Abstract
Na+-dependent Mg2+ efflux activity was studied with the fluorescent Mg2+ indicator furaptra in the presence of various potential antagonists known to inhibit other transporters and channels. Among the compounds tested, KB-R7943, an inhibitor of Na+/Ca2+ exchange, most potently inhibited the Na+/Mg2+ exchange with half inhibitory concentrations (IC50) of 21 μM (25°C) and 16 μM (35°C). These IC50 values were a factor of three to four lower than those of imipramine, a widely used inhibitor of Na+/Mg2+ exchange. Apart from the inhibitory effect on Na+/Mg2+ exchange, relatively high concentrations of KB-R7943 (100 μM at 25°C and ≥20 μM at 35°C), in combination with prolonged UV-illumination, caused cell shortening, probably because of the phototoxicity of the compound and the formation of rigor crossbridges. We conclude that KB-R7943 may be a useful tool to study cellular Mg2+ homeostasis if care is taken to minimize its phototoxicity.
Keywords: Na+/Mg2+ exchange, Na+/Ca2+ exchange, Inhibitors, Imipramine, Phototoxicity
Introduction
Extracellular Na+-dependent Mg2+ efflux (putative Na+/Mg2+ exchange) operates powerfully in various types of cells [1] and is thought to be one of the major mechanisms in the regulation of intracellular Mg2+ concentration ([Mg2+]i) in cardiac myocytes [2, 3]. Among the major intracellular and extracellular ions (Na+, K+, Ca2+, Mg2+), Mg2+ efflux seems to be activated only by intracellular Mg2+ and extracellular Na+ with half maximum activation at 1.5 and 55 mM, respectively, whereas transport is significantly inhibited by intracellular Na+ and extracellular Mg2+ only, with half inhibitory concentrations (IC50) at ~40 and 10 mM, respectively [4, 5]. In addition to gradients of Na+ and Mg2+, the absolute necessity of ATP has been suggested; a decrease in intracellular ATP below ~0.4 mM diminishes Mg2+ efflux [6]. After prolonged ischemia or hypoxia, the breakdown of ATP causes release of Mg2+ from Mg-ATP and a rise in [Mg2+]i. Intracellular depletion of ATP and Na+ accumulation (owing to inhibition of the Na+/K+ pump) strongly inhibit Mg2+ efflux, and are likely to contribute to maintenance of [Mg2+]i at high levels, which is thought to counter Ca2+ overload of the cells and may play a protective role.
Further examination to elucidate the physiological and pathological roles of putative Na+/Mg2+ exchange has been hampered by the lack of potent inhibitors of the transport. Féray and Garay [7] screened tri-cyclic anti-depressant agents in human red cells, and found that imipramine, an inhibitor of noradrenaline and serotonin uptake transporters, inhibits the Na+-dependent Mg2+ efflux with the lowest IC50 value among the compounds tested. Although the IC50 value of imipramine for Mg2+ transport is one to two orders of magnitude higher than typical serum concentrations of the drug in therapeutic use, imipramine has been widely used in a variety of tissues as a relatively potent inhibitor of Na+/Mg2+ exchange [8–10]. The objective of this study was to find a more potent inhibitor of Na+/Mg2+ exchange. After testing various compounds, we report that KB-R7943, which is known as an inhibitor of Na+/Ca2+ exchange, is the most potent inhibitor of Na+/Mg2+ exchange reported to date.
A preliminary version of the results has appeared in abstract form [11].
Methods
General
All experimental procedures involving animals were approved in advance by the Institutional Animal Care and Use Committee of Tokyo Medical University, and were performed in accordance with the “Guidelines for Proper Conduct of Animal Experiments” approved by the Science Council of Japan. The experimental procedure and set-up used in this study have been described previously [3, 12]. In brief, single ventricular myocytes enzymatically dissociated from Wistar rat hearts (9–12 weeks) were placed in a chamber on the stage of an inverted microscope (TE300; Nikon, Tokyo, Japan) and superfused with normal Tyrode’s solution containing (mM): 135 NaCl, 5.4 KCl, 1.0 CaCl2, 1.0 MgCl2, 0.33 NaH2PO4, 5.0 glucose, and 10 HEPES (pH 7.40 at 25°C by NaOH). After measurement of background fluorescence and indicator loading by incubation with furaptra-AM in normal Tyrode’s solution at room temperature, the AM ester was washed out with Ca-free Tyrode’s solution (which contained 0.1 mM K2EGTA in place of the 1.0 mM CaCl2 of normal Tyrode’s solution). Subsequent measurement of the indicator fluorescence was carried out in Ca-free conditions, unless otherwise stated.
For fluorescence measurements, the intracellular indicator was alternately excited with 350 and 382 nm light beams at 10 ms intervals, and fluorescence at 500 nm was detected from the entire volume of single cells. At each excitation wavelength, the background fluorescence measured for each cell before indicator loading was subtracted from the total fluorescence measured after indicator loading to yield indicator fluorescence intensity.
Measurements and analyses of furaptra signals
Calibration of furaptra signals was carried out as described previously [3]. Briefly, the ratio of furaptra fluorescence intensities excited at 382 and 350 nm [R = F(382)/F(350)] was converted to [Mg2+]i by use of the equation:
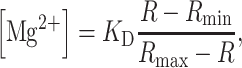 |
1 |
where K D is the dissociation constant, and R min and R max are the normalized R values at zero [Mg2+] and saturating [Mg2+], respectively. We used the values previously estimated in rat ventricular myocytes at 25°C: K D = 5.30 mM, R min = 0.969, and R max = 0.223 [13].
Although the initial screening of the inhibitors was performed at 25°C (near room temperature frequently used for single-cell experiments), more detailed analyses were also performed at 35°C (near body temperature at which the drugs’ effect may be of more physiological relevance). Because fluorescence measurements were carried out at both 25 and 35°C, we examined the effects of temperature on furaptra fluorescence with a spectrofluorimeter (FP6500, Jasco) by measuring F(382)/F(350) of 0.5 µM furaptra in 1-cm quartz cells (Fig. 1). As temperature was elevated from 15 to 35°C, furaptra’s K D was significantly reduced, with a temperature coefficient (Q 10) of 0.639 (Fig. 1A), whereas R max and R min were only slightly reduced with Q 10 of 0.778 and 0.985, respectively (Fig. 1B). Therefore, for experiments carried out at 35°C we corrected the in-vivo values using these Q 10 values estimated in vitro; the K D, R min and R max values used for intracellular furaptra were, respectively, 3.387 mM, 0.955, and 0.174 for 35°C.
Fig. 1.

Effects of temperature on K D (A), and R min and R max (B) of furaptra measured in vitro in a 1-cm quartz cell at solution temperatures between 15 and 35°C. The solutions contained 0–150 mM KCl, 0–50 mM MgCl2, 0.1 mM EGTA, 0.5 µM furaptra, and 10 mM MOPS, and the pH of the solution was adjusted by addition of KOH for each temperature. From a set of R values obtained at 0, 0.5, 1, 2, 5, 10, 20, and 50 mM Mg2+ concentrations, estimates of K D (circles), R min (triangles), and R max (crosses) were obtained by use of the nonlinear least-squares fitting technique with Eq. 1. For each data set, the solid line was drawn by nonlinear least-squares fitting with a function of the form indicated in the graph (solid lines)
Experimental procedure and solutions
The cells were first loaded with Mg2+ by incubation in solution containing high-Mg2+ and low-Na+ concentrations (Mg-loading solution) for 3–5 h at ~25°C. Mg-loading solution was prepared by substitution of 135 mM NaCl of Ca2+-free Tyrode’s solution with 101 mM NMDG-Cl (N-methyl-d-glucamine titrated with HCl to pH 7.40), 16.9 mM MgCl2, plus 6.0 mM MgMS2 (pH 7.40 adjusted by HCl and NaOH, final [Mg2+] = 24 mM and [Na+] = 1.6 mM). After [Mg2+]i was elevated from the resting level (~0.9 mM) to ~1.5 mM, Mg2+ efflux was induced by superfusion with the Ca2+-free Tyrode’s solution that contained 140 mM Na+ (described above). The initial rate of decrease in [Mg2+]i (initial Δ[Mg2+]i/Δt) was estimated by linear regression of data points spanning 180 s (for 25°C) or 120 s (for 35°C), unless otherwise stated, after the addition of extracellular Na+, and was analyzed as an index of the rate of Mg2+ efflux. As the initial Δ[Mg2+]i/Δt values depend strongly on the initial [Mg2+]i levels [10], for precise comparison the estimated initial Δ[Mg2+]i/Δt values must be adjusted for variations in initial [Mg2+]i. For this purpose, we used the standard relationship between initial Δ[Mg2+]i/Δt and initial [Mg2+]i established previously (solid curve in Fig. 2 of Ref. [12]); all values of the initial Δ[Mg2+]i/Δt were normalized to the value on the standard curve at a given initial [Mg2+]i to calculate relative values of the initial Δ[Mg2+]i/Δt (relative Δ[Mg2+]i/Δt).
In some experiments, [Mg2+]i of the Mg2+-loaded cells was maintained at a high level by incubation of the cells in essentially Na+-free solution (NMDG-Tyrode’s solution). For NMDG Tyrode’s solution, NaCl of the Ca2+-free Tyrode’s solution was isosmotically replaced by NMDG-Cl (N-methyl-d-glucamine titrated with HCl to pH 7.40) to give final values of [Na+] = 0.33 mM. The pH of all superfusion solutions was adjusted to 7.40 at the same temperature as the fluorescence measurements.
Patch-clamp experiments
Whole-cell patch-clamp was conducted at 25 and 37°C. The patch electrodes were prepared from borosilicate glass capillaries and had a resistance of 1.7–2.2 MΩ when filled with a pipette solution (see below for composition). Series resistance (<10 MΩ) was compensated by 70% to minimize voltage errors. Current and voltage signals were recorded using an Axopatch 200B amplifier (Molecular Devices, Union City, CA, USA) coupled to a DigiData 1321A A/D and D/A converter (Molecular Devices). Current signals measured under the voltage-clamp mode were filtered at 1 kHz using a four-pole Bessel filter and were digitized at 10 kHz. Membrane potential measured under the current-clamp mode was low-pass filtered at 10 Hz and was digitized at 50 Hz. The pipette (intracellular) solution consisted of (in mM): 150 K-gluconate, 5.0 Na2ATP, 5.29 MgCl2, 0.1 K2EGTA, and 10 HEPES (pH 7.1 at 37°C, pH 7.27 at 25°C). All voltage data were corrected for a liquid junction potential of −16 mV found between the pipette solution and the Ca2+-free Tyrode’s solution.
Chemicals
Furaptra (tetra-potassium salt of mag-fura-2) and furaptra AM (mag-fura-2 AM) were purchased from Invitrogen (Carlsbad, CA, USA). Ethylisopropyl amiloride (EIPA) was obtained from Sigma–Aldrich (St. Louis, MO, USA). Imipramine hydrochloride, propranolol hydrochloride, and ouabain were from Nacalai Tesque (Kyoto, Japan). 2-[2-[4-(4-Nitrobenzyloxy)phenyl]ethyl]isothiourea (KB-R7943) mesylate was purchased from Tocris Bioscience (Bristol, UK; purity >99% according to the manufacturer). 2-[4-[(2,5-Difluorophenyl)methoxy]phenoxy]-5-ethoxyaniline (SEA0400) was a generous donation from Taisho Pharmaceutical (Tokyo, Japan). KB-R7943 and SEA0400 were dissolved in DMSO, and EIPA was dissolved in ethanol to make concentrated stock solutions. The final concentration of the solvent was ≤0.1% which did not affect the fluorescence and electrophysiological measurements.
We have previously reported that the direct effect of 200 µM imipramine on furaptra fluorescence is negligible [9]. To test the direct effects of KB-7943, SEA0400, and EIPA on furaptra fluorescence, 100 µM KB-R7943, 10 µM SEA0400, or 5 µM EIPA was dissolved in solutions that contained 150 mM KCl, 0–4 mM MgCl2, 0.1 mM EGTA, 50 µM furaptra, and 10 mM 3-morpholinopropanesulfonic acid (MOPS), pH adjusted to 7.2 by addition of KOH; furaptra R was measured in quartz capillaries (internal diameter ~50 µm) containing furaptra solutions of Mg2+ concentration 0, 0.5, 1, 2, or 4 mM. None of these compounds significantly affected furaptra R at any [Mg2+] tested.
Furapta binds Ni2+ and Gd3+ with affinities much higher than that for Mg2+, and undergoes large fluorescence changes. Permeation of these ions into the cell would therefore disturb [Mg2+]i measurements with furaptra. However, when furaptra R was monitored in myocytes bathed in Ca2+-free Tyrode’s solution, extracellular addition of neither 5 mM NiCl2 nor 200 µM GdCl3 significantly changed furaptra R for up to 30 min. This suggests that Ni2+ and Gd3+ enter the cell at very limited rates, and the interference caused by these ions reacting with furaptra is, if any, very minor within the time range of these experiments.
Data analysis
Linear and nonlinear least-squares fitting were performed with the software Origin (Ver. 8, OrginLab, Northampton, MA, USA). Statistical data are reported as mean ± SEM for the number of cells indicated. The statistical significance of a difference between means was evaluated with Student’s two-tailed t test.
Results
Effects of various transporter/channel inhibitors on the rate of Mg2+ efflux at 25°C
We studied the effects of known inhibitors of Na+/Ca2+ exchange (KB-R7943, SEA0400, and Ni2+) on Na+-dependent Mg2+ efflux and compared their effects with that of imipramine, a widely used inhibitor of Na+/Mg2+ exchange [7–9]. For this purpose, one of the drugs was added to the Mg-loading solution for the final 6 min of Mg2+ loading, and Mg2+ efflux was induced by superfusion with Ca2+-free Tyrode’s solution in the continuous presence of the drug.
KB-R7943 at 30 µM and higher concentrations strongly inhibited the rate of Mg2+ efflux (Fig. 2B, C). We also unexpectedly found during the fluorescence measurement that 100 µM KB-R7943 caused an abrupt rise of [Mg2+]i to high levels (2–3 mM) that was followed by shortening of cell length (Fig. 2C). Because the cell had been depleted of Ca2+ and the extracellular Ca2+ concentration was low with 0.1 mM EGTA, cell shortening was likely to be because of impaired cellular metabolism and the formation of rigor crossbridges [6], rather than Ca2+ overload. The cells out of the optical field showed little or no sign of shortening, suggesting that the combination of KB-R7943 (at high concentrations) and UV-illumination caused toxicity (i.e., phototoxicity). SEA0400, even at 10 µM, the highest concentration of this compound, resulted in little inhibition of the rate of Na+/Mg2+ exchange (Fig. 2D). Higher concentrations of SEA0400 could not be dissolved in the Tyrode’s solution with 0.1% DMSO, and were not used. Ni2+ (5 mM) significantly inhibited Na+/Mg2+ exchange (Fig. 2F). As a reference, we confirmed that 200 µM imipramine strongly inhibited Na+/Mg2+ exchange (Fig. 2E).
Fig. 2.

Records from six separate experiments in which Mg2+ efflux was induced by addition of 140 mM Na+, at the times indicated by the arrows, in the absence of the inhibitor (A) or in the presence of 30 µM KB-R7093 (B), 100 µM KB-R7943 (C), 10 µM SEA0400 (D), 200 µM imipramine (E), or 5 mM Ni2+ (F) at 25°C. In this figure and Fig. 5, [Mg2+]i traces have been smoothed with adjacent averaging of 51 data points (10 s) to reduce noise of the graphic display. In each panel, a solid line was drawn by the least-squares fit to unsmoothed data points; the initial Δ[Mg2+]i/Δt estimated from the slope is indicated (µM/s) near the trace
We also tested inhibitors of other transporters/channels. For screening, we set concentrations of the inhibitors to be high enough to exert nearly full inhibition of their target transporters/channels: 200 µM gadolinium (an inhibitor of stretch-activated channels and some trp channels) [14, 15], 5 µM EIPA (an inhibitor of the Na+/H+ exchanger) [16], 1 mM ouabain (an inhibitor of the Na+/K+ pump) [17], and 100 µM propranolol (an antiarrhythmic drug with β-adrenoceptor blockade and general membrane stabilizing actions) [18]. None of these drugs had a significant effect on the Na+/Mg2+ exchange (Fig. 3). [Note: Higher concentrations of EIPA were not tested, because we found that the compound had significant absorbance at wavelengths <400 nm (the molar extinction coefficient ~2.2 × 104 M −1 cm−1 at 380 nm), which might interfere with measurements of furaptra fluorescence with excitation at 350 and 382 nm (see above).]
Fig. 3.
Effects of various inhibitors on relative Δ[Mg2+]i/Δt. Initial Δ[Mg2+]i/Δt was estimated at 25°C in the absence of the inhibitor (control, the leftmost column) or in the presence of (from left to right) 100 µM KB-R7943 (KB-R), 10 µM SEA0400 (SEA), 5 mM NiCl2 (Ni 2+), 200 µM GdCl3 (Gd 3+), 200 µM imipramine (Imip), 5 µM EIPA, 1 mM ouabain (Oua), or 100 µM propranolol (Prop). All values of initial Δ[Mg2+]i/Δt were normalized to those expected for the initial [Mg2+]i to calculate relative Δ[Mg2+]i/Δt. Columns show mean ± SEM from 3–8 cells. Data for ouabain were taken from Fig. 7 of Ref. [4]. **P < 0.01 versus control (the left-most column)
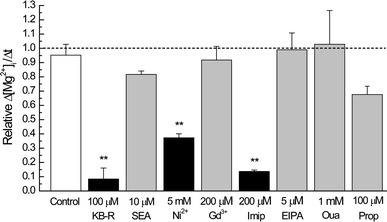
To further study the kinetics of the inhibitory effect of KB-R7943, the drug (30 µM) was applied in Ca2+-free Tyrode’s solution that induced Mg2+ efflux without preincubation (Fig. 4A). The rates of decrease in [Mg2+]i (Δ[Mg2+]i/Δt) were estimated by linear regression of data points spanning 60 s with 60-s intervals after simultaneous addition of extracellular Na+ and KB-R7943. Figure 4B shows that relative Δ[Mg2+]i/Δt values quickly decreased to a steady state level with a time constant of about 1 min, indicating rapid onset of the inhibitory effect.
Fig. 4.
A An example of [Mg2+]i measurements in which Mg2+ efflux was induced by 140 mM extracellular Na+ with simultaneous application of 30 µM KB-R7943 at the time indicated by the arrow (25°C). B The onset of the inhibitory effect of KB-R7943 obtained from the experiments of the type shown in A. KB-R7943 (30 µM) was applied simultaneously with 140 mM Na+ to the Mg2+-loaded cells at time zero on the abscissa, and the values of relative Δ[Mg2+]i/Δt were plotted as a function of time after application of the drug. The first point at 60 s was obtained by linear regression of [Mg2+]i data between 30 and 90 s after application of KB-R7943, and the following points were similarly obtained with 60-s intervals. Each symbol represents a mean value from 3 cells. A solid line was drawn by nonlinear least-squares fitting with an exponential (plus constant) function of the form and values as indicated in the graph
Effects of KB-R7943 at 35°C
After Mg2+ loading at room temperature (~25°C), the temperature of the superfusion solution was set at 35°C, and the fluorescence measurement runs were carried out at this temperature (Fig. 5). In the absence of any inhibitor, the initial Δ[Mg2+]i/Δt was, on average, −1.94 ± 0.236 µM/s (n = 6) (Fig. 5A, E). This average value is 67% greater than that obtained at 25°C (1.16 ± 0.049 µM/s, n = 8) at comparable initial [Mg2+]i (1.51 ± 0.083 mM at 25°C and 1.50 ± 0.060 at 35°C). The relative Δ[Mg2+]i/Δt calculated by normalization of the initial Δ[Mg2+]i/Δt to the value on the standard curve (see above) was 1.56 ± 0.139 at 35°C and 0.95 ± 0.076 at 25°C, giving a Q 10 value of 1.64.
Fig. 5.

A–D Measurements of initial Δ[Mg2+]i/Δt in four separate experiments at 35°C, in which Mg2+ efflux was induced by 140 mM extracellular Na+ (Ca2+-free Tyrode’s solution) at the times shown by the arrows. Estimated values of the initial Δ[Mg2+]i/Δt (the slopes of the solid lines) are indicated near the traces. The cell was continuously illuminated by UV light throughout the run (~20 min) in the absence (A) and in the presence (B) of 20 µM KB-R7943. C The cell was intermittently illuminated, except for a 3-min period for the initial Δ[Mg2+]i/Δt measurement with 20 µM KB-R7943 present throughout the run. D The Mg2+-loaded cell was treated with 20 μM KB-R7943 for 6 min in the Mg-loading solution (35°C), and then KB-R7943 was washed out by superfusion with NMDG Tyrode’s solution for 7 min before UV-illumination started for the initial Δ[Mg2+]i/Δt measurement. EColumns a–e summarize relative Δ[Mg2+]i/Δt estimated at 35°C with or without continuous UV-illumination (Cont. illum. − or +, respectively) and with various combinations of extracellular Ca2+ and KB-R7943 concentrations, as shown below each column. Columns a, b, c and e show results obtained from the experiments of the type shown in A, B, C and D, respectively. Columns represent mean ± SEM from 3–8 cells. **P<0.01 versus control (column a)
Similar to the findings at 25°C, KB-R7943 strongly inhibited Na+/Mg2+ exchange at 35°C (Fig. 5A, B); Fig. 5B clearly shows that 20 µM KB-R7943 slows Mg2+ efflux. Also shown in Fig. 5B is that [Mg2+]i rapidly rises to 2.4 mM with a late onset. In all three cells that were continuously UV-illuminated in the presence of 20 µM KB-R7943, the rise of [Mg2+]i was observed and visual observation confirmed shortening of the cell length at the end of the fluorescence measurement runs. When UV-illumination was minimized, on the other hand, neither the [Mg2+]i rise nor cell shortening was observed in the three cells (Fig. 5C), suggesting that the phototoxic effect was induced by lower concentrations of KB-R7943 at higher temperatures (20 µM at 35°C vs. 100 µM at 25°C). None of the 4 cells treated with 10 µM KB-R7943 at 35°C showed any sign of the phototoxic effect even with continuous UV-illumination (not shown). Importantly, even with little sign of the phototoxic effect, Mg2+ efflux was significantly slowed by KB-R7943 (Fig. 5C); in the presence of 20 µM KB-R7943, the rates measured with or without continuous illumination were not significantly different (Fig. 5E, columns b and c). We also examined the effect of extracellular Ca2+ on the inhibitory effect of KB-R7943, and found that addition of 1 mM Ca2+ did not significantly change the Mg2+ efflux rate at 20 µM KB-R7943 (Fig. 5E, column d). The results indicate that, although most experiments were carried out under Ca2+-free conditions in this study, KB-R7943 is effective at physiological levels of extracellular (and presumably intracellular) Ca2+.
Finally, we assessed the reversibility of the effect of KB-R7943. The Mg2+-loaded cell was treated with 20 µM KB-R7943 for 6 min in the Mg-loading solution (35°C), and then KB-R7943 was washed out by superfusion with NMDG Tyrode’s solution for 7 min before the run was started for the initial Δ[Mg2+]i/Δt measurement with continuous UV-illumination (Fig. 5D). The initial Δ[Mg2+]i/Δt values measured in cells previously treated with 20 µM KB-R7943 were not significantly different from those of untreated cells (Fig. 5E, columns e and a). Thus, the inhibitory effect of 20 µM KB-R7943 seems to be reversed by washout of the compound.
Effects of KB-R7943 on membrane potential
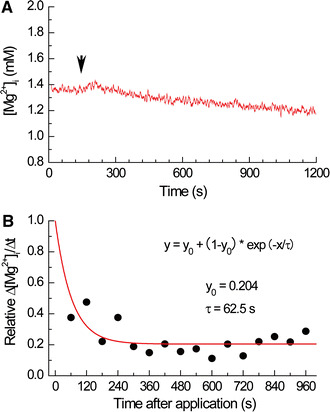
Because it has been shown that large membrane depolarization significantly affects Na+-dependent Mg2+ efflux [3, 19], a part of the inhibitory effect of KB-R7943 described above may be caused by a change in membrane potential, i.e., hyperpolarization, rather than the drug’s direct effect on the transport. To examine this possibility, the myocytes were patch-clamped, and the membrane potential was measured under the current-clamp mode (Fig. 6). We found that the cell membrane was depolarized after application of 20 µM KB-R7943, and washout of the drug was followed by nearly full reversal of the effect (Fig. 6A, upper). The mean depolarization was 22.5 mV at 37°C (Fig. 6B) and 26.1 mV at 25°C (6 cells, not shown). This depolarization was likely to be because of inhibition of the inward rectifier K+ current reported earlier [20, 21]. We also confirmed that the inwardly rectifying current was strongly and reversibly inhibited by 20 µM KB-R7943 (Fig. 6Aa–Ac and C).
Fig. 6.
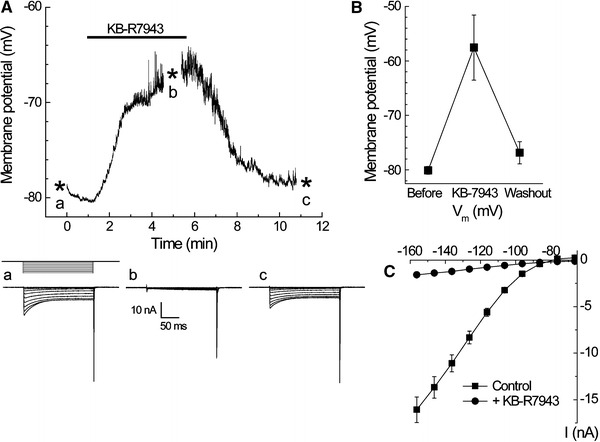
Effects of KB-R7943 on membrane potential and membrane currents at 37°C. A Upper a typical trace of membrane potential measured in the current-clamp mode. The myocyte was perfused with Ca-free Tyrode’s solution, and 20 μM KB-R7943 was added to the perfusate as shown above by a horizontal bar. At times indicated by asterisks (a, b and c), the myocyte was voltage-clamped to measure membrane currents. Lower traces in a, b and c show whole-cell currents recorded under voltage-clamp at asterisks marked a, b and c, respectively, in the upper panel. The holding potential was −66 mV (after correction of the junction potential, see text) and 200-ms hyperpolarizing pulses were applied in 10-mV increments as shown at the top of a. B Summary of membrane potential recorded in experiments of the type shown in A. Membrane potential was averaged over 30 s at the beginning of the current-clamp measurement (Before), just before the voltage-clamp run in the presence of KB-R7943 (KB-R7943) and at the steady level after washout of KB-R7943 (Washout). C Current–voltage relationships of whole-cell currents before (squares) and during (circles) application of KB-R7943. Current amplitudes at 61 ms after the onset of the hyperpolarizing pulses were plotted as a function of membrane potential. Note inwardly going rectification of the current. In B and C, each symbol represents mean ± SEM values from 7 cells
Concentration–response relationships
Figure 7 summarizes the effects of 4 compounds on Na+/Mg2+ exchange at 25 and 35°C. KB-R7943 inhibited Na+/Mg2+ exchange in a concentration-dependent manner with IC50 values of 21 µM at 25°C and 16 µM at 35°C (Fig. 7A), whereas Ni2+ only weakly inhibited Na+/Mg2+ exchange with IC50 values of 3.0 mM at 25°C and 1.7 mM at 35°C with shallow slopes of the concentration–response relationships (Fig. 7B). SEA0400 in the concentration range of 1–10 µM caused no significant inhibition (Fig. 7C). Imipramine was used as a reference inhibitor of Na+/Mg2+ exchange. IC50 values for imipramine were 59 µM at 25°C and 67 µM at 35°C (Fig. 7D); these values were similar to, but slightly lower than, the value obtained previously at 25°C in ionomycin-treated cardiac myocytes, 79 µM [9].
Fig. 7.
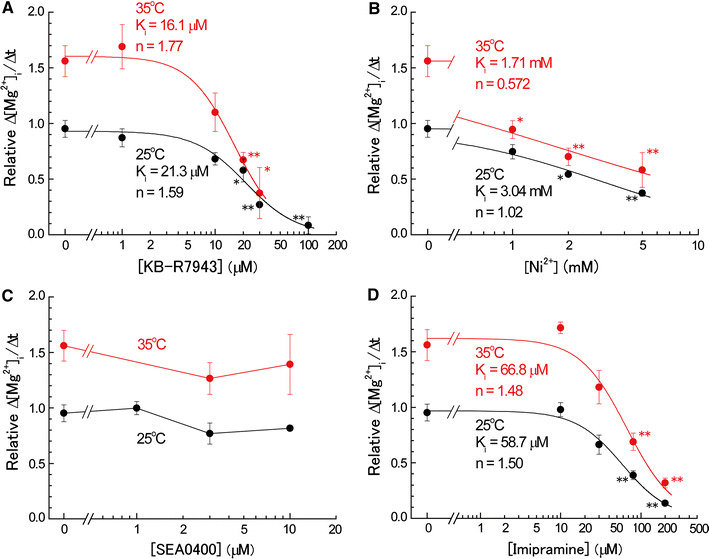
Concentration–response curves for KB-R7943 (A), Ni2+ (B), SEA0400 (C) and imipramine (D) at 25°C (black) or 35°C (red). In A–D, each symbol represents mean ± SEM from 3–8 cells. At 20 µM KB-R7943 and 35°C in A, the initial Δ[Mg2+]i/Δt values were similar in the three cells that were continuously illuminated by UV light throughout the run (column b of Fig. 5E) and the other three cells that were intermittently illuminated (column c of Fig. 5E). We therefore treated data from all 6 cells as a single group for the statistical analysis. Similarly, at 30 µM KB-R7943 and 35°C in A, we calculated statistical values from three cells; two with intermittent illumination and the other with continuous illumination. In A, B, and D the solid lines indicate the least-squares fit of the data set by the Hill-type curve with the values shown in the panel: 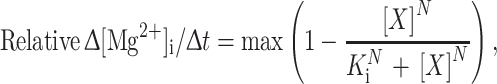 where max is relative Δ[Mg2+]i/Δt in the absence of the inhibitor X, N is the Hill coefficient, and K
i is IC50. *0.01 ≤ P < 0.05; **P < 0.01 versus relative Δ[Mg2+]i/Δt in the absence of the inhibitor
where max is relative Δ[Mg2+]i/Δt in the absence of the inhibitor X, N is the Hill coefficient, and K
i is IC50. *0.01 ≤ P < 0.05; **P < 0.01 versus relative Δ[Mg2+]i/Δt in the absence of the inhibitor
Discussion
Inhibitors of Na+/Mg2+ exchange have been most extensively studied in red cells. Féray and Garay [7, 22] reported that imipramine and quinidine inhibited Na+/Mg2+ exchange in human red cells with IC50 values of, respectively, 25 and 50 µM. In ferret red cells, on the other hand, higher concentrations of imipramine or quinidine (IC50 of 200–600 µM) seemed to be required for substantial inhibition of Na+-dependent Mg2+ efflux [8]. Na+/Mg2+ exchange was also inhibited by high concentrations of amiloride (IC50 ~600 µM) in chicken red blood cells [23].
KB-R7943 has been widely used as a tool to study Na+/Ca2+ exchanger. Initial reports suggested that the compound inhibits the reverse mode of the Na+/Ca2+ exchange current (i.e., Ca2+ influx) much more effectively than the forward mode (i.e., Ca2+ efflux) [20, 24]. Apparent IC50 values for the reverse (Ca2+ influx) mode varied between 0.3 µM [20] and 1.6–2.4 µM [24], whereas the corresponding values for the forward (Ca2+ efflux) mode were 17 µM [20] or even ≥30 µM [24]. However, later experiments with cardiac myocytes under conditions allowing bidirectional currents indicated direction-independent inhibition of the Na+/Ca2+ exchange current by KB-R7943 with an IC50 of ≤1 µM [25]. It has been reported that KB-R7943, at high concentrations, also blocks other transporters, ion channels, and receptors [20, 21].
Matsuda et al. [26] introduced SEA0400, a more potent and selective inhibitor of the Na+/Ca2+ exchanger. It has been shown, under bidirectional conditions, that SEA0400 inhibits the Na+/Ca2+ exchange current in both directions (direction-independent block) with IC50 of <100 nM [21]. The Na+/Ca2+ exchange current is also blocked by heavy metal cations, for example La3+, Cd2+, Mn2+, and Ni2+ [27]. Ni2+ inhibits Na+/Ca2+ exchange with IC50 ~200 µM [28], and the Na+/Ca2+ exchange current is nearly completely abolished by 5 mM Ni2+ [27, 29].
Among these inhibitors of Na+/Ca2+ exchange (KB-R7943, SEA0400, and Ni2+) and other transporters/channels tested in this study, we found that KB-R7943 strongly inhibited Na+/Mg2+ exchange at both 25 and 35°C, with IC50 values a factor of three to four lower than those of imipramine, one of the most potent inhibitors of Na+/Mg2+ exchange known. The question whether the compound affects K m ([Mg2+]i for half maximal activation) or V max (the transport rate at infinitely high [Mg2+]i) or both of the transports requires further study.
Many antiarrhythmic drugs that have membrane-stabilizing action inhibit multiple ion channels and transporters, and may also inhibit Na+/Mg2+ exchange. However, we have previously reported that verapamil (30 µM) had little effect on Na+/Mg2+ exchange [3], and in this study, propranolol (100 µM) did not significantly inhibit relative Δ[Mg2+]i/Δt (Fig. 3). It is thus unlikely that antiarrhythmic drugs, in general, inhibit Na+/Mg2+ exchange as a result of their membrane-stabilizing action. It should be noted, however, that propranolol may affect Mg2+ efflux in vivo via blockade of β-adrenoceptors, because it has been demonstrated that β-adrenergic stimulation causes a significant increase in Mg2+ efflux from isolated hearts and ventricular myocytes [30, 31].
The inhibitory effect of KB-R7943 on Mg2+ efflux had rapid onset (with a time constant of approximately 1 min, Fig. 4) and was reversible upon washout, as reported for Na+/Ca2+ exchange transport [24, 25, 32]. For inhibition of the Na+/Ca2+ exchange, KB-R7943 preferentially acts from the external surface of the cell membrane [20, 32]. Because the inhibitory effect of KB-R7943 seems to develop with similar time courses for Na+/Ca2+ exchange and Na+/Mg2+ exchange, the side-dependence of the drug effect may be also observed for Na+/Mg2+ exchange and should be determined in future studies.
We also found that 20 µM KB-R7943 caused depolarization of, on average, 23 mV (37°C) and 26 mV (25°C) within a few minutes, probably because of inhibition of the inward rectifier K+ current (Fig. 6). In our previous study at 25°C [3], the rate of Na+-dependent Mg2+ efflux was significantly accelerated by membrane depolarization from −80 to −0 mV, although the effect was not significant at −40 mV. Almulla et al. [19] also found significant facilitation of Mg2+ efflux by depolarization from −80 to 0 mV at 37°C. This suggests that membrane depolarization of about 20–30 mV caused by KB-R7943 may, if it has any effect, accelerate Mg2+ efflux somewhat, which consequently may mask the drug’s inhibitory effect on the transport; the inhibitory effect of KB-R7943 found in this study should be even more potent under conditions in which the membrane potential is maintained constant. Thus, IC50 values might be slightly overestimated in this study, and should be regarded as upper limits.
Because of nonspecific effects of KB-R7943 (see above), inhibition of Na+/Mg2+ exchange by KB-R7943 does not necessarily mean that Na+/Ca2+ exchange and Na+/Mg2+ exchange share the same transporter molecules. Rather, evidence against a significant contribution of the Na+/Ca2+ exchanger (NCX1) to Mg2+ efflux has been reported in rat ventricular myocytes; Na+-dependent Mg2+ efflux was unaffected even under conditions in which the intracellular activator Ca2+ required for activity of the Na+/Ca2+ exchanger was strongly buffered by millimolar concentrations of BAPTA [5]. Also, the results of our current study did not support, even indirectly, involvement of the Na+/Ca2+ exchanger in Mg2+ transport. First, the Q10 value of 1.6 found for Na+/Mg2+ exchange was much lower than the ~4 reported for the Na+/Ca2+ exchange current [27]. Second, Ni2+ only weakly inhibited Na+/Mg2+ exchange, with IC50 values ~10 times higher than those for the Na+/Ca2+ exchange current at both 25 and 35°C.
The results of this study are apparently in contrast with those reported by Almulla et al. [19], who found little effect of 20 µM KB-R7943 on the time constants of the Na+-dependent fall in [Mg2+]i in Mg2+-loaded rat ventricular myocytes at 37°C. It should be noted, however, that Almulla et al. [19] loaded the cells with Mg2+ to reach, on average, 4.8 mM [Mg2+]i which was much higher than the level achieved in this study (~1.5 mM). The apparent discrepancy could be explained, if KB-R7943 inhibits Na+/Mg2+ exchange by increasing K m with little change in V max; with [Mg2+]i levels much higher than K m of the transport, the transport rate approaches V max, and the small shift of K m by KB-R7943 could be masked. Although the factor that causes the discrepancy is still unclear, the results of this study clearly indicate that KB-R7943 reduces the rate of Na+-dependent Mg2+ efflux at [Mg2+]i in a concentration-dependent manner at near physiological levels.
Thus, KB-R7943 may be useful as a tool to investigate Na+/Mg2+ exchange under experimental conditions in which the effects of KB-R7943 on other membrane transporters are considered insignificant for cellular Mg2+ homeostasis. Sensitivity to KB-R7943 could be used as one of the “signatures” of the Na+/Mg2+ exchanger to identify still unknown molecules responsible for the transport. For use of KB-R7943 as an inhibitor of Na+/Ca2+ exchange, on the other hand, it should be noted that inhibition of Mg2+ efflux by high concentrations of KB-R7943 may change [Mg2+]i and thereby may influence Na+/Ca2+ exchange activity. Although the molecular mechanism for the phototoxic effect of KB-R7943 is not fully understood, UV-illumination should be minimized when this compound is used with fluorescence measurements for studies of either Na+/Ca2+ exchange or Na+/Mg2+ exchange transport.
Acknowledgments
The authors thank Taisho Pharmaceutical Co. for kindly providing a sample of SEA0400. We also thank Shinobu Tai for technical assistance and Mary Shibuya for reading the manuscript. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 20590219).
References
- 1.Flatman PW. Mechanisms of magnesium transport. Ann Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- 2.Handy RD, Gow IF, Ellis D, Flatman PW. Na-dependent regulation of intracellular free magnesium concentration in isolated rat ventricular myocytes. J Mol Cell Cardiol. 1996;28:1641–1651. doi: 10.1006/jmcc.1996.0154. [DOI] [PubMed] [Google Scholar]
- 3.Tashiro M, Tursun P, Miyazaki T, Watanabe M, Konishi M. Effects of membrane potential on Na+-dependent Mg2+ extrusion from rat ventricular myocytes. Jpn J Physiol. 2002;52:541–551. doi: 10.2170/jjphysiol.52.541. [DOI] [PubMed] [Google Scholar]
- 4.Tashiro M, Tursun P, Konishi M. Intracellular and extracellular concentrations of Na+ modulate Mg2+ transport in rat ventricular myocytes. Biophys J. 2005;89:3235–3247. doi: 10.1529/biophysj.105.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tashiro M, Tursun P, Miyazaki T, Watanabe M, Konishi M. Effects of intracellular and extracellular concentrations of Ca2+, K+, and Cl− on the Na+-dependent Mg2+ efflux in rat ventricular myocytes. Biophys J. 2006;91:244–254. doi: 10.1529/biophysj.106.082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashiro M, Inoue H, Konishi M. Metabolic inhibition strongly inhibits Na+-dependent Mg2+ efflux in rat ventricular myocytes. Biophys J. 2009;96:4941–4950. doi: 10.1016/j.bpj.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Féray JC, Garay R. Demonstration of a Na+:Mg2+ exchange in human red cells by its sensitivity to tricyclic antidepressant drugs. Naunyn Schmiederberg’s Arch Pharmacol. 1988;338:332–337. doi: 10.1007/BF00173409. [DOI] [PubMed] [Google Scholar]
- 8.Flatman PW, Smith LM. Magnesium transport in ferret red cells. J Physiol (Lond) 1990;431:11–25. doi: 10.1113/jphysiol.1990.sp018318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tashiro M, Konishi M. Sodium gradient-dependent transport of magnesium in rat ventricular myocytes. Am J Physiol. 2000;279:C1955–C1962. doi: 10.1152/ajpcell.2000.279.6.C1955. [DOI] [PubMed] [Google Scholar]
- 10.Tashiro M, Konishi M, Iwamoto T, Shigekawa M, Kurihara S. Transport of magnesium by two isoforms of the Na+–Ca2+ exchanger expressed in CCL39 fibroblasts. Pflügers Arch. 2000;440:819–827. doi: 10.1007/s004240000384. [DOI] [PubMed] [Google Scholar]
- 11.Tashiro M, Inoue H, Tai S, Konishi M. Effects of Na+/Ca2+ exchange inhibitors on the Na+-dependent Mg2+ efflux activity. J Physiol Sci. 2010;60:S113. doi: 10.1007/s12576-010-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tursun P, Tashiro M, Konishi M. Modulation of Mg2+ efflux from rat ventricular myocytes studied with the fluorescent indicator furaptra. Biophys J. 2005;88:1911–1924. doi: 10.1529/biophysj.104.055517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe M, Konishi M. Intracellular calibration of the fluorescent Mg2+ indicator furaptra in rat ventricular myocytes. Pflügers Arch. 2001;442:35–40. doi: 10.1007/s004240000499. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YH, Youm JB, Sung HK, Lee SH, Ryu SY, Lee S-H, Ho W-K, Earm YE. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J Physiol (Lond) 2000;523:607–619. doi: 10.1111/j.1469-7793.2000.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwanyanya A, Amuzescu B, Zakharov SI, Macianskiene R, Sipido KR, Bolotina VM, Vereecke J, Mubagwa K. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation. J Physiol (Lond) 2004;559:761–776. doi: 10.1113/jphysiol.2004.067637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusakari Y, Hongo K, Kawai M, Konishi M, Kurihara S. The mechanism of increasing Ca2+ responsiveness by α1-adrenoceptor stimulation in rat ventricular myocytes. Jpn J Physiol. 2002;52:531–539. doi: 10.2170/jjphysiol.52.531. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka N, Fielding AJ, Berlin JR. Na pump current can be separated into ouabain-sensitive and -insensitive components in single rat ventricular myocytes. Jpn J Physiol. 1996;46:215–223. doi: 10.2170/jjphysiol.46.215. [DOI] [PubMed] [Google Scholar]
- 18.Ha TNV, Fryer MW. Inhibitory effects of (±)-propranolol on excitation-contraction coupling in isolated soleus of the rat. Br J Pharmacol. 1997;122:463–468. doi: 10.1038/sj.bjp.0701405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almulla HA, Bush PG, Steele MG, Flatman PW, Ellis D. Sodium-dependent recovery of ionized magnesium concentration following magnesium load in rat heart myocytes. Pflügers Arch. 2006;451:657–667. doi: 10.1007/s00424-005-1501-8. [DOI] [PubMed] [Google Scholar]
- 20.Watano T, Kimura J, Morita T, Nakanishi H. A novel antagonist, No. 7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac cells. Br J Pharmacol. 1996;119:555–563. doi: 10.1111/j.1476-5381.1996.tb15708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka H, Nishimaru K, Aikawa T, Hirayama W, Tanaka Y, Shigenobu K. Effect of SEA0400, a novel inhibitor of sodium-calcium exchanger, on myocardial ionic currents. Br J Pharmacol. 2002;135:1096–1100. doi: 10.1038/sj.bjp.0704574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Féray JC, Garay R. An Na+-stimulated Mg2+ transport system in human red blood cells. Biochim Biophys Acta. 1986;856:76–84. doi: 10.1016/0005-2736(86)90012-X. [DOI] [PubMed] [Google Scholar]
- 23.Günther T, Vormann J. Mg2+ efflux is accomplished by an amiloride-sensitive Na+/Mg2+ transport. Biochim Biophys Res Comm. 1985;130:540–545. doi: 10.1016/0006-291X(85)90450-4. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- 25.Kimura J, Watano T, Kawahara M, Sakai E, Yatabe J. Direction-independent block of bi-directional Na+/Ca2+ exchange current by KB-R7943 in guinea-pig cardiac myocytes. Br J Pharmacol. 1999;128:969–974. doi: 10.1038/sj.bjp.0702869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hanano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A. SEA0400, a novel and selective inhibitor of the Na+–Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- 27.Kimura J, Miyamae S, Noma A. Identification of sodium–calcium exchange current in single ventricular cells of guinea-pig. J Physiol (Lond) 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bers DM. Excitation–contraction coupling and cardiac contractile force. 2. Dordrecht: Kluwer; 2001. [Google Scholar]
- 29.Hobai IA, Bates JA, Howarth FC, Levi AJ. Inhibition by external Cd2+ of Na/Ca exchange and L-type Ca channel in rabbit ventricular myocytes. Am J Physiol. 1997;272:H2164–H2172. doi: 10.1152/ajpheart.1997.272.5.H2164. [DOI] [PubMed] [Google Scholar]
- 30.Romani A, Scarpa A. Hormonal control of Mg2+ transport in the heart. Nature. 1990;346:841–844. doi: 10.1038/346841a0. [DOI] [PubMed] [Google Scholar]
- 31.Romani A, Marfella C, Scarpa A. Regulation of magnesium uptake and release in the heart and in isolated ventricular myocytes. Circ Res. 1993;72:1139–1148. doi: 10.1161/01.res.72.6.1139. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto T, Kita S, Uehara A, Inoue Y, Taniguchi Y, Imanaga I, Shigekawa M. Structural domains influencing sensitivity to isothiourea derivative inhibitor KB-R7943 in cardiac Na+/Ca2+ exchanger. Mol Pharmacol. 2001;59:524–531. doi: 10.1124/mol.59.3.524. [DOI] [PubMed] [Google Scholar]


