Abstract
We investigated if blood flow restriction (BFR, cuff pressure 20 mmHG below individual occlusion pressure) increases metabolic stress, hormonal response, release of muscle damage markers, and muscle swelling induced by moderate-intensity eccentric contractions. In a randomized, matched-pair design, 20 male subjects (25.3 ± 3.3 years) performed four sets of unilateral eccentric knee extensions (75% 1RM) to volitional failure with (IG) or without (CG) femoral BFR. Despite significant differences of performed repetitions between IG (85.6 ± 15.4 repetitions) and CG (142.3 ± 44.1 repetitions), peak values of lactate (IG 7.0 ± 1.4 mmol l−1, CG 6.9 ± 2.7 mmol l−1), growth-hormone (IG 4.9 ± 4.8 ng ml−1, CG 5.2 ± 3.5 ng ml−1), insulin-like growth factor 1 (IG 172.1 ± 41.9 ng ml−1, CG 178.7 ± 82.1 ng ml−1), creatine-kinase (IG 625.5 ± 464.8 U l−1, CG 510.7 ± 443.5 U l−1), the absolute neutrophil count (IG 7.9 ± 1.3 103 µl−1, CG 8.7 ± 2.0 103 µl−1), induced muscle swelling of rectus femoris and vastus lateralis and perceived pain did not differ. The present data indicate that BFR is suitable to intensify eccentric exercises.
Keywords: Lactate, Human growth hormone, Creatine kinase, Muscle damage
Introduction
When compared to concentric training, eccentric training has been reported to be associated with greater strength and mass gains in healthy individuals [1]. It has been proposed that the capacity to exert higher forces during eccentric contractions are responsible for this observation [1], indicating that the mechanical stimulus is a key anabolic signal in resistance training. It has further been speculated that the associated exercise-induced muscle damage (EIMD), which results in a temporary decline of muscle performance after eccentric exercises, affects the gene expression in muscle fibers and is required for long-term hypertrophic adaptations [2]. By contrast, Damas et al. [3] reported that EIMD and the associated protein synthesis was the highest after the initial bouts of a resistance training program, whereas the protein synthesis was only related to hypertrophy after the third and tenth week of training when muscle damage was attenuated. This indicates that high mechanical forces but not the EIMD are the anabolic signal of the eccentric training. Meanwhile, it is well established that the signaling pathway used by the organism for mechanically induced muscle growth activates the mammalian target of rapamycin (mTOR), resulting in increased protein synthesis and hypertrophy of muscle fibers [4, 5]. A recently published study has shown that the anabolic signaling was greater after eccentric than concentric contractions [6], which supports the outcome of the meta-analysis above, published by Roig et al. [1]. In summary, it seems unquestionable that mechanical stress represents a key anabolic stimulus for skeletal muscles.
However, there is a growing body of evidence that neither EIMD nor high forces are compulsory for exercise-induced mass and strength gains [2, 7]. For example, it has been demonstrated that moderate-intensity training regimens are associated with greater hypertrophic responses of musculature when compared to high-force protocols [8–10]. Even intensities of only 30% of the one repetition maximum (1RM) have been reported to induce similar responses of the muscular protein synthesis, when compared to high intensities (90% 1RM), provided that both protocols are performed until muscle failure [11]. In fact, a recent study suggests that a maximal voluntary contraction can induce similar strength gains when compared to a high load [12]. This indicates that other signals than high mechanical forces must be involved that can trigger the anabolic signaling cascade of skeletal muscle fibers.
Several lines of evidence suggest that metabolic stress, a result of metabolite accumulation, especially of lactate and H+, represents such a trigger and may explain structural and functional adaptations in the absence of meaningful muscle tension (defined as training intensities below ~60% of the 1RM) [13]. Support for this assumption comes from studies in which the metabolic stress is artificially increased by blood flow restriction (BFR). Intriguingly, this type of training has been shown to induce mass and strength gains in muscles that were trained at low intensities as little as ~20% 1RM with a predetermined amount of total repetitions [14, 15]. Even lower intensities in the form of walking with BFR have been reported to be associated with hypertrophic adaptations after a period of just 3 weeks [16]. The underlying mechanisms by which metabolic stress exerts its anabolic effects on muscle tissue remain unclear. However, it has been speculated that differences in the pattern of fiber recruitment, greater hormonal responses, reactive oxygen species (ROS), cellular swelling, and altered myokine production could explain the anabolic potential of the exercise-induced metabolic stress [13].
In summary, both eccentric exercises and BFR training have been proven effective training modalities for eliciting muscle strength and mass gains, though by different mechanisms. Therefore, it seems plausible that combining both modalities provides additive benefits. However, previous data on this issue are scarce and conflicting. Sudo et al. [17] reported that combining high-intensity eccentric contractions with BFR enhanced the hypertrophic signaling in exercised muscles of Wistar rats, while the amount of muscle damage was much lower compared to the unrestricted condition. In contrast, in another study the combination of BFR and eccentric contractions induced less hypertrophy and strength gains in a 6-week, low-intensity training intervention, when compared to the combination of BFR and concentric contractions [18]. However, participants in that study did not perform each set to volitional failure but until a predetermined amount of repetitions. In consequence, the rate of perceived exertion was greater with the concentric-BFR combination compared to the eccentric-BFR protocol. Thus, it is likely that in the case of the latter combination, more repetitions could have been performed by the participants and that potential benefits of combining BFR with eccentric contractions were masked by the premature termination of the exercise. To shed more light on the effects of BFR during eccentric contractions, the present study investigated if this combination increases the metabolic stress, the response of anabolic hormones, and the amount of muscle damage, compared to eccentric contractions without BFR, when performed until muscle failure. Further, we assessed if muscle swelling, the range of motion, and the number of circulating inflammatory cells (neutrophils) differed between restricted and non-restricted blood flow conditions.
Methods
Subjects
Twenty healthy active male sports students (25.1 ± 3.1 years; 185.5 ± 6.5 cm; 81.4 ± 10.0 kg) were recruited as a sample of convenience from our university. All participants were engaged in at least 4 h of physical training per week and familiar with leg extension machines to avoid the effects of naïve exercise. Exclusion criteria were defined as any cardiovascular or orthopedic pathologies reported by the participants. Further, any medication was considered an exclusion criterion and all participants were instructed to refrain from taking any nutrition or other performance enhancing substances. To ensure similar groups regarding at least one key measurement, all participants were matched according to their knee extension one-repetition maximum (1RM). Matched pairs were then randomly assigned to an intervention (IG) or a control group (CG). The participants were asked to refrain from any strenuous physical exercises for 48 h before the 1RM tests and the intervention. Written informed consent was obtained from all participants before the start of the intervention. All procedures of the present study were in accordance with the Declaration of Helsinki and were approved by the local ethics board.
One repetition maximum (1RM)
One week before the intervention, the unilateral 1RM for knee extensions was assessed for the dominant leg according to the protocol of Baechle and Earle [19]. Briefly, participants were seated on a leg extension machine (Miltronic, Milon, Emersacker, Germany). Back pad position and lever arm length were adjusted to participants’ anthropometrics. The range of motion was set to 90° for all participants. All of these settings were saved on a chip card for each participant to ensure that the same settings were used during the exercise protocol. After a warm-up period, consisting of 15 submaximal repetitions, we started at 20% of a conservatively estimated 1RM so that the athletes could easily complete 3–5 reps. Each trial was followed by a 3-min resting period. We then increased the weight by 20%. As long as the athlete completed the following trials of one repetition per set successfully, we increased the weight by 10% until the athlete could no longer perform a repetition. We then decreased the weight by 5% until a 1RM was determined. All participants were advised to grasp the handles of the machine during the attempt. Every attempt was supervised to guarantee sufficient execution. Each maximal attempt was separated by a 3-min rest period. The highest weight that participants were able to lift in a controlled manner throughout the full test range of motion (10–100° inner knee angle) was defined as the 1RM.
Cuff pressure
BFR training is supposed to occlude the venous, but not the arterial blood flow. This results in a venous pooling without inducing a complete ischemia. The latter would significantly increase the risk of negative side effects and may reduce the favorable muscular adaptations [20]. In line with recommendations of Loenneke et al. [21], the applied cuff pressure in the present study was oriented on the individual blood pressure instead of using a fixed cuff pressure for all participants.
For that purpose, the arterial occlusion pressure was measured for all participants before the intervention, using an ultrasound Doppler system (Xario XG, Toshiba Medical Systems, Germany). To take account for orthostatic effects, participants were positioned on an examination bench with an upright back pad similar to the leg extension machine, which was used for the exercise protocol. In this position, a 13-cm-wide cuff (Riester Komprimeter Nr. 5255, Rudolf Riester GmbH, Jungingen, Germany) was wrapped around the proximal thigh. Posterior to the medial malleolus, the arteria tibialis posterior was displayed with the ultrasound device, and a Doppler was used to assess the blood flow within the vessel. After that, the cuff was inflated until no further blood flow was detectable. This pressure was defined as the individual occlusion pressure. Though 10 mmHg below this pressure, some blood flow in the arteria tibialis posterior was detected in all participants, we chose to err on the side of caution and used 20 mmHg below the occlusion pressure during the training protocol to guarantee arterial blood flow in the trained leg. The average occlusion pressure for the BFR group was 197 ± 33.7 mmHg.
Exercise protocol
All participants performed four sets of unilateral eccentric knee extensions with their dominant leg until volitional muscle failure. The latter was defined as the inability to perform another repetition while maintaining the predefined movement velocity. The ROM was set to 90° (100–10° knee flexion). To ensure a proper technique, all of the subjects participated in a familiarization training 1 week before the intervention. The resistance for this eccentric phase of the movement was set to 75% of the predetermined concentric 1RM, as it was shown previously that this moderate-intensity eccentric resistance was equally effective in inducing strength gains when compared to higher external loads of 125% of the concentric 1RM [22]. Further, this intensity aimed to increase the maximum number of possible repetitions per set to increase the metabolic stress. The concentric phase of the movement was performed only with the opposite leg. During that phase of the movement, the resistance was automatically lowered by the machine to 50% of the eccentric load (corresponding to 37.5% of the 1RM). The inter-set rest period was set to 30 s, as is frequently used for BFR training protocols [23]. A visual biofeedback system was used to ensure that repetitions were performed at a standardized movement velocity of 1.0 and 2.0 s for the concentric and the eccentric phase of the movement.
Blood sampling and analysis
Venous blood samples were drawn from the antecubital vein of participants before (pre), immediately after (post), 20 min, 2 h, and 24 h after the exercise protocol. We opted for these time intervals, as we wanted to determine short-term and intermediate hormonal responses on the one side and to keep the number of blood samples for the participants as low as possible. Furthermore, previous studies indicated that the markers investigated in the present study (see below) change within 24 h after eccentric contractions and BFR training [24–26]. The blood samples were drawn into Serum Separation Tubes™ (SST) and ethylenediaminetetraacetate (EDTA)-coated tubes. The latter were used for an absolute neutrophil count (ANC), which was performed immediately after taking the sample using an automated hematology analyzer (Sysmex KX-21N, Sysmex Deutschland GmbH, Norderstedt, Germany). The SSTs were stored at room temperature for 30 min before they were centrifuged for 10 min at 1861 × g and 4° C (Rotixa 50RS, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany). Until further analysis, samples were frozen and stored at −80 °C. About 4 weeks after the intervention, samples were unfrozen and analyzed for creatine kinase (CK) activity and human growth hormone (hGH) concentration by an external laboratory. CK was determined with the clinical chemistry system Advia 1800 (Siemens Healthcare Diagnostics GmbH, Eschborn, Germany), whereas hGH was determined with a two-stage chemiluminescent immunometric assay using the immunoassay analyzer Immulite 2000 (Siemens Healthcare Diagnostics GmbH, Eschborn, Germany). Capillary blood samples were taken from the earlobe before (pre), immediately after (post), 2, 4, and 6 min after the intervention to determine the lactate concentrations using the EBIOplus (EKF Diagnostic Sales, Magdeburg, Germany). The IGF-1 concentration was determined by using an ELISA-Kit (IGF-1, Quantikine ELISA, Cat. no.: 173 DG100, R&D Systems, Minneapolis, MN, USA).
Muscle thickness
To assess exercise-induced muscle swelling, the same experienced physician measured muscle thicknesses of the rectus femoris and the vastus intermedius at pre, post, 20 min, 2 h, and 24 h. During the examination, care was taken to provide a standardized position for all subjects. That is, participants were lying supine on an examination table with hips and knees extended and muscles completely relaxed, which is in accordance to previously published research [29, 30]. The ultrasound sensor (Xario XG, Toshiba Medical Systems, Germany) was placed at 50% of the total muscle length, measured as the distance between the spina iliaca anterior superior and the proximal margin of the patella. To increase the repeatability, this point was marked with water-resistant ink and subject positioning was kept constant for all follow-up measurements. Muscle thickness was defined as the distance between the lower margin from the upper fascia and the upper margin of the lower fascia of the respective muscles, measured perpendicular to the surface.
Perceived muscle pain
Before the first and immediately after every set, as well as 20 min, 2 h, and 24 h after the last set, participants were asked to rate their perceived muscle pain on a visual analog scale. The scale consisted of a 10-cm-long line, with only the beginning and the end of the line being labeled with “no pain” and “worst conceivable pain”, respectively.
Range of motion (ROM)
Exercise-induced muscle damage (EIMD) is known to limit the range of motion of adjacent joints due to an increased longitudinal stiffness of the muscle and is therefore frequently used to quantify the EIMD [27]. The ROM was tested at the same intervals like the perceived muscle pain, and the same physician performed all procedures. The ROM of the knee joint was measured while participants were lying prone on a flat examination bench with their hips and knees fully extended. From this position, the leg that performed the eccentric contractions was passively flexed until a noticeable resistance occurred. The inner knee angle in that position was measured with a goniometer (Bauerfeind AG, Zeulenroda-Triebes, Germany). After that, the same procedure was performed with the opposite leg.
Statistical analysis
Data in the tables are presented as mean values, SDs, and statistical significance levels for specific comparisons. A two-way mixed ANOVA was used to compare changes in measures over time between both groups. If ANOVA revealed significant results, a Bonferroni post hoc analysis was used to examine which of the within- and between-treatment differences were significant. The critical level of significance in the present study was set to p < 0.05. The IBM software package SPSS Statistics (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp, USA) was used for all analyses.
Results
All of the enrolled participants completed the entire study protocol, and none of them was injured. The matching of the participants resulted in well-balanced groups regarding the unilateral knee extension 1RM (IG 68.3 ± 11.5 kg; CG 66.2 ± 8.1 kg). There was a significant group by time interaction for the number of performed repetitions F(3, 18) = 6.66, p < 0.001 (Fig. 1a). While the number of performed repetitions significantly (p = 0.02) differed between both groups at the first set, no such difference was found for the second to fourth set. As can be seen in Fig. 1, the number of performed repetitions substantially decreased from the first to the second set in both groups, whereas only slight decreases were found from set 2 to 3 and from set 3 to 4. Overall, the participants of the CG performed 39.9 ± 34.9% more repetitions with 75% of the 1RM than participants in the IG, resulting in a total load (=total number of repetitions × load) of 9438 ± 357 kg in the CG and 5849 ± 176 kg in the IG.
Fig. 1.
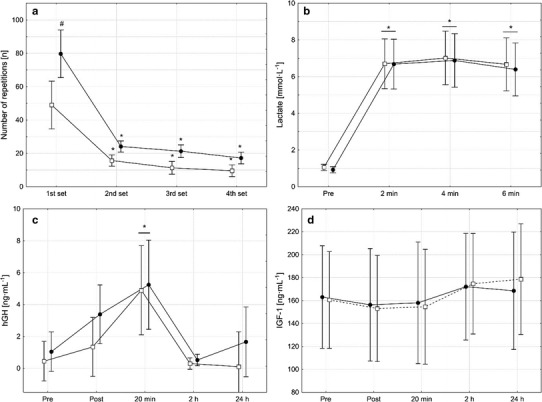
Number of completed repetitions (a), lactate (b), growth hormone (hGH; c), and insulin-like growth factor 1 (IGF-1; d) concentrations assessed prior to and after four sets of unilateral eccentric knee extensions that were performed until volitional muscle failure either with (open squares) or without (filled circles) blood flow restriction. Data are presented as means with 95% confidence intervals. Asterisks indicate significant difference (p < 0.05) from pre, pound keys indicate significant difference (p < 0.05) between groups. In cases where no significant group by time interactions were observed, asterisks above horizontal lines spanning adjacent bars indicate this measurement time point was significantly different (p < 0.05) from pre, collapsed across groups
Before the intervention commenced, lactate concentration (IG 1.1 ± 0.3 mmol l−1; CG 0.9 ± 0.2 mmol l−1), CK activity (IG 181.8 ± 105.7 U l−1; CG 170.2 ± 79.2 U l−1), ANC (IG 5.6 ± 0.9 103 µl−1; CG 5.8 ± 1.3 103 µl−1), IGF-1 (IG 163.0 ± 46.1 ng ml−1, CG 160.5 ± 68.9 ng ml−1), and hGH concentration (IG 0.5 ± 0.9 ng ml−1; CG 1.0 ± 2.5 ng ml−1) did not differ between both groups and despite pronounced differences in performed repetitions and total load, no group or group by time interactions was observed for these blood parameters (Figs. 1, 2). However, there was a significant main effect of time for lactate F(3, 18) = 126.2, p < 0.001, CK F(4, 18) = 12.4, p < 0.001, ANC F(4, 18) = 76.5, p < 0.001, and hGH F(4, 18) = 10.4, p < 0.001, but not for IGF-1 F(4, 18) = 6.66, p < 0.001. No significant group by interaction was found for the perceived muscle pain F(7, 18) = 138.68, p = 0.09. However, there was a main effect of time (Fig. 2c).
Fig. 2.
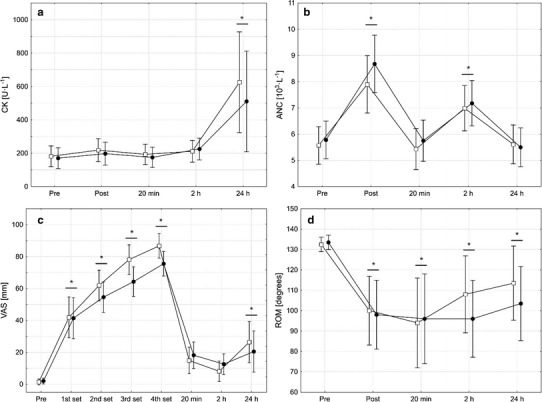
Activity of creatine kinase (CK; a), absolute neutrophil count (ANC; b), perceived muscle pain measured using the visual analogue scale (VAS; c), and the range of motion of the knee joint (ROM; d) assessed prior to and after four sets of unilateral eccentric knee extensions that were performed until volitional muscle failure either with (open squares) or without (filled circles) blood flow restriction. Data are presented as means with 95% confidence intervals. No significant group by time interactions were observed for CK, ANC, VAS, and ROM. Therefore, asterisks above horizontal lines spanning adjacent bars indicate that this measurement time point significantly different from pre, collapsed across groups
The perceived muscle pain significantly increased from pre values in both groups with a peak after the fourth set and declined after that (Fig. 2c). A second increase was observed 24 h after the intervention. No significant difference was found between groups. No group by time interaction F(4, 18) = 0.8, p = 0.5 or a main effect for group F(1, 18) = 0.18, p = 0.7 was found for the ROM values (Fig. 2d). However, a significant main effect of time emerged from the ANOVA, F(4, 18) = 19.42, p < 0.001; meaning that the ROM of the knee joint was similarly impaired in the IG and the CG. After the intervention, the ROM values remained significantly (p < 0.001) decreased over the entire observation period.
There was no significant group by time interaction on the muscle thickness of the rectus femoris F(4, 18) = 0.62, p = 0.6 or the vastus intermedius F(3, 18) = 0.07, p = 0.9 (Fig. 3). However, a significant main effect of time was observed for both muscles (rectus femoris, F(4, 18) = 59.4, p < 0.001; vastus intermedius, F(4, 18) = 33.5, p < 0.001). That is, in both groups, the thickness of the rectus femoris and the vastus medialis increased from pre to post. While this increase remained statistically significant (p < 0.001) until 24 h after the intervention for the rectus femoris, the muscle thickness of the vastus intermedius presented a bimodal pattern. Only the post (p < 0.001), 20 min (p = 0.004), and 24 h (p < 0.001) values were significantly greater than the respective pre-value, while the 2 h value did not differ from pre (p = 0.5).
Fig. 3.
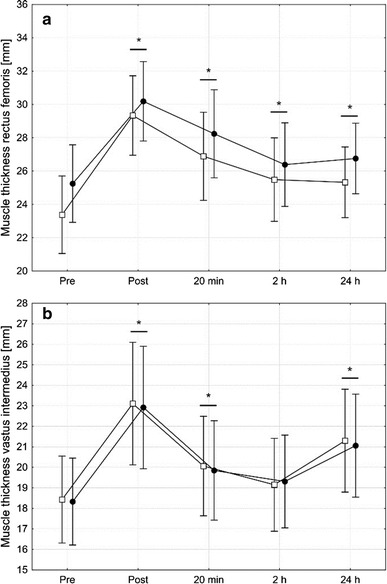
Muscle thickness of the rectus femoris (a) and the vastus intermedius (b) assessed prior to and after four sets of unilateral eccentric knee extensions that were performed until volitional muscle failure either with (squares) or without (circles) blood flow restriction. Data are presented as means with 95% confidence intervals. No significant group by time interactions were observed for the muscle thickness of the rectus femoris and the vastus intermedius. Therefore, asterisks above horizontal lines spanning adjacent bars indicate that this measurement time point significantly different from pre, collapsed across groups
Discussion
The main finding of the present study was that the metabolic stress, the endocrine response and indirect markers of muscle damage did not differ between the IG and the CG, despite the pronounced differences regarding the number of performed repetitions and the total load lifted. That is, fewer repetitions were needed to evoke a comparable response pattern of the selected markers when the blood flow to the leg muscles was restricted during moderate intensity eccentric knee extensions.
It can be speculated that the BFR-mediated effects are based on an altered recruitment pattern. Studies on low-intensity exercises in conjunction with BFR indicate that the reduced oxygen supply of the working muscles results in a premature fatigue of slow twitch fibers (STFs), which is counterbalanced by an early recruitment of fast twitch fibers (FTFs) to maintain the necessary force output [28]. Because a recruitment of large and high-threshold motor units is thought to be crucial for this adaptation [31], some authors assume that this early FTF recruitment is the key factor for the hypertrophic responses seen after a BFR training regimen [32, 33]. Since the energy production of FTFs primarily relies on anaerobic glycolysis, their early recruitment results in a rapid buildup of metabolites, associated with the release of anabolic hormones [23, 34]. However, FTFs are naturally recruited early during eccentric contractions [34]. Therefore, it is unlikely that an altered recruitment pattern is responsible for the results of the present study. Nevertheless, the reduced blood flow likely aggravated the metabolic situation in all recruited muscle fibers and resulted in the early fatigue of the knee extensors in the IG. This is indicated by the lactate concentrations that were similar between both conditions even thought the time under tension and the total load lifted was markedly lower in the IG than in the CG (Fig. 1b).
Lactate, hGH, and IGF-1
Though the exact mechanisms underlying the exercise-induced hGH release remain unclear, a metabolite buildup with a concomitant reduction in muscle pH may be involved in triggering the hGH release from the pituitary gland. In this context, Häkkinen and Pakarinen [35], who compared two different high-intensity resistance training protocols, reported that the hGH release is linked to the grade of exercise-induced muscle fatigue. Endings of myelinated group III and unmyelinated group IV nerves residing in the interstitium of skeletal muscles denoted as metaboreceptors [36, 37], are thought to play a significant role in this metabolic stress-dependent cascade [38, 39]. Since both groups in the present study performed all sets to muscle failure and reached comparable lactate concentrations, the resulting metaboreflex may explain the comparable hGH response in the IG and CG.
Eccentric contractions, either with constant external load [40] or isokinetically [41, 42], are known to induce lower hGH responses in the post-exercise phase than concentric contractions. This phenomenon likely results from the fact that eccentric contractions induce less metabolic stress when compared to concentric contractions performed at the same total load [40, 43]. By contrast, low-intensity resistance training protocols in combination with BFR have been demonstrated to result in marked hGH elevations in the blood [26, 44, 45]. For example, Pierce et al. [46] found a ninefold hGH increase from baseline after unilateral knee extension at 20% MVC with BFR. Takarada et al. [26] even found a 290-fold increase from baseline, following five sets of low-intensity (20% 1RM) blood flow-restricted bilateral knee extensions performed until failure. These studies delineate that aggravating the blood supply for muscle fibers during exercise via BFR results in greater hGH responses. In the present study, hGH increased fivefold in the CG and tenfold in the IG (BFR: pre: 0.5 ± 0.94; post: 4.9 ± 4.83 ng/ml, p = 0.03; CG: pre: 1.0 ± 2.5 ng/ml; post: 5.24 ± 3.47 ng/ml, p = 0.01) on average. However, this difference was not statistically significant. Collapsed across both groups, the hGH increased 6.8-fold from pre to 20 min after the intervention, which is in line with the study presented by Pierce et al. [46]. The fact that the hGH response did not differ between both groups despite the significant difference in the number of performed repetitions, indicates that the intensification of exercises via BFR seems not only to apply for low intensity (20–50% 1RM) protocols, as demonstrated previously [26, 46], but also for moderate-intensity (75% 1RM) eccentric contractions. The relevance of this finding for chronic adaptations remains to be determined by future research.
It has been debated for decades whether exercise-induced elevations of hGH affect the process of muscle hypertrophy [47]. On one side it has been stated that administration of recombinant hGH does not induce muscle growth in healthy subjects [48–50] and it was further demonstrated that exercise-induced muscle hypertrophy was independent of concentrations of circulating anabolic hormones [51]. On the other side, the greatest hGH elevations are usually seen in response to “hypertrophy protocols” that are characterized by high volume and short interset rest periods, whereas relatively small hGH increases are associated with strength training protocols with low repetitions and longer rest periods [52]. While some authors suggest that the anabolic potential of hGH mainly arises from IGF-1 releasing effect [53], others are convinced that hGH and IGF-1 effects on muscle anabolism are additive and rely on different signaling pathways [54]. Nevertheless, the circulating IGF-1 remained virtually unaffected in both groups of the present study and may therefore play a minor role for potential muscular adaptations arising from the combination of eccentric contractions and BFR. However, a recently published review points out that hGH-mediated effects on skeletal muscle fibers may be based on a local IGF-1 synthesis that is not detected by plasma analyses and might explain why several studies failed to detect a link between muscular adaptations and circulating IGF-1 concentrations [55].
Muscle swelling
Muscle swelling has been hypothesized to be involved in metabolic stress-mediated hypertrophy [13]. It has been suggested that an increased intracellular pressure against the cytoskeleton activates integrin-associated osmosensors within the muscle fibers, triggering anabolic and anticatabolic processes [56]. Since an intracellular metabolite accumulation seems to be the driving force for the transmembrane fluid shift [13], the same degree of muscle swelling in both groups of the present study seems plausible based on the comparable lactate concentrations that were found in the IG and the CG.
It is interesting to note that the muscle thickness of the rectus femoris remained increased over the entire follow-up period of 24 h and that the muscle thickness of the vastus intermedius presented a bimodal response with a second increase after 24 h. While the initial swelling likely results from the metabolite build up, as described above, the second increase in muscle thickness may be related to an EIMD-induced inflammation that is known to occur with some delay [10]. If cell swelling provides an anabolic signal, this signal seems to be active for a prolonged period after exhausting eccentric contractions, no matter if the time until exhaustion was shortened by BFR or not. However, the duration of the muscle swelling is unlikely based on metabolites as these should be cleared early from the muscles after the intervention [57]. Therefore, it is reasonable to assume that factors other than the metabolite buildup contributed to the muscle swelling. One of these factors could be inflammation-induced edema, involving an accumulation of neutrophil granulocytes. Neutrophils are known to release proteolytic enzymes and produce reactive oxygen species, resulting in an increased capillary leakage and may further worsen an edema by inducing a vasoconstriction or a capillary plugging [58].
EIMD and circulating neutrophils
Muscle damage is known to result predominantly from unaccustomed lengthening contractions. Over the past decades, most research has emphasized that mechanical stress acting on the muscle fibers during eccentric contractions induces membrane damage [59]. The resulting clefts allow large molecules such as muscle enzymes to leak into the extracellular space. These molecules are therefore used as indirect markers of muscle damage. However, the observation that large molecules also occur in the bloodstream after exhausting exercises that virtually lack any mechanical stress [60, 61] led to the recently published hypothesis on metabolic stress-associated membrane disturbances [62]. Accordingly, we hypothesized that the metabolic stress resulting from the reduced oxygen supply during the eccentric contractions leads to greater membrane damage in the IG. This damage may result from reactive oxygen species that are formed in consequence of an ischemia–reperfusion sequence or by invading neutrophils [63–65].
However, in the present study, the CK response, the perceived muscle pain, and the ANC did not differ between groups. This observation is consistent with previous investigations reporting that BFR training is neither associated with high ROS concentrations [26, 66] nor with high concentrations of muscle damage markers in the blood [67, 68]. Further, in line with the literature [69], the present data do not indicate that BFR aggravated the eccentric exercise-induced longitudinal stiffness of the quadriceps, as the ROM of the knee joint was similarly affected in both groups. Nevertheless, the indirect markers of muscle damage (CK, ANC, and ROM) assessed in the present study should be interpreted in the light of the pronounced group differences regarding the performed repetitions and the total load lifted. That is, less mechanical load in the IG induced a comparable amount of EIMD.
Limitations
The findings of the present study are subject to at least four limitations. First, we did not investigate a third group that was matched to the total load performed in the IG. Though we are convinced that the acute effects of such a group would have been much lower than those seen in the IG, we are unable to scale this difference. Secondly, the present study is entirely based on the investigation of blood parameters, measures of muscle fatigue, and perceived muscle pain. However, it would have been interesting to know from muscle biopsies if the protein accretion and induced muscle damage is similar after both protocols. Thirdly, it is important to note that it is hard to estimate long-term effects of new training modalities on the grounds of acute responses. To the best knowledge of the authors, only one study to date has investigated chronic muscular adaptations to blood flow-restricted eccentric contractions [18]. In that study, muscle growth was greater for concentric BFR contractions when compared to eccentric BFR contractions. However, the low resistance used in that study (i.e., 30% of the concentric 1RM) and the fact that movements were not performed until muscle failure, clearly reduce the comparability to the present study. Therefore, future investigations are needed to clarify if the addition of BFR to moderate-intensity eccentric contractions is associated with beneficial effects on functional and structural adaptations when applied over several weeks. Finally, it needs to be taken into account that all participants performed not only eccentric but also concentric contractions to return the lever arm of the knee extensor machine back into the starting position. Although the load for this part of the movement was reduced, it is likely that the concentric contractions affected the response of the investigated parameters. However, since the CG performed much more contractions (eccentric and concentric) than the IG, this rather supports our conclusion that blood flow restriction reduced the number of contractions needed to induce the observed effects.
Conclusions
To the extent of our knowledge, the present study was the first to investigate the effect of BFR on the acute metabolic, hormonal, inflammatory, and muscle damage response to a single bout of moderate-intensity eccentric contractions performed until exhaustion. Despite a considerably lower number of repetitions that were performed under the blood flow-restricted condition, we observed similar responses of lactate, hGH, ANC, CK, and ROM when compared to the unrestricted condition. Further, the exercise-induced muscle swelling of the rectus femoris and vastus intermedius did not differ between both groups. The conclusion that can be drawn from the present data is that BFR is capable of intensifying the exercise stimulus of moderate-intensity eccentric contractions in terms that fewer repetitions (and total load) are needed to induce a similar response pattern of selected parameters. However, it needs to be determined by future investigations if muscular adaptations are comparable between both protocols when applied chronically.
Acknowledgements
We wish to thank Dr. Silvia Achtzehn for her support in the ELISA analysis.
Author contributions
MB: conception and design of the work; acquisition, analysis and interpretation of the data; writing and critically revising the manuscript critically for important intellectual content. LH: acquisition, analysis, and interpretation of the work; writing the manuscript; critically revising the manuscript for important intellectual content. JL: analysis and interpretation of the data for the work; writing the manuscript; critically revising the manuscript for important intellectual content. JM: conception and design of the work; critically revising the manuscript for important intellectual content.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Roig M, O’Brien K, Kirk G, et al. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009;43(8):556–568. doi: 10.1136/bjsm.2008.051417. [DOI] [PubMed] [Google Scholar]
- 2.Schoenfeld B. Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy? J Strength Cond Res. 2012 doi: 10.1519/JSC.0b013e31824f207e. [DOI] [PubMed] [Google Scholar]
- 3.Damas F, Phillips SM, Libardi CA, et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol. 2016;594(18):5209–5222. doi: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle. 2006;5(1538–4101):1391–1396. doi: 10.4161/cc.5.13.2921. [DOI] [PubMed] [Google Scholar]
- 5.Goodman CA. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol. 2014;166:43–95. doi: 10.1007/112_2013_17. [DOI] [PubMed] [Google Scholar]
- 6.Gehlert S, Suhr F, Gutsche K, et al. High force development augments skeletal muscle signalling in resistance exercise modes equalized for time under tension. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1579-y. [DOI] [PubMed] [Google Scholar]
- 7.Flann KL, LaStayo PC, McClain DA, et al. Muscle damage and muscle remodeling: no pain, no gain? J Exp Biol. 2011;214(Pt 4):674–679. doi: 10.1242/jeb.050112. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson PM, Byrnes WC, McCormick KM, et al. Muscle soreness and serum creatine kinase activity following isometric, eccentric, and concentric exercise. Int J Sports Med. 1986;7(3):152–155. doi: 10.1055/s-2008-1025753. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–520. [PubMed] [Google Scholar]
- 10.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(11 Suppl):S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 11.Burd NA, West Daniel W D, Staples AW, et al. Low-load high-volume resistance exercise stimulates muscle protein synthesis more than high-load low-volume resistance exercise in young men. PLoS One. 2010;5(8):e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Counts BR, Buckner SL, Dankel SJ, et al. The acute and chronic effects of “NO LOAD” resistance training. Physiol Behav. 2016;164(Pt A):345–352. doi: 10.1016/j.physbeh.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013;43(3):179–194. doi: 10.1007/s40279-013-0017-1. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Brechue WF, Kurita K, et al. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J KAATSU Train Res. 2008;4(1):1–8. doi: 10.3806/ijktr.4.1. [DOI] [Google Scholar]
- 15.Abe T, Yasuda T, Midorikawa T, et al. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J KAATSU Train Res. 2005;1(1):6–12. doi: 10.3806/ijktr.1.6. [DOI] [Google Scholar]
- 16.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100(5):1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 17.Sudo M, Ando S, Poole DC, et al. Blood flow restriction prevents muscle damage but not protein synthesis signaling following eccentric contractions. Physiol Rep. 2015;3(7):e12449. doi: 10.14814/phy2.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda T, Loenneke JP, Thiebaud RS, et al. Effects of blood flow-restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS One. 2012;7(12):e52843. doi: 10.1371/journal.pone.0052843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baechle TR, editor. Essentials of strength training and conditioning. Champaign: Human Kinetics; 1994. [Google Scholar]
- 20.Loenneke JP, Wilson JM, Wilson GJ, et al. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011;21(4):510–518. doi: 10.1111/j.1600-0838.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 21.Loenneke JP, Thiebaud RS, Abe T, et al. Blood flow restriction pressure recommendations: the hormesis hypothesis. Med Hypotheses. 2014;82(5):623–626. doi: 10.1016/j.mehy.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder ET, Hawkins SA, Jaque SV. Musculoskeletal adaptations to 16 weeks of eccentric progressive resistance training in young women. J Strength Cond Res. 2004;18(2):227–235. doi: 10.1519/00124278-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Scott BR, Loenneke JP, Slattery KM, et al. Blood flow-restricted exercise for athletes: a review of available evidence. J Sci Med Sport. 2015 doi: 10.1016/j.jsams.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Raastad T, Bjøro T, Hallén J. Hormonal responses to high- and moderate-intensity strength exercise. Eur J Appl Physiol. 2000;82(1–2):121–128. doi: 10.1007/s004210050661. [DOI] [PubMed] [Google Scholar]
- 25.Malm C, Nyberg P, Engström M, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529(1):243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takarada Y, Nakamura Y, Aruga S, et al. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 2000;88(1):61–65. doi: 10.1152/jappl.2000.88.1.61. [DOI] [PubMed] [Google Scholar]
- 27.Yanagisawa O, Sakuma J, Kawakami Y, et al. Effect of exercise-induced muscle damage on muscle hardness evaluated by ultrasound real-time tissue elastography. Springerplus. 2015;4:308. doi: 10.1186/s40064-015-1094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loenneke JP, Wilson GJ, Wilson JM. A mechanistic approach to blood flow occlusion. Int J Sports Med. 2010;31(1):1–4. doi: 10.1055/s-0029-1239499. [DOI] [PubMed] [Google Scholar]
- 29.Strasser EM, Draskovits T, Praschak M, et al. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 2013;35(6):2377–2388. doi: 10.1007/s11357-013-9517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arts IMP, Pillen S, Schelhaas HJ, et al. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41(1):32–41. doi: 10.1002/mus.21458. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JM, Lowery RP, Joy JM, et al. Practical blood flow restriction training increases acute determinants of hypertrophy without increasing indices of muscle damage. J Strength Cond Res. 2013;27(11):3068–3075. doi: 10.1519/JSC.0b013e31828a1ffa. [DOI] [PubMed] [Google Scholar]
- 32.Loenneke JP, Fahs CA, Wilson JM, et al. Blood flow restriction: the metabolite/volume threshold theory. Med Hypotheses. 2011;77(5):748–752. doi: 10.1016/j.mehy.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Meyer RA. Does blood flow restriction enhance hypertrophic signaling in skeletal muscle? J Appl Physiol. 2006;100(5):1443–1444. doi: 10.1152/japplphysiol.01636.2005. [DOI] [PubMed] [Google Scholar]
- 34.Manini TM, Yarrow JF, Buford TW, et al. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm IGF Res. 2012;22(5):167–172. doi: 10.1016/j.ghir.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Häkkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol. 1993;74(2):882–887. doi: 10.1152/jappl.1993.74.2.882. [DOI] [PubMed] [Google Scholar]
- 36.Sterns DA, Ettinger SM, Gray KS, et al. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84(5):2034–2039. doi: 10.1161/01.CIR.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 37.Scott AC, Davies LC, Coats Andrew J S, et al. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci. 2002;102(1):23–30. doi: 10.1042/cs1020023. [DOI] [PubMed] [Google Scholar]
- 38.Viru M, Jansson E, Viru A, et al. Effect of restricted blood flow on exercise-induced hormone changes in healthy men. Eur J Appl Physiol Occup Physiol. 1998;77(6):517–522. doi: 10.1007/s004210050369. [DOI] [PubMed] [Google Scholar]
- 39.Wahl P, Hein M, Achtzehn S, et al. Acute metabolic, hormonal and psychological responses to cycling with superimposed electromyostimulation. Eur J Appl Physiol. 2014;114(11):2331–2339. doi: 10.1007/s00421-014-2952-4. [DOI] [PubMed] [Google Scholar]
- 40.Durand RJ, Castracane VD, Hollander DB, et al. Hormonal responses from concentric and eccentric muscle contractions. Med Sci Sports Exerc. 2003;35(6):937–943. doi: 10.1249/01.MSS.0000069522.38141.0B. [DOI] [PubMed] [Google Scholar]
- 41.Kraemer WJ, Dudley GA, Tesch PA, et al. The influence of muscle action on the acute growth hormone response to resistance exercise and short-term detraining. Growth Horm IGF Res. 2001;11(2):75–83. doi: 10.1054/ghir.2000.0192. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Blaudow R, Artale L, et al. The relationship of growth hormone to isokinetic exercise: concentric vs. eccentric. Med Sci Sports Exerc. 1999;31(Supplement):S229. doi: 10.1097/00005768-199905001-01086. [DOI] [Google Scholar]
- 43.Beaven CM, Willis SJ, Cook CJ, et al. Physiological comparison of concentric and eccentric arm cycling in males and females. PLoS One. 2014;9(11):e112079. doi: 10.1371/journal.pone.0112079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95(1):65–73. doi: 10.1007/s00421-005-1389-1. [DOI] [PubMed] [Google Scholar]
- 45.Tanimoto M, Madarame H, Ishii N. Muscle oxygenation and plasma growth hormone concentration during and after resistance exercise: comparison between “KAATSU” and other types of regimen. Int J KAATSU Train Res. 2005;1(2):51–56. doi: 10.3806/ijktr.1.51. [DOI] [Google Scholar]
- 46.Pierce JR, Clark BC, Ploutz-Snyder LL, et al. Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol. 2006;101(6):1588–1595. doi: 10.1152/japplphysiol.00585.2006. [DOI] [PubMed] [Google Scholar]
- 47.Schoenfeld BJ. Postexercise hypertrophic adaptations: a reexamination of the hormone hypothesis and its applicability to resistance training program design. J Strength Cond Res. 2013;27(6):1720–1730. doi: 10.1519/JSC.0b013e31828ddd53. [DOI] [PubMed] [Google Scholar]
- 48.Lange Kai Henrik, Wiborg Andersen JL, Beyer N, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87(2):513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- 49.Yarasheski KE, Campbell JA, Smith K, et al. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol. 1992;262(3 Pt 1):E261–E267. doi: 10.1152/ajpendo.1992.262.3.E261. [DOI] [PubMed] [Google Scholar]
- 50.Yarasheski KE, Zachwieja JJ, Campbell JA, et al. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol. 1995;268(2 Pt 1):E268–E276. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- 51.West Daniel W D, Burd NA, Tang JE, et al. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol. 2010;108(1):60–67. doi: 10.1152/japplphysiol.01147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 53.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154(3):557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotiropoulos A, Ohanna M, Kedzia C, et al. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci USA. 2006;103(19):7315–7320. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frystyk J. Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc. 2010;42(1):58–66. doi: 10.1249/MSS.0b013e3181b07d2d. [DOI] [PubMed] [Google Scholar]
- 56.Schoenfeld BJ, Contreras B. The muscle pump. Strength Conditioning J. 2014 doi: 10.1519/SSC.0000000000000021. [DOI] [Google Scholar]
- 57.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 58.Walden DL, McCutchan HJ, Enquist EG, et al. Neutrophils accumulate and contribute to skeletal muscle dysfunction after ischemia-reperfusion. Am J Physiol. 1990;259(6 Pt 2):H1809–H1812. doi: 10.1152/ajpheart.1990.259.6.H1809. [DOI] [PubMed] [Google Scholar]
- 59.Yu JG, Liu JX, Carlsson L, et al. Re-evaluation of sarcolemma injury and muscle swelling in human skeletal muscles after eccentric exercise. PLoS One. 2013;8(4):e62056. doi: 10.1371/journal.pone.0062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Serfass RC, Apple FS. Alterations in the expression and activity of creatine kinase-M and mitochondrial creatine kinase subunits in skeletal muscle following prolonged intense exercise in rats. Eur J Appl Physiol. 2000;81(1–2):114–119. doi: 10.1007/PL00013783. [DOI] [PubMed] [Google Scholar]
- 61.Haralambie G, Senser L. Metabolic changes in man during long-distance swimming. Eur J Appl Physiol Occup Physiol. 1980;43(2):115–125. doi: 10.1007/BF00422442. [DOI] [PubMed] [Google Scholar]
- 62.Behringer M, Montag J, Franz A, et al. Exhaustive exercise—a near death experience for skeletal muscle cells? Med Hypotheses. 2014 doi: 10.1016/j.mehy.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Renzi CP, Tanaka H, Sugawara J. Effects of leg blood flow restriction during walking on cardiovascular function. Med Sci Sports Exerc. 2010;42(4):726–732. doi: 10.1249/MSS.0b013e3181bdb454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker PM. Ischemia/reperfusion injury in skeletal muscle. Ann Vasc Surg. 1991;5(4):399–402. doi: 10.1007/BF02015307. [DOI] [PubMed] [Google Scholar]
- 65.Gillani S, Cao J, Suzuki T, et al. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(6):670–675. doi: 10.1016/j.injury.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Goldfarb AH, Garten RS, Chee PDM, et al. Resistance exercise effects on blood glutathione status and plasma protein carbonyls: influence of partial vascular occlusion. Eur J Appl Physiol. 2008;104(5):813–819. doi: 10.1007/s00421-008-0836-1. [DOI] [PubMed] [Google Scholar]
- 67.Loenneke JP, Thiebaud RS, Abe T. Does blood flow restriction result in skeletal muscle damage? A critical review of available evidence. Scand J Med Sci Sports. 2014;24(6):e415–e422. doi: 10.1111/sms.12210. [DOI] [PubMed] [Google Scholar]
- 68.Thiebaud RS, Yasuda T, Loenneke JP, et al. Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv Med Appl Sci. 2013;5(2):53–59. doi: 10.1556/IMAS.5.2013.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thiebaud RS, Loenneke JP, Fahs CA, et al. Muscle damage after low-intensity eccentric contractions with blood flow restriction. Acta Physiol Hung. 2014;101(2):150–157. doi: 10.1556/APhysiol.101.2014.2.3. [DOI] [PubMed] [Google Scholar]


