the fact the participants consumed enough carbohydrates at breakfast and lunch to replenish the glycogen degradation by the overnight fast. Consequently, it was found that liver glycogen fluctuations during the day differ depending on exercise timing. A shortage in liver glycogen directly facilitates lipolysis in white adipose tissue by activating a liver–brain–adipose neurocircuitry, this demonstrates the presence of a “glycogen depletion signal” [20, 40]. The fat utilization signal may continue to increase fat oxidation after exercise in the morning as decreased liver glycogen levels remain severely depressed after breakfast [15]. In addition to fat metabolism, plasma glucose clearance rates are higher in the morning compared with the evening in response to similar glucose intake [34]. Since increased liver glycogen synthesis leads to improved glucose tolerance [29], a significant transient decrease in liver glycogen by exercise may be beneficial in improving glucose tolerance. It has been suggested that optimizing the timing of exercise is beneficial for improving glucose homeostasis [9] and diurnal variation in liver glycogen with different timing of exercise would provide useful insights for carbohydrate/fat metabolism.
Muscle glycogen levels did not decrease after overnight fasting in the morning (+ 2%) and afternoon (+ 3%) trials compared with each baseline value. Diurnal muscle glycogen variation has been observed to be markedly small on sedentary days [18, 35] because muscle glycogen does not contribute to the maintenance of blood glucose. Thus, there were no significant differences in pre-exercise muscle glycogen levels compared to baseline levels in each study, but the diurnal variation differed significantly depending on the timing of exercise. The respiratory exchange ratio during the experimental exercise was significantly lower in the morning trial than in the afternoon trial (Table 1). Nonetheless, the net change in muscle glycogen due to experimental exercise did not differ between trials. Previous research indicates that net muscle glycogen breakdown is similar between post-absorptive and post-prandial exercise, but there is a significant increase fat oxidation and decrease in the intramyocellular triglyceride in type I muscle after post-absorptive compared to post-prandial exercise [8]. It has been suggested that intramyocellular triglyceride content is a marker or mediator of muscle insulin resistance [22]. Furthermore, muscle glycogen is not only a carbohydrate energy reserve, but also a molecular sensor capable of activating signaling pathways in response to exercise, including the nuclear translocation of AMPK and upregulation of genes responsible for fat oxidation [26]. Additionally, exercise-induced muscle glycogen depletion can increase subsequent insulin sensitivity and prevent glucose from being diverted to de novo lipogenesis [21]. When considering carbohydrate/fat metabolism and glucose tolerance, it is potentially beneficial to know the timing of exercise and muscle glycogen fluctuations.
In summary, the diurnal fluctuations in liver and muscles glycogen varied depending on the timing of exercise. Previous studies suggest that the timing of exercise may affect energy metabolism over 24 h by altering the pattern of glycogen fluctuations [15, 16, 31]. Furthermore, acute [8, 32] and chronic [24, 38] post-absorptive exercise have been observed to induce different metabolic adaptations than post-prandial exercise, and have been associated with diurnal variation of glycogen storage. Future studies are needed to evaluate the effect of the timing of exercise on glycogen fluctuations in the liver and muscles over a longer period.
One limitation of the present study is that it did not measure liver and muscle glycogen levels after exercise in the afternoon trial, that is, the effect of supper and sleep on post-exercise glycogen fluctuation. Thus, the effect of morning or afternoon exercise on the circadian rhythm of glycogen variability remains unclear. Although the nocturnal energy expenditure and substrate did not differ depending on the timing of exercise [15, 17], it would be interesting to investigate liver and muscle glycogen levels the following morning after supper and sleep to examine the relationship between glycogen fluctuations and linked physiological indicators. Another limitation is that the post-exercise carbohydrate intake timing might not have been optimal for glycogen resynthesis. Consuming carbohydrates immediately after exercise is better for glycogen resynthesis than consuming carbohydrates 2 h after exercise [19]. For the purpose of glycogen measurement, participants in this study consumed breakfast 90 min after exercise rather than immediately in the morning trial. However, differences in intake timing do not affect glycogen synthesis when evaluated over 8 h or 24 h [25]. Therefore, we believe that the effect of dietary intake timing in this study on eventual glycogen storage is negligible. Finally, the intensity and duration of the experimental exercise may be too intense for the general population. The energy expenditure during exercise, which is feasible for many people, may be lower than in the experimental exercise of the present study, and consequently, the variability in glycogen may be smaller.
Conclusions
Exercise performed in the morning before breakfast was associated with a relative decrease in liver and muscle glycogen during the day compared to exercise performed in the afternoon. The effect of the timing of exercise on glycogen fluctuation, which has been shown to be related to a variety of metabolic signals, will be useful in future studies of energy metabolism. The results of this study may also be applied to consider the optimal exercise timing for patients with diabetes.
Acknowledgements
Not applicable.
Authors’ contributions
KI and FT conceived and designed research. KI analyzed data, interpreted results of experiments and prepared figures; KI, YT, and TO performed experiments; KI and HT drafted manuscript, edited and revised manuscript. All authors approved final version of manuscript.
Funding
This study was supported by the Japan Society for the Promotion of Science (Grant-in-Aid for Research Activity start-up 16H07476).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Japan Institute of Sports Sciences (IRS-2017-048). Written informed consent was obtained from all participants.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anon . Dietary reference intakes for Japanese (2015) Tokyo: Ministry of Health Labour and Welfare of Japan; 2015. [Google Scholar]
- 2.Bachman JJ, Deitrick RW, Hillman AR. Exercising in the fasted state reduced 24-hour energy intake in active male adults. J Nutr Metab. 2016;2016:1984198. doi: 10.1155/2016/1984198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blankenship JM, Rosenberg RC, Rynders CA, Melanson EL, Catenacci VA, Creasy SA. Examining the role of exercise timing in weight management: a review. Int J Sports Med. 2021;42:967–978. doi: 10.1055/a-1485-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behavior. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor Patterns in human walking and running. J Neurophysiol. 2006;95:3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 6.Chtourou H, Souissi N. The effect of training at a specific time of day: a review. J Strength Cond Res. 2012;26:1984–2005. doi: 10.1519/JSC.0b013e31825770a7. [DOI] [PubMed] [Google Scholar]
- 7.Coyle EF, Coggan AR, Hemmert MK, Lowe RC, Walters TJ. Substrate usage during prolonged exercise following a preexercise meal. J Appl Physiol. 1985;59:429–433. doi: 10.1152/jappl.1985.59.2.429. [DOI] [PubMed] [Google Scholar]
- 8.De Bock K, Richter EA, Russell AP, Eijnde BO, Derave W, Ramaekers M, Koninckx E, Leger B, Verhaeghe J, Hespel P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564:649–660. doi: 10.1113/jphysiol.2005.083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabriel BM, Zierath JR. Circadian rhythms and exercise - re-setting the clock in metabolic disease. Nat Rev Endocrinol. 2019;15:197–206. doi: 10.1038/s41574-018-0150-x. [DOI] [PubMed] [Google Scholar]
- 10.Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity. 2013;21:2249–2255. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein I, Hager GL. Transcriptional and chromatin regulation during fasting—the genomic era. Trends Endocrinol Metab. 2015;26:699–710. doi: 10.1016/j.tem.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez JT, Fuchs CJ, Betts JA, van Loon LJC. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am J Physiol. 2016;311:E543–E553. doi: 10.1152/ajpendo.00232.2016. [DOI] [PubMed] [Google Scholar]
- 13.Hara H, Mihara M, Narushima M, Todokoro T, Yamamoto T, Koshima I. Blood glucose measurement for flap monitoring to salvage flaps from venous thrombosis. J Plast Reconstr Aesthetic Surg. 2012;65:616–619. doi: 10.1016/j.bjps.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol. 1997;273:E768–E775. doi: 10.1152/ajpendo.1997.273.4.E768. [DOI] [PubMed] [Google Scholar]
- 15.Iwayama K, Kawabuchi R, Park I, Kurihara R, Kobayashi M, Hibi M, Oishi S, Yasunaga K, Ogata H, Nabekura Y, Tokuyama K. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J Appl Physiol. 2015;118:80–85. doi: 10.1152/japplphysiol.00697.2014. [DOI] [PubMed] [Google Scholar]
- 16.Iwayama K, Kurihara R, Nabekura Y, Kawabuchi R, Park I, Kobayashi M, Ogata H, Kayaba M, Satoh M, Tokuyama K. Exercise increases 24-h fat oxidation only when it is performed before breakfast. EBioMed. 2015;2:2003–2009. doi: 10.1016/j.ebiom.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwayama K, Ogawa A, Tanaka Y, Yajima K, Park I, Ando A, Ogata H, Kayaba M, Zhang S, Tanji F, Nabekura Y, Yamamoto K, Tokuyama K. Effects of exercise before breakfast on plasma free fatty acid profile and 24-h fat oxidation. Metabol Open. 2020;8:100067. doi: 10.1016/j.metop.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwayama K, Onishi T, Maruyama K, Takahashi H. Diurnal variation in the glycogen content of the human liver using 13C MRS. NMR Biomed. 2020;33:e4289. doi: 10.1002/nbm.4289. [DOI] [PubMed] [Google Scholar]
- 19.Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol. 1988;64:1480–1485. doi: 10.1152/jappl.1988.64.4.1480. [DOI] [PubMed] [Google Scholar]
- 20.Izumida Y, Yahagi N, Takeuchi Y, et al. Glycogen shortage during fasting triggers liver–brain–adipose neurocircuitry to facilitate fat utilization. Nat Commun. 2013;4:2316. doi: 10.1038/ncomms3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–346. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- 23.Mancilla R, Krook A, Schrauwen P, Hesselink MKC. Diurnal regulation of peripheral glucose metabolism: potential effects of exercise timing. Obesity. 2020;28:S38–S45. doi: 10.1002/oby.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nybo L, Pedersen K, Christensen B, Aagaard P, Brandt N, Kiens B. Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiol. 2009;197:117–127. doi: 10.1111/j.1748-1716.2009.01996.x. [DOI] [PubMed] [Google Scholar]
- 25.Parkin JA, Carey MF, Martin IK, Stojanovska L, Febbraio MA. Muscle glycogen storage following prolonged exercise: effect of timing of ingestion of high glycemic index food. Med Sci Sport Exerc. 1997;29:220–224. doi: 10.1097/00005768-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Philp A, Hargreaves M, Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab. 2012;302:E1343–E1351. doi: 10.1152/ajpendo.00004.2012. [DOI] [PubMed] [Google Scholar]
- 27.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robusto KM, Trost SG. Comparison of three generations of ActiGraph activity monitors in children and adolescents. J Sport Sci. 2012;30:1429–1435. doi: 10.1080/02640414.2012.710761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ros S, Zafra D, Valles-Ortega J, Garcia-Rocha M, Forrow S, Dominguez J, Calbo J, Guinovart JJ. Hepatic overexpression of a constitutively active form of liver glycogen synthase improves glucose homeostasis. J Biol Chem. 2010;285:37170–37177. doi: 10.1074/jbc.M110.157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada K, Yamamoto Y, Iwayama K, Nakamura K, Yamaguchi S, Hibi M, Nabekura Y, Tokuyama K. Effects of post-absorptive and postprandial exercise on 24 h fat oxidation. Metabolism. 2013;62:793–800. doi: 10.1016/j.metabol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Stocks B, Dent JR, Ogden HB, Zemp M, Philp A. Postexercise skeletal muscle signaling responses to moderate- to high-intensity steady-state exercise in the fed or fasted state. Am J Physiol. 2019;316:E230–E238. doi: 10.1152/ajpendo.00311.2018. [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld BJ, Aragon AA, Wilborn CD, Krieger JW, Sonmez GT. Body composition changes associated with fasted versus non-fasted aerobic exercise. J Int Soc Sports Nutr. 2014;11:54. doi: 10.1186/s12970-014-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications. 2014;28:836–843. doi: 10.1016/j.jdiacomp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Kamei A, Osawa T, Kawahara T, Takizawa O, Maruyama K. 13C MRS reveals a small diurnal variation in the glycogen content of human thigh muscle. NMR Biomed. 2015;28:650–655. doi: 10.1002/nbm.3298. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Ogata H, Kayaba M, Ando A, Park I, Yajima K, Araki A, Suzuki C, Osumi H, Zhang S, Isihara A, Takahashi K, Shoda J, Nabekura Y, Satoh M, Tokuyama K. Effect of a single bout of exercise on clock gene expression in human leukocyte. J Appl Physiol. 2020;128:847–854. doi: 10.1152/japplphysiol.00891.2019. [DOI] [PubMed] [Google Scholar]
- 37.Terada T, Wilson BJ, Myette-Cote E, Kuzik N, Bell GJ, McCarggar LJ, Boule NG. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolim. 2016;65:599–608. doi: 10.1016/j.metabol.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Van Proeyen K, Szlufcik K, Nielens H, Ramaekers M, Hespel P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J Appl Physiol. 2011;110:236–245. doi: 10.1152/japplphysiol.00907.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira AF, Costa RR, Macedo RCO, Coconcelli L, Kruel LFM. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr. 2016;116:1153–1164. doi: 10.1017/S0007114516003160. [DOI] [PubMed] [Google Scholar]
- 40.Yahagi N. Hepatic control of energy metabolism via the autonomic nervous system. J Atheroscler Thromb. 2017;24:14–18. doi: 10.5551/jat.RV16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi U, Nishide S, Honma S, Honma K. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol. 2015;309:R1112–R1121. doi: 10.1152/ajpregu.00127.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.