Abstract
This article reviews 40 years of research (1970–2010) into the capability of the efferent sympathetic nervous system to display differential responsiveness. Discovered first were antagonistic changes of activity in sympathetic filaments innervating functionally different sections of the cardiovascular system in response to thermal stimulation. During the subsequent four decades of investigation, a multitude of differential sympathetic efferent response patterns were identified, ranging from opposing activity changes at the level of multi-fiber filaments innervating different organs to the level of single fibers controlling functionally different structures in the same organ. Differential sympathetic responsiveness was shown to be displayed in response to exogenous or artificial stimulation of afferent sensory fibers transmitting particular exogenous stimuli, especially those activating peripheral nociceptors. Moreover, sympathetic differentiation was found to be characteristic of autonomic responses to environmental changes by which homeostasis in the broadest sense would be challenged. Heat or cold loads or their experimental equivalents, altered composition of inspired air or changes in blood gas composition, imbalances of body fluid control, and exposure to agents challenging the immune system were shown to elicit differential efferent sympathetic response patterns which often displayed a high degree of specificity. In summary, autonomic adjustments to changes of biometeorological parameters may be considered as representative of the capability of the sympathetic nervous system to exert highly specific efferent control of organ functions by which bodily homeostasis is maintained.
Keywords: Sympathetic nervous system, Sympathetic differential response, Homeostatic control, Autonomic reflex, Environmental physiology, Cardiovascular physiology
Introduction
Sympathetic innervation represents the most widespread section by which autonomic nervous control of bodily functions is accomplished. Its generalized activation as a response to stress was early on recognized as an essential function of sympathetic innervation [6], causing more or less diffuse transmitter release from its postganglionic nerve endings in the periphery, as well as activation of adreno-medullary hormone release. Especially for sympathetic innervation of the cardiovascular system, its tendency to respond en masse was long considered as its predominant mode of action. Its potential to produce more complex response patterns was, for a long time, not in the focus of neurophysiological research, although it had been increasingly taken into account as a possibility by circulation physiologists. Analysis of a variety of reflex circulatory responses suggested differential reflex changes of vasomotor tone in diverse parts of the peripheral vascular bed ([43], for references). Different fractions of baroreceptor-dependent and -independent sympathetic efferents supplying various sections of the vascular system were disclosed as the neurophysiological correlate of quantitative non-uniformity of the baroreflex actions on regional vasomotor activity [60].
Circulation physiologists early on proposed higher degrees of differential cardiovascular sympathetic innervation to explain more complex modes of cardiovascular adjustments. It was concluded as a general view from indirect evidence that “many similarities with the organization of the complex control of somatomotor activity will be revealed by future work; this implies that the old concept that the sympathetic nervous system was largely diffuse in its activity, is far from correct” [9]. For cardiovascular adjustments observed in temperature regulation, it had been stated earlier that “the thermoregulatory responses of the skin vessels are not consistent with the idea of a widespread, diffuse action of the sympathetic system” [2]. Indeed, its capability to display qualitative non-uniformity or “qualitative differentiation”, i.e., opposing activity changes in different efferent fibers, was discovered first when thermoregulatory cardiovascular adjustments were analyzed [45, 81], and regional qualitative sympathetic differentiation was shown to be the autonomic contribution to a genuinely homeostatic response. The strategy to investigate autonomic nervous responses in conditions when bodily homeostasis was challenged has subsequently disclosed a variety of differential sympathetic response patterns.
Due to progress in electrophysiological methodology, neurophysiological investigation of stimulus–response relationships has become the predominant experimental approach to elucidate patterns of sympathetic efferent non-homogeneity. Analysis of autonomic reflex response components and of evoked responses to local central nervous, electrical, or pharmacological stimulations have convincingly confirmed that qualitative differentiation of sympathetic efferent fiber activities is an essential property of the autonomic nervous outflow. In target-specific sympathetic efferent control, differential response patterns are demonstrable for the entire range, extending from sympathetic innervation of functionally different sections of the cardiovascular system to the level of single postganglionic fibers controlling different end organs, and, moreover, may involve different neuro-effector transmitters [34–37, 54]. In addition to electrophysiological analysis of sympathetic nervous outflow, neurochemical labeling and viral retrograde tracing have been increasingly employed to elucidate the degree to which tissue specificity of postganglionic innervation reflects specificity of sympathetic preganglionic neurons and of their control by cerebrospinal pre-motor autonomic neurons ([54], for references).
Challenges to homeostasis: seminal experiments
Thermal homeostasis
Simultaneous recordings from two multi-fiber preparations innervating a cutaneous and a visceral vascular bed revealed regionally antagonistic changes of activity, when deep-body thermosensors in the spinal cord were adequately stimulated in cats (Fig. 1) and rabbits [81]. These observations were in line with results obtained by regional blood flow measurements in dogs subjected to the same deep-body thermosensor stimulation [45]. Subsequent studies investigated regional responses to stimulation of deep-body thermosensors in the hypothalamus as well as responses to stimulation of peripheral skin thermosensors in parallel neurophysiological and hemodynamic studies. The results were basically identical with those obtained by spinal cord cooling and heating. In studies in which cardiac sympathetic activity was recorded, it was found to parallel the changes of visceral sympathetic innervation (for references see: [29, 30, 76]).
Fig. 1.
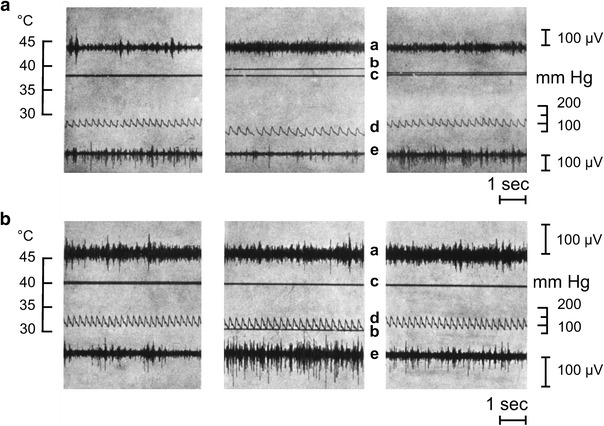
Original recordings from two multi-fiber preparations in a cat. Trace a sympathetic nerve filament innervating the spleen (visceral); trace e postganglionic filament emerging from the superior cervical ganglion and supplying the skin at the lateral head and ear. Line c deep-body temperature; line b temperature in the peridural space of the vertebral canal. Temperature within the vertebral canal was changed by means of a water-perfused tube inserted into the peridural space to warm (a) or cool (b) the spinal cord. Trace d arterial pressure. Middle sections recordings during spinal cord warming and cooling. Left-hand sections recordings prior to thermal stimulation. Right-hand sections post-stimulation recordings. The cat was anesthetized and artificially ventilated because neuromuscular transmission had been blocked in order to suppress cold shivering in response to spinal cord cooling. Warming: splenic nerve activity is enhanced and cutaneous sympathetic activity is inhibited. Cooling: splenic nerve activity is slightly but visibly reduced and cutaneous nerve activity is distinctly enhanced. From Walther et al. [81]
There is evidence that the feedback loops controlling thermoregulatory effectors are segmentally organized, with the hypothalamus as the highest level of coordination [72]. The integrative function of the hypothalamus in temperature regulation may be conceptually separated from its function as a deep-body temperature sensor [73], of which the latter function is quantitatively predominant in mammals, but not in birds [75]. Feedback loops that are subordinate to those of the hypothalamus exist along the central nervous axis. The same applies to deep-body thermosensors along the central nervous axis and to the segmentally organized thermosensory input from the skin. As a result, even the spinal cord alone is capable of generating thermoregulatory effector activities such as shivering [44] and of reducing and enhancing, respectively, skin blood flow in response to spinal cord cooling and warming [83]. Figure 2 shows that specificity of the cutaneous vasomotor response in chronically spinalized rabbits was confirmed by the observation that the drop of ear skin temperature, indicative of enhanced vasoconstrictor tone in the skin in response to spinal cord cooling, was accompanied by reduced activity of splanchnic sympathetic efferents [82]. In line with the presumed segmental organization of the thermoregulatory feedback system were observations in decerebrated rabbits, in which warm and cold stimulation of spinal temperature sensors produced the same antagonism between cutaneous efferent sympathetic activity on the one hand and cardiac and visceral sympathetic efferent activities on the other hand (Fig. 3), as they had been observed in intact animals [27].
Fig. 2.
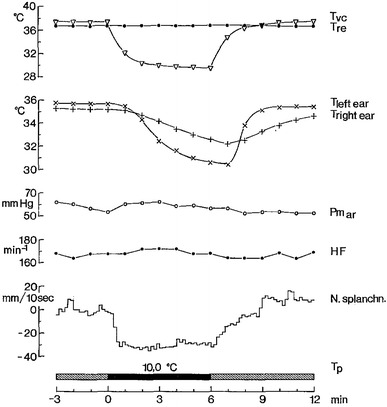
Results obtained during cold stimulation of deep-body thermosensors in the spinal cord of a rabbit 4 days after spinal transection at C6, i.e., below the origin of the phrenic nerve. The thoracic section of the vertebral canal was cooled by means of a water-perfused thermode tube inserted into the thoracic peridural space. T p water temperature at the entrance into the thermode during cooling (black bar). The anesthetized animal was kept in a warm-thermoneutral environment and artificially ventilated under neuromuscular blockade which suppressed cold shivering in response to spinal cord cooling [44]. T re deep-body (rectal) temperature, Tvc peridural space temperature, T left ear, T right ear ear skin temperatures as skin blood flow indicator. N splanchn. multi-fiber activity recorded from a splanchnic nerve branch. Postganglionic sympathetic activity is shown as the change of output, relative to average activity level during pre-cooling period, after analog integration of multi-fiber discharge. Cooling of spinal deep-body thermosensors clearly depressed splanchnic sympathetic activity, whereas decreases in ear skin temperature indicated reduced skin blood flow attributable to activation of vasoconstrictor efferents to the ear skin. No distinct changes of arterial pressure (Pm ar) and heart rate (HF). From Walther et al. [82]
Fig. 3.
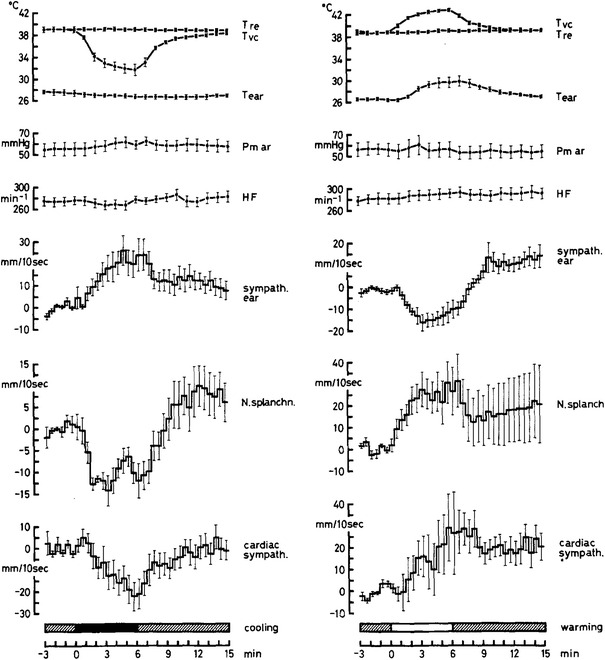
Regionally differential sympathetic responses to cooling (black bar) and warming (white bar) deep-body temperature sensors in the spinal cord, using a water-perfused tube inserted into the vertebral peridural space of anesthetized, artificially ventilated rabbits under neuromuscular blockade in the condition of midcollicular decerebration (means ± standard errors, n = 8). Shown are sympathetic activity changes, relative to average activity levels during pre-stimulation periods, after analog integration of sympathetic discharges recorded from multi-fiber preparations of a sympathetic filament containing spontaneously active fibers innervating the ear skin (sympath. ear), from a filament of a splanchnic nerve branch (N. splanchn.) and from a filament separated from a sympathetic branch innervating the heart (cardiac sympath.). Further shown are courses of deep-body temperature (T re) and peridural vertebral space temperature (Tvc), arterial pressure (Pm ar) and heart rate (HF). Cooling activates sympathetic innervation to the skin and causes parallel reductions of visceral and cardiac sympathetic efferent activities. Warming produces the opposite effects: reduction of cutaneous sympathetic activity reflected by rising ear skin temperature (T ear) and parallel activation of visceral and cardiac sympathetic efferent activities. From Iriki and Kozawa [27]
Experimental fever as a challenge to thermal homeostasis has been shown to elicit differential changes of regional sympathetic activities. The first phase of the fever syndrome [24] displays the characteristic of a cold defense response, i.e., skin vasoconstriction and/or activation of metabolic cold defense cause a rise of deep-body temperature. Activities of efferents to the skin, heart, spleen, and kidney were repeatedly studied. Regional sympathetic activities induced by systemic or intracerebroventricular injections of pyrogens, fever-inducing cytokines, and PGE2 as the ultimate central mediator of the fever response characteristically displayed patterns of differentiation, which changed with the time course of fever and displayed pattern variations, depending on the fever-inducing agent and on whether activities were recorded in the presence or absence of anesthesia ([28, 61], for references).
Notable are recent studies on animal models in which infection with live bacteria produced more extended defense responses to pyrogenic agents (Fig. 4). Relevant for the topic of this review are recordings of cardiac and renal sympathetic activities over extended periods of time (32 h) in conscious sheep suffering from a presumably non-lethal septic shock [66]. Recordings showed substantial increases in cardiac and opposing decreases in renal sympathetic activity during the first hours after infection when arterial pressure remained stable. These changes of regional sympathetic activities were reflected by rising heart rate and by enhanced renal blood flow determined in parallel experiments. With the onset of arterial hypotension, which persisted throughout the observation period, renal sympathetic activity increased, presumably due to reduced baroreflex inhibition. Maintenance of elevated renal blood flow despite increased renal sympathetic activity indicated secondary modulatory influences which may be attributable to enhanced local levels of nitric oxide.
Fig. 4.
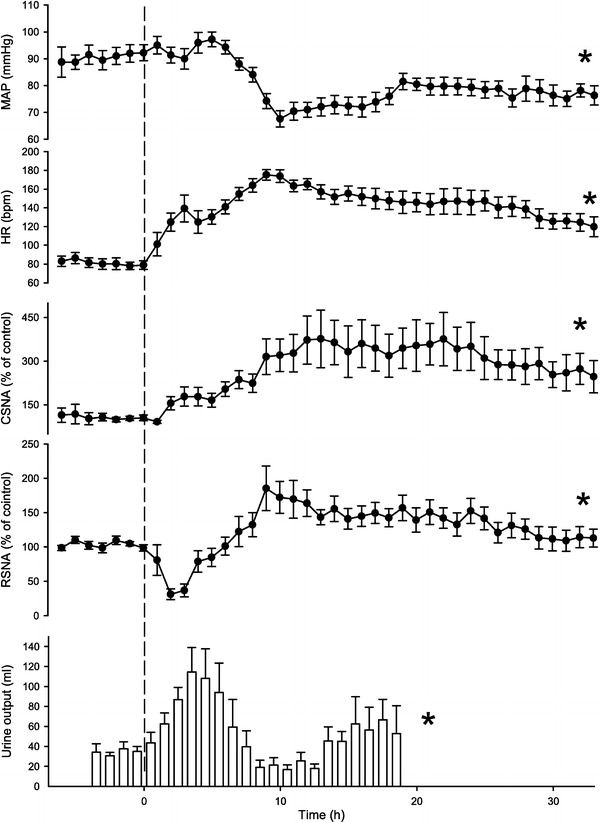
Changes (means with standard errors) of cardiac (CSNA) and renal (RSNA) sympathetic activities induced by injection of live Escherichia coli (time 0) to produce a presumably non-lethal septic shock in conscious sheep equipped with chronically implanted recording electrodes. Note opposing changes of CSNA and RSNA during the early hours of septicemia when mean arterial pressure (MAP) had not yet changed. Heart rate (HR) approximately follows CSNA. Initial depression of renal sympathetic activity is accompanied by an increase in renal blood flow determined in separate experiments (not shown) which persists, however, during the subsequent re-increase of renal sympathetic activity to an elevated level. During the first 10 h after infection, urine output seems to be inversely correlated with the level of renal sympathetic activity. Asterisks indicate significant effects over time. From Ramchandra et al. [66]
The spleen offers favorable conditions to study neuroimmunomodulation of the innate immune system induced by experimental fever or in response to centrally acting cytokines, because the splenic nerve is easily accessible and virtually devoid of a sensory innervation. According to available evidence, splenic efferent sympathetic activity is increased during experimental fever and may be combined with a biphasic decrease of renal sympathetic activity [57]. Comparison of natural killer cell (NK) cytotoxicity from sham-denervated and denervated spleen [79], measurements of norepinephrine release from the spleen [71] and effects of splenic nerve electrostimulation [40] consistently showed inhibition of NK cytotoxicity by enhanced splenic nerve activity, which is probably mediated by beta-adrenergic receptors ([22], for references).
Beyond the scope of this review is the greatly expanding field of research on the role of autonomic regulation of the immune system in chronic inflammatory processes, including autoimmune diseases. The importance of sympathetic efferents has been convincingly documented [41]. Different from the generally suppressive action of adrenergic transmitters on innate immune activity, acquired immune defense may be stimulated or inhibited [56]. Sympathetic innervation may interact locally with certain afferent fibers to modulate neurogenic inflammatory processes caused by the peripheral release of sensory neuropeptides [47, 63]. In the course of chronic inflammatory processes, structural changes may permanently alter relationships between sympathetic efferent and afferent innervation at the site of inflammation [78].
Blood gas homeostasis
Simultaneous recordings from two multifiber preparations, innervating a cutaneous and a visceral vascular bed in anesthetized artificially ventilated rabbits with neuromuscular blockade, revealed regionally antagonistic changes of activity during temporary respiratory arrest, i.e., asphyxia (Fig. 5), causing a decrease of blood oxygen tension and an increase of blood CO2 tension [31]. Further analysis showed that qualitatively identical patterns of differential sympathetic responses could be induced by hypoxia alone, as well as by combined normoxia and hypercapnia, although rather high arterial CO2 tensions were required to induce distinct effects. In studies in which cardiac sympathetic activity was recorded during hypoxia and hypercapnia, its course followed that of cutaneous sympathetic activity. Figure 6 shows that, under hypoxia, cutaneous and cardiac sympathetic activities were depressed, opposite to splanchnic sympathetic activity. Under primary tissue hypoxia, depression of cutaneous sympathetic activity persisted, but now cardiac sympathetic activity increased, similar to splanchnic sympathetic activity ([26, 30], for references).
Fig. 5.
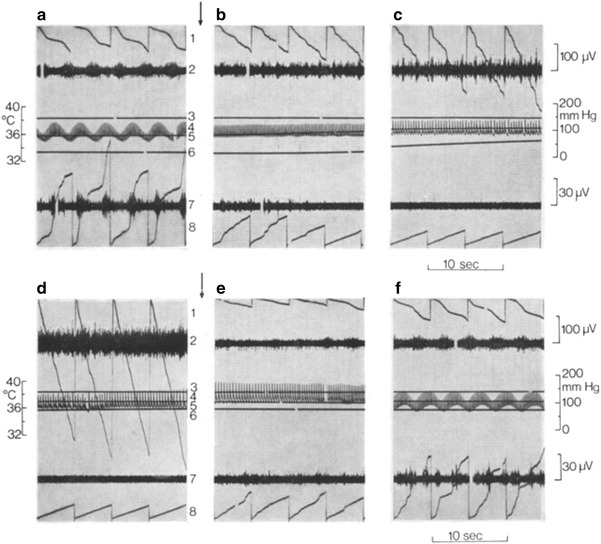
Regionally antagonistic changes of sympathetic activity induced in an anesthetized artificially ventilated rabbit under neuromuscular blockade by respiration pump arrest for 2 min (asphyxia, start and end indicated by arrows). Sections from original recordings of multi-fiber discharges in a filament of a splanchnic nerve branch (upper two traces) and of a sympathetic branch to the ear skin (lower two traces) before (a), at different times during 2 min of asphyxia (b 20–40 s, c 40–60 s, d 100–120 s), and after restarting the respiratory pump (e 10–20 s, f 160–180 s later). Traces numbered in a and d: 1 analog integration in 5 s intervals (downward deflections) of splanchnic sympathetic activity; 2 original trace of splanchnic activity; 3 core temperature; 4 arterial pressure; 5, 6 skin temperatures of right and left ear; 7 original trace of cutaneous sympathetic activity; 8 its analog integration in 5 s intervals (upward deflection). Almost total suppression of cutaneous sympathetic activity during asphyxia results in rises of ear skin temperatures. From Iriki et al. [31]
Fig. 6.
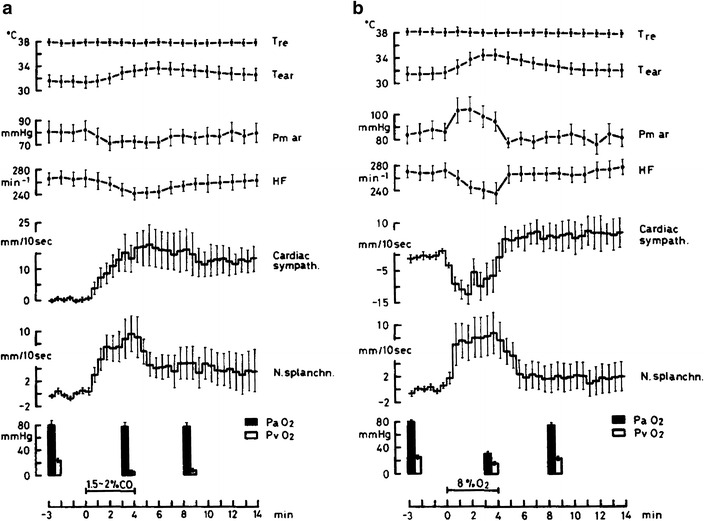
Cardiac and intestinal sympathetic efferent activities shown after analog integration of sympathetic discharges during ventilation (4 min) with a 1.5–2 % carbon monoxide in air (primary tissue hypoxia), and b 8 % oxygen and 92 % nitrogen (arterial hypoxia) in anesthetized artificially ventilated rabbits under neuromuscular blockade (means ± standard errors, n = 8). In each condition, core (rectal, T re) temperature did not change; ear skin temperature (T ear) increased during both primary tissue and arterial hypoxia, due to suppression of cutaneous sympathetic activity, as confirmed by separate recordings in supplementary experiments (not shown). Changes relative to pre-stimulation activity levels are shown after analog integration of multi-fiber activities from splanchnic (N. splanchn.) and cardiac (cardiac sympath.) sympathetic nerve branches. Effects of primary tissue hypoxia differed from those of arterial hypoxia with respect to the cardiac sympathetic response: during primary tissue hypoxia its increase in activity accompanied that of splanchnic sympathetic activity without significant changes in arterial pressure (Pm ar) and heart rate (HF). During arterial hypoxia, cardiac sympathetic activity became reduced, as reflected also by reduction of heart rate, in contrast to the increase in splanchnic activity, and arterial pressure increased. Columns indicate changes in arterial (PaO 2) and venous (PvO 2) oxygen tension. From Iriki and Kozawa [26]
Afferent sites monitoring especially changes in the O2 content of arterial blood are chemoreceptors of the glomus caroticum or vagal chemoreceptors near the aortic arch, which transmit their signals by glossopharyngeal and vagal afferents to the medullary nucleus of the solitary tract (NST), which provides inputs to the medullary vasomotor center controlling sympathetic efferent activity. Sino-aortic denervation and vagotomy will abolish the peripheral O2-sensitive input. CO2- and/or pH-sensitive structures existing within the medulla will remain functional, however, and Fig. 7 shows for this condition that they are sufficient alone to generate the typical pattern of sympathetic differentiation in response to hypercapnia.
Fig. 7.
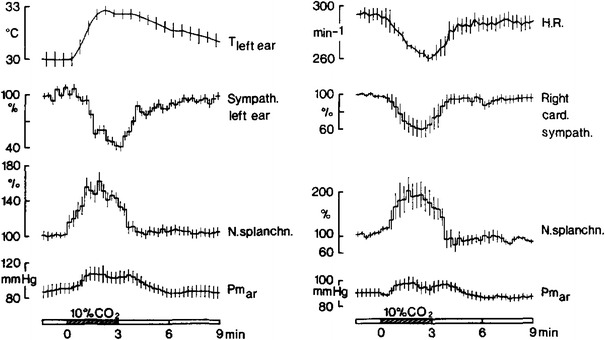
Average differential cutaneous (sympath. left ear), cardiac (right card. sympath.) and visceral (N. splanchn.) sympathetic activities (shown as percent changes, relative to average pre-stimulation activities, after analog integration of sympathetic discharges) in rabbits after sino-aortic denervation and vagotomy to eliminate peripheral chemoreceptors and arterial baroreceptors. Animals (two series with n = 6) were anesthetized and artificially ventilated under neuromuscular blockade. Shown are changes of regional sympathetic activities in response to ventilation with 10 % CO2 in 21 % oxygen and 69 % nitrogen (black bars). The differential sympathetic response pattern during hypercapnia is qualitatively identical with those during asphyxia or arterial hypoxia. Its induction must be attributed to medullary CO2 or pH chemoreceptors. Further shown are ear skin temperature (T left ear) and heart rate (HR) which change according to the changes of regional sympathetic innervation. Mean arterial pressure (Pm ar) rises during hypercapnia. From Simon and Riedel [76]
Figure 8 shows results of studies in rabbits with midcollicular decerebration. Here, the regionally antagonistic response pattern to respiratory hypoxia was replaced by uniform activation of cutaneous, cardiac, and splanchnic sympathetic efferents [27]. In this condition, the O2-sensitive afferent input and the vasomotor efferent output of the medullary reflex loop of cardiovascular control remained non-severed. Thus, differential responses to changes in blood gas composition seem to be critically dependent on suprapontine neuronal circuits. The observation supports conclusions drawn from regional blood flow changes and cardiac responses to hypoxia of rabbits with brain lesions at different levels [43, 80]. Abolition of the differential response to changes in blood gas composition after severing connections to the hypothalamus is in line with the proposal that chemoreceptor signals are conducted by excitatory and inhibitory projections from the NST to the posteromedial hypothalamus, which, in turn, controls descending pathways by which, in particular, inhibitory influences on cardiovascular reflexes are exerted [5].
Fig. 8.
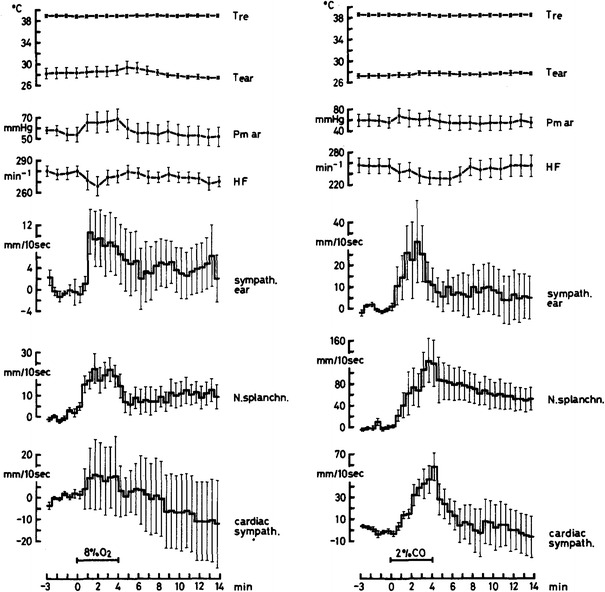
Average courses of cutaneous (sympath. ear), visceral (N. splanchn.) and cardiac (cardiac sympath.) nerve activities shown as changes, relative to average pre-stimulation activities, after analog integration of sympathetic discharges recorded in anesthetized rabbits with midcollicular decerebration being artificially ventilated under neuromuscular blockade. Stimulations were: ventilation with 8 % O2 in 92 % N2 (arterial hypoxia, left, n = 8) or 2 % CO in air (primary tissue hypoxia, right, n = 7), each for 4 min. Also shown are core temperature (T re), average ear skin temperature (T ear), arterial pressure (Pm ar) and heart rate (HF). The differential sympathetic response, which is typically elicited by arterial hypoxia, is completely abolished after midcollicular decerebration. In line with the equidirectional sympathetic responses to respiratory and to tissue hypoxia the tendency for ear skin vasodilatation (see Fig. 6) is lost. From Iriki and Kozawa [27]
Stimulus–response analysis in the sympathetic nervous periphery
With successively improved recording techniques, single sympathetic efferents have become increasingly accessible to analysis in nerve preparations containing a few or only one postganglionic fibers. In combination with the identification of their putative targets, response characteristics of single postganglionic fibers can be elucidated under the influence of a variety of peripheral and central stimulations. Results have been reviewed in detail [32, 35, 37]. Most important as a general result has been the high degree of stimulus specificity among the frequently observed differential response patterns displayed by sympathetic efferent fibers innervating different organs and/or cellular targets. Attention will be focused here on a few examples which seem relevant from the viewpoint of reflex organization or function specificity.
Reflex analysis
Differential noradrenergic sympathetic reflex responses can be elicited by stimulating defined afferents. An exemplary differential reflex is presented in Fig. 9: inhibition of vasoconstrictor efferents to the skin and activation of vasoconstrictors innervating skeletal muscle elicited by stimulation of peripheral (skin) nociceptors [19]. Mechanical noxious stimulation (pinching of the ipsilateral paw skin) and thermal noxious stimulation (immersing the contralateral hindleg in hot water) induced the same antagonism between skin and muscle vasoconstrictors. The differential vasomotor reflex induced by skin nociceptor stimulation was qualitatively preserved in the state of chronic spinalization, i.e., the reflex loop was primarily spinal [20]. Skin vasoconstrictor inhibition was strongest when the stimulus was applied ipsilaterally to the skin region innervated by the investigated vasoconstrictor fibers, whereas a contralateral stimulus and stimulation of more remote skin areas caused lesser inhibition. In contrast, enhanced vasoconstrictor activity of sympathetic efferents supplying muscle vessels was less dependent on the site of skin nociceptor stimulation.
Fig. 9.
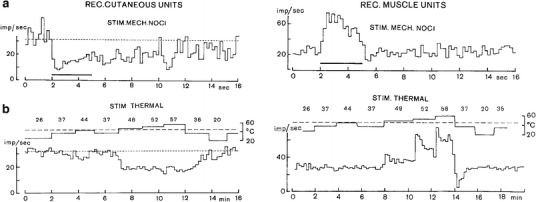
Responses of sympathetic postganglionic fibers innervating the hindleg skin (left) and muscle (right) to noxious mechanical stimulation (horizontal bar) applied to the ipsilateral hindfoot skin (a) and to noxious thermal stimulation (b) applied by immersing the contralateral hindleg in a temperature controlled water bath (upper line with numbers, °C). Analyzed were spontaneously active (hence vasoconstrictor) fibers in anesthetized artificially ventilated cats under neuromuscular blockade. Nerve filament preparations contained a few active fibers. a Mechanical noxious stimulations of a skin area depress skin vasoconstrictor activity and activate muscle vasoconstrictor activity. b Graded heat stimulation applied to the skin is ineffective, as long as the pain threshold temperature (≈44 °C) is not exceeded. Stimulating heat nociceptors with further stepwise increases of temperature induce graded opposing responses of skin and muscle vasoconstrictor fibers that are equidirectional to those following mechanical noxious stimulation. From Horeyseck and Jänig [19]
An example for a differential sympathetic reflex response to a visceral mechanical stimulus (Fig. 10, left diagram) are opposing effects of a pressure increase in the urinary bladder on muscle and skin vasoconstrictors [15]. Differentiation is lost, however (Fig. 10, right diagram) in the state of chronic spinalization [33, 46]. The importance of supraspinal contributions to control of bladder function and to cardiovascular reflex responses elicited by bladder filling corresponds to clinical observations in paraplegic patients.
Fig. 10.
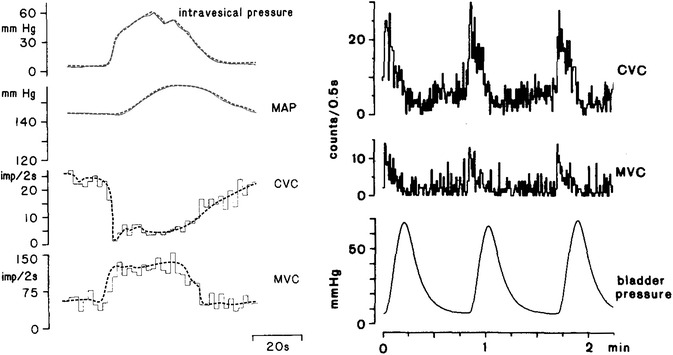
Reflex responses of sympathetic vasoconstrictor efferents innervating cutaneous (CVC) and muscular (MVC) vascular beds to experimental distension of the urinary bladder (intravesical or bladder pressure). Left-hand diagram bladder distension reflex in a cat with intact spinal cord. Lines show opposing courses of CVC and MVC (lines redrawn for better visualization). Further shown is the course of mean arterial pressure (MAP). Data from Häbler et al. [15]. Right-hand diagram bladder distension reflex of a cat in the state of chronic spinalization. In response to 3 phases of bladder distension CVC and MVC are uniformly activated. Figure redrawn from Kümmel [46]
For homeostatic adjustments, the reflex analysis approach confirmed qualitative differentiation of efferent cardiovascular innervation under the influence of central thermal stimulation. In chronic spinalized cats, spinal cord cooling enhanced and spinal cord heating reduced vasoconstrictor outflow to the skin [39]. It may be assumed that the opposing visceral vasoconstrictor response was also preserved, since a change in visceral sympathetic activity opposite to that in the skin was shown (see Fig. 2) to be a component of the response to spinal cord cooling in chronically spinalized rabbits [83]. Results would suggest, at first sight, to categorize the opposing differential responses to spinal cord cooling and heating as spinal reflexes, but it has to be kept in mind that regional sympathetic differentiation as part of a homeostatic response may go beyond the scope of the reflex response category.
The autonomic component of the defense reaction has long been considered to consist in “en masse” excitatory responses of the cardiovascular sympathetic innervation mediated by the “defense area” in the brain stem, but the discovery of differential sympathetic responsiveness suggests that this view should be reconsidered. The defense area is an extended brainstem region with the dorsomedial hypothalamus (DMH) as the “defense center”. Apart from typical flight–fight behavior displayed by conscious animals, electrical or chemical stimulation of the DMH or adjacent regions typically induce general activation of efferent sympathetic outflow, which is accompanied by increased muscle blood flow due to muscle vasodilator activation. The vasodilator transmitter involved may [49] or may not be [84] cholinergic, depending on the species. Typically, vasoconstrictor and cardioacceleratory activation caused by DMH stimulation reflect general resetting of the operating point of baroreceptor control to a higher blood pressure level [51]. Experimentally induced patterns of regional sympathetic activity as well as of arterial pressure and of heart rate changes are quite variable. If muscle vasodilatation is taken as a criterion for the defense response, it may be stated—as an approximate summary of the defense vasoconstrictor response pattern—that skin vasoconstrictors are activated more or less uniformly, whereas muscle vasoconstrictor fibers may show a more complex response pattern starting with enhanced vasoconstrictor activity which subsides to different extents during the subsequent period of stimulation. The involvement of vasodilator sympathetic innervation which accounts for enhanced muscle blood flow despite an increased rather than reduced muscle vasoconstrictor activity is important (Fig. 11). Sympathetic vasodilator efferents to the muscles are generally silent in control conditions but become strongly activated during electro-stimulation in the defense area (Fig. 12). Taken together, variations in the degrees and time courses of excitation of sympathetic efferents innervating functionally different vascular beds [21] are indicative of their differential central control even in the “defense state” of an animal. The diversity of sympathetic response patterns, as well as the variability of hemodynamic and cardiac responses in the course of a defense response [7], reflect the complexity of the defense reaction as a graded behavioral and autonomic response.
Fig. 11.
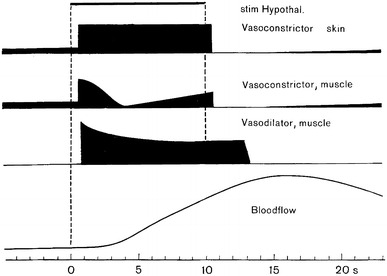
Schematic presentation of the average changes of activity of 3 different types of sympathetic efferents in response to electrostimulation in the defense area (stim Hypothal.). Skin vasoconstrictors (vasoconstrictor skin), displaying spontaneous activity before stimulation are generally activated during stimulation. Muscle vasoconstrictors (vasoconstrictor, muscle), which are also spontaneously active before stimulation, are activated more variably and display, on the average, a temporally modulated response. Muscle vasodilator fibers (vasodilator, muscle) do not show spontaneous activity during pre- and post-stimulation periods. They are strongly activated during defense area electrostimulation (see Fig. 12). Change of blood flow (electromagnetic flow probe) in the lumbar aorta (bloodflow) is mainly attributable to the change in skeletal muscle blood flow which is dominated by the sympathetic vasodilator innervation. From data of Horeyseck et al. [21]
Fig. 12.
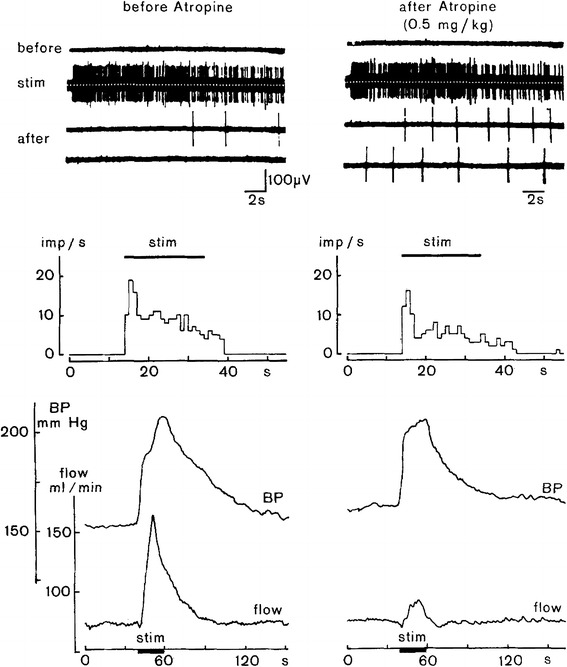
Response of vasodilator fibers to the skeletal muscle to electrostimulation within the defense area as an essential component of the defense reaction. Further shown are accompanying changes in arterial pressure (BP) and blood flow measured in the lumbar aorta (flow, electromagnetic flow probe). Recording traces and diagrams without atropine treatment (left) and atropine treatment to block muscarinic cholinergic transmission (right). Upper 4 traces show activity in a sympathetic filament innervating skeletal muscle in which no spontaneous activity exists during the pre-stimulation phase (before). During stimulation (stim) the filament shows strong activation with some decrement in the course of stimulation, and after stimulation (after, two subsequent traces covering ≈40 s) activity returns to silence more or less rapidly. For the diagrams below, the period of electrostimulation within the defense area is indicated by horizontal bars (stim). Middle diagrams represent the discharge rates of the recorded vasodilator fibers being silent before stimulation and becoming silent again with some delay after the end of stimulation. Lower diagrams show stimulus-induced comparable rises of arterial pressure without (left) and with (right) cholinergic blockade with atropine. Stimulus-induced blood flow increase in the lumbar aorta (left) was almost completely suppressed after atropine treatment (right), whereas vasodilator fiber activity was not affected (upper right-hand recording traces). From Horeyseck et al. [21], modified
Postganglionic non-vasoconstrictor neurons
Vasodilator innervation of muscle vessels
As reported above, activation of sympathetic muscle vasodilator innervation as part of the defense reaction occurs in combination with variable patterns of skin and muscle vasoconstrictor activation. An atropine-sensitive vasodilator mechanism was early on proposed ([1], for references) as an important component of the cardiovascular defense response. According to Fig. 12, the cat provides an example for cholinergic neuroeffector transmission of the sympathetic vasodilatory signal to skeletal muscle [21]. In rats, a non-cholinergic, possibly adrenergic transmitter was assumed to mediate sympathetically induced muscle vasodilation in response to stimulation of the defense area [84].
Non-vasoconstrictor, autonomic efferent innervation of the skin
Among sympathetic efferent fibers innervating the hairy and hairless skin of the cat hindpaw sudomotor, pilomotor, and presumably vasodilator neurons were identified according to their responsiveness to stimulation and to corresponding effects exerted upon their targets. As a rule, these fibers are silent in the resting non-stimulated animal, in contrast to vasoconstrictor neurons which are spontaneously active.
Enhanced sudomotor activity of sweat glands in the hairless cat paw skin is indicated by enhanced skin conductance. The response is elicited by activation of a set of normally silent postganglionic efferents. Vibration as a stimulus to the skin was found to be particularly effective in activating sudomotor efferents [38]. In response to warming and cooling of hypothalamus or spinal cord as deep-body thermosensors, skin vasoconstrictor activity was adequately inhibited and activated, respectively. In contrast, sudomotor activity was not stimulated by warming deep-body thermosensors in the spinal cord or hypothalamus, but tended to be activated by cooling, and, thus, does not seem to be involved in temperature regulation of the cat [13]. Well established, in contrast, is thermoregulatory sweating in humans, which is controlled by cholinergic sympathetic efferents, whereas adrenergic thermoregulatory sweating has been demonstrated for several large mammals [4].
Pilomotor neurons supplying the cat tail were silent in anesthetized cats, and probably also in awake cats, under thermoneutral and emotionally neutral conditions. In anesthetized cats, a set of normally silent neurons was found to display enhanced activity accompanied by piloerection only in response to asphyxia [14]. Piloerection is a component of the defense reaction. It remains to be determined whether the same sympathetic efferent pilomotor pathway mediates general piloerection as a response to cold exposure. Pilomotor muscles respond directly with contraction to local cooling, the response being enhanced by catecholamines [18].
As putative mediators of active vasodilation in the skin, a set of neurons was discovered in sympathetic fiber preparations supplying the hairy or non-hairy skin of the cat paw (Fig. 13). Fibers were silent at thermoneutral conditions and responded in a graded manner to heat stimulation of deep-body thermosensors in the spinal cord, while vasoconstrictor fibers were inhibited in a graded manner [12]. The same thermal stimulus also activated vasodilator fibers in spinalized cats [39]. This fiber type could not be activated by any other peripheral or central natural stimulus. Resistance to hexamethonium of pre-post-ganglionic signal transmission has raised some concern about the origin and course of the putative vasodilator fibers. On the other hand, differences in sensitivity to hexamethonium of ganglionic transmission of sudomotor and even vasoconstrictor neurons had to be taken into account [3]. Apart from this pharmacological problem, thermoregulatory heat dissipation from the skin is presumably less effective in cats than in humans and in a variety of other experimental animals, especially dogs, in which the feet are effective heat sinks, due to a high density of arterio-venous anastomoses in their foot pads. Indeed, support for “active vasodilation” as a thermoregulatory response was provided by measurements of blood flow to the foot of dogs, in which vasoconstrictor control had been abolished by reserpine pretreatment depleting the catecholamine stores (Figs. 14, 15). Electro-stimulation of the lumbar sympathetic chain of catecholamine-depleted dogs elicited vasodilator responses in the ipsilateral hindfoot, and heating deep-body temperature sensors in the spinal cord as well as in the hypothalamus of catecholamine-depleted dogs distinctly enhanced skin blood flow [62, 70]. Severing the ipsilateral sympathetic chain or ganglionic blockade abolished the thermally induced ipsilateral skin vasodilation. The vasodilatory response was atropine-resistant and, thus, is non-adrenergic and non-cholinergic (NANC). In humans, active vasodilatation in the skin, i.e., increases in skin blood flow exceeding those caused by abolition of vasoconstrictor activity, is well documented; it is under cholinergic control. It is not yet decided whether active vasodilation in humans is a primary thermoregulatory response induced by a particular set of vasodilator neurons to enhance heat transfer from the body core to the skin or, alternatively, whether it is an effect secondary to cholinergic thermoregulatory sweating.
Fig. 13.
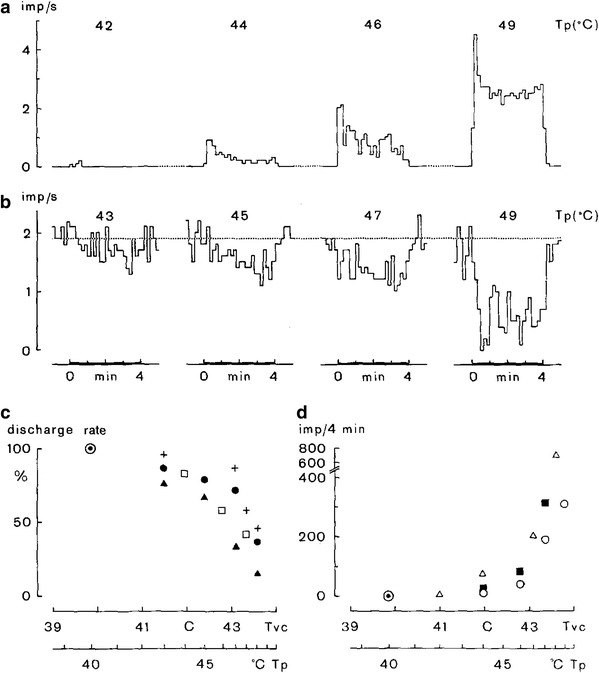
A single presumably postganglionic neuron which is silent at normal body temperature (a) responds by graded activation in response to graded heating of the spinal cord by means of a thermode tube placed in the peridural space. Since the neuron did not respond to any other afferent stimulus it fulfilled the criteria of a vasodilatory sympathetic efferent fiber. A spontaneously active neuron, presumably a vasoconstrictor neuron (b), displays graded inhibition in response to graded spinal cord heating. Numbers above recordings indicate temperature of the water perfusing the thermode (T p). Lower diagrams show temperature-response relationships of vasoconstrictor neurons (c) and of putative vasodilator neurons (d) with different symbols for individual experiments. Average relationship between T p and temperature measured in the vertebral peridural space (Tvc) is indicated at the abscissa. Note that the opposing responses of vasoconstrictor and putative vasodilator neurons to temperature increases are graded and start at non-noxious peridural space temperatures. From Gregor et al. [12]
Fig. 14.
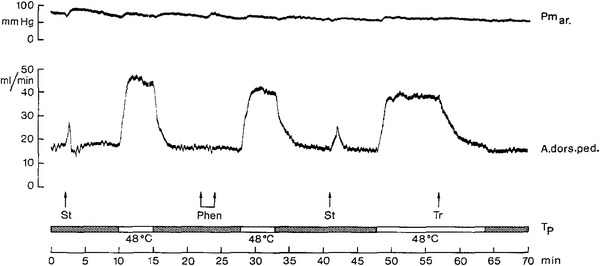
Blood flow (electromagnetic flow probe measurement) to hindfoot (A. dors. ped.) in a catecholamine-depleted (reserpinized) anesthetized dog. Blood flow is distinctly increased by spinal cord warming, using a water-perfused thermode located in the peridural space. Water temperature at entrance of thermode (T p, white bars). Absence of effective vasoconstrictor innervation is indicated by the vasodilator response to electro-stimulation (St) of the ipsilateral lumbar sympathetic chain. As expected, phenoxybenzamine (Phen) as a blocker of alpha-adrenergic transmission prevailing in skin blood vessel vasoconstrictor innervation is ineffective and leaves thermally induced blood flow increase unimpaired. Enhanced blood flow during spinal cord warming is abolished by transection (Tr) of the ipsilateral lumbar sympathetic chain. Arterial pressure (Pm ar) does not change. From Schönung et al. [70]
Fig. 15.
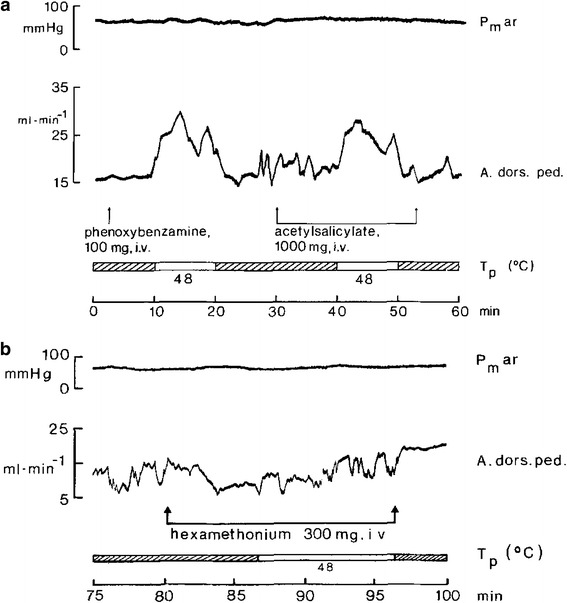
Blood flow (electromagnetic flow probe measurement) to hindfoot (A. dors. ped.) in a catecholamine-depleted (reserpinized) anesthetized dog. a Adrenergic blockade by intravenous (i.v.) phenoxybenzamine injection has no effect on resting blood flow. Warming the anterior hypothalamus with a steretoaxically implanted thermode (water temperature at thermode entrance (T p, white bars) distinctly increased blood flow to the hindfoot, despite α-adrenergic blockade. Persistence of thermally induced vasodilation during acetylsalicylate infusion excluded synthesis of prostaglandin as a putative transmitter. β-adrenergic (propranolol) and cholinergic (atropine) blockade did not abolish thermally induced vasodilation (not shown). b During continuous iv. infusion of hexamethonium (rate ≈ 1 mg/kg/min) resting blood flow was unchanged but the vasodilator response was abolished when hypothalamic heating was started 6 min after start of ganglion blockade. Arterial pressure (Pm ar) does not change. From Peter and Riedel [62]
Sympathetic control of the adrenal medulla
A highly specific pattern of differential sympathetic activity controlling endocrine epinephrine and norepinephrine release was recently disclosed [54, 55], and documents that sympathetic differentiation may extend to the level of single cells (Fig. 16). Sympathetic efferent fibers innervating either ephinephrine secreting or norepinephrine secreting chromaffine cells can be distinguished from each other by making use of the fact that systemic interference with glucose metabolism by administering 2-deoxy-glucose (2DG) specifically stimulates the release of epinephrine. Preganglionic sympathetic fibers were identified as epinephrine release stimulating efferents (EPI-ADR) by their activation in response to 2DG or as norepinephrine release stimulating efferents (NE-ADR) by their non-responsiveness to 2DG. Peri-stimulus histograms clearly documented that circumscribed electrostimulation in the rostroventral lateral medulla produced differential responses with a more sustained excitatory response of EPI-ADR fibers and brief excitation with subsequent depression of NE-ADR fiber activity. Aortic nerve electrostimulation mimicking enhanced baroreceptor input barely affected EPI-ADR fiber activity but reduced the activity of NE-ADR fibers.
Fig. 16.
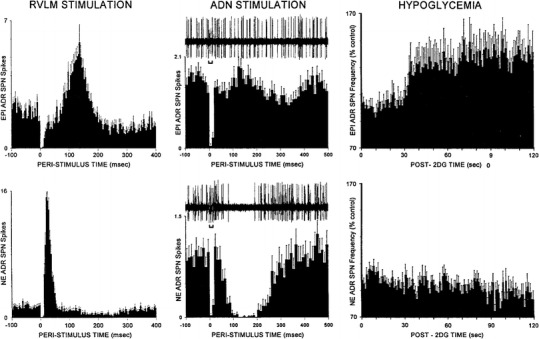
Discharge activities of sympathetic efferent fiber filaments (shown as peri- or post-stimulation activities) innervating epinephrine secreting cells (EPI ADR, upper panel) or nor-epinephrine secreting cells (NE ADR, lower panel) of the adrenal medulla. Right-hand diagrams identification according to response to tissue hypoglycemia (HYPOGLYCEMIA) induced by administration of 2-deoxy-glucose (2DG) which activates EPI ADR fibers and inhibits NE ADR fibers. Left-hand diagrams electrostimulation of the rostroventral lateral medulla (RVLM STIMULATION) at time 0, with peri-stimulus histograms showing delayed activation of fibers innervating EPI ADR cells which persists for ≈0.2 s after stimulation, while activity of fibers innervating NE ADR cells is increased instantaneously, though briefly, and subsequently depressed for >0.2 s after the end of stimulation. Middle diagrams with original recordings of fiber discharges (upper traces) show activity changes caused by stimulation of the aortic depressor nerve (ADN STIMULATION, bars below upper traces). With some delay activity of fibers innervating NE ADR cells is strongly reduced temporarily, while activity of fibers innervating EPI ADR cells does not change distinctly. The response pattern is reflected by the peri-stimulus histograms. From Morrison [54]
Angiotensinergic modulation of regional sympathetic efferent activity
Angiotensin II (ANGII)—as a systemic hormone—acts in the periphery upon vascular smooth muscles as a vasoconstrictor peptide. However, its actions on certain brain targets from the viewpoint of sympathetic differentiation are important, in particular on ANGII-sensitive neurons of the preoptic and anterior hypothalamic (POAH) region at sites where the blood–brain barrier (BBB) is absent. Particular brain structures without a BBB are the so-called circumventricular organs (CVOs) which exist especially in the POAH region but also at the medullary level. Moreover, a brain-intrinsic renin–angiotensin system exists, including sets of “angiotensinergic” neurons containing ANGII as a synaptic transmitter or modulator. While it is particularly prominent in the POAH region, the brain-intrinsic renin–angiotensin system and angiotensinergic neurons are distributed along the brain stem neuraxis.
In cardiovascular pathophysiology, the roles of ANGII and of related compounds in the generation and maintenance of arterial hypertension have been a focus of interest during the past two decades, and various animal models are currently under investigation. From the viewpoint of sympathetic differentiation, responses of cardiovascular innervation to ANGII are of particular interest. If ANGII is applied intravenously at doses which do not exert peripheral hypertensive actions, the hormone may, nevertheless, induce centrally mediated cardiovascular effects by acting at CVOs. Neurons in these structures and in the adjacent neuropil are endowed with various angiotensin receptor (AT) subtypes. In studies with local central applications of ANGII or related compounds at different levels from the lateral cerebral ventricles to the caudal medulla, strong cardiovascular effects were elicited which varied, however, depending on the site of application and the animal model under investigation. Central neurogenic hypertensive actions of ANGII are assumed to be predominantly transmitted to the periphery by the sympathetic nervous system. Because of the apparent clinical relevance of neurogenic hypertension, the involvement of sympathetic cardiovascular innervation in the hypertensive actions has been frequently studied with a multitude of experimental approaches. ANGII and related compounds, and, respectively, their antagonists acting nonspecifically or specifically upon various angiotensin receptors, were administered systemically or locally into various brain stem sites in animals receiving low-salt or high-salt diets. The results unanimously document strong effects on blood pressure homeostasis. Because of manifold inconsistencies which still need to be clarified ([48], for references), it would seem currently inadequate to review the data extensively from the viewpoint of to which extent differential sympathetic responses are contributing to the observed ANGII-induced cardiovascular responses. However, the observation (Fig. 17) of opposing changes of hindlimb and visceral vascular conductance when ANGII was infused into the fourth cerebral ventricle of rabbits was suggestive as indirect evidence of differential ANGII actions on regional sympathetic effects [17]. Also indicative of differential regional sympathetic responses were results of chronic monitoring of multifiber activity in filaments of renal and lumbar sympathetic activities over 3-week periods in chronically ANGII-infused rats fed a high salt-diet [85]. Figure 18 shows that, under this treatment, arterial hypertension developed and attained a maximum after 5 days of the 10-day infusion period. During chronic ANGII infusion, renal sympathetic activity decreased to a minimum towards the end of the infusion period. Sympathetic control of hindlimb muscle blood flow, represented by lumbar sympathetic nerve activity, did not change, on average, during chronic ANGII infusion. Thus, at least quantitative differential control of the vascular beds of hindlimb muscle and of the kidney was confirmed. Enhanced venomotor sympathetic tone, i.e., enhanced activity of a set of efferents to the visceral vascular bed, was tentatively considered as the ultimate cause of sustained elevation of arterial pressure. Indeed, as indirect hemodynamic evidence, enhanced mean circulatory filling pressure was found to develop during chronic ANGII infusion in rats on a high-salt diet, suggesting sympathetically mediated enhanced visceral venous tone as the cause for the development of hypertension. Its neurogenic origin was confirmed by temporary decreases of both visceral filling pressure and arterial pressure in response to ganglionic blockade induced by single injections of hexamethonium [42]. The authors point out that “sympathetic nervous activation demonstrated in the ANGII-infused group fed a high-salt diet seemed to be regionally heterogeneous”.
Fig. 17.
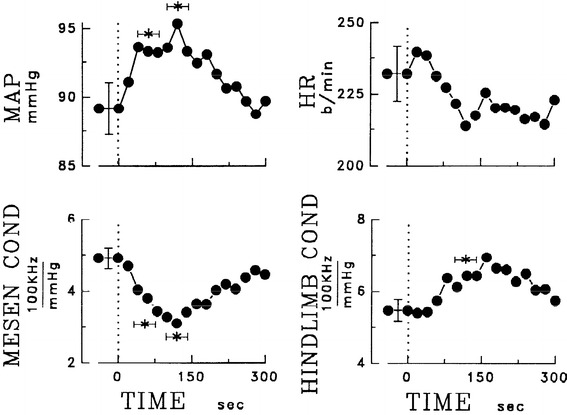
Averaged time course of mean arterial pressure (MAP), heart rate (HR), and calculated changes of mesenteric (MESEN) and hindlimb (HINDLIMB) vascular conductance (COND) after 4th ventricle injection of ANGII in 5 experiments on conscious rabbits. Conductance changes were derived from blood pressure gradients and regional blood flow rates determined with implanted Doppler flow probes. Vertical error bars represent ± SE from analysis of variance. Asterisks indicate significant deviations of measurements from control for either 40–80 or 110–140 s (horizontal bars indicating time interval). Dose-dependence of changes in MAP, HR, MESEN COND and HINDLIMB COND was similar for ANGI, ANGII, and ANGIII (not shown). From Head and Williams [17]
Fig. 18.
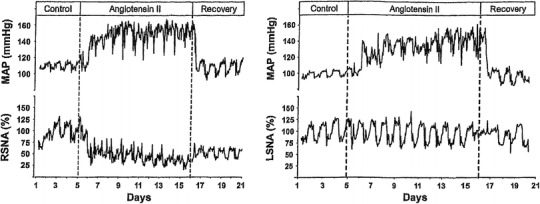
Examples of percent changes, relative to pre-stimulation average activities, in chronic recordings of renal sympathetic (RSNA, left) and lumbar sympathetic (LSNA, right) nerve activities together with mean arterial pressure (MAP) in rats on high salt diet as influenced by chronic systemic ANGII infusion (angiotensin II) with preceding control period (control) and subsequent recovery period (recovery). Gradual development of arterial hypertension indicates its neurogenic origin induced by central actions of ANGII at sites where the blood–brain barrier is permeable for the peptide. Courses of renal and lumbar sympathetic activities differ quantitatively and do not explain alone how hypertension is generated. The involvement of enhanced visceral sympathetic activity enhancing venoconstrictor tone is tentatively considered according to hemodynamic evidence provided by [42]. Diagrams taken from Yoshimoto et al. [85]
Currently, no studies seem to exist in which multiple functionally different sympathetic efferents were studied in conditions of experimental hypertension induced by neurogenic actions of ANGII with or without high salt intake, as would be required to demonstrate differential vasomotor control of functionally different sections of the cardiovascular system. Analysis of neurogenic ANGII actions on circulatory control proceeding from the “baroreflex resetting” concept will be discussed in a subsequent section.
In salt and fluid balance, the role of ANGII, both as a circulating hormone as well as a brain-intrinsic mediator, is known to consist of powerful stimulation of thirst and of sodium intake [8]. POAH brain structures have been identified and characterized as sites of central perception of circulating ANG II and of osmoreception. Moreover, the POAH-intrinsic angiotensinergic system was shown to be closely involved in central signal processing by which control of body fluid volume and of its tonicity are established [53]. Because salt and fluid intake and output are predominantly under behavioral and hormonal control, respectively, contributions of the sympathetic efferent system have so far been little considered. However, the results of a recent analysis of the effects of central osmotic stimulation observed in conscious sheep point to sympathetically controlled adjustments in the cardiovascular system as a component of body fluid homeostasis [50]. As shown by the left-hand diagram of Fig. 19, intracerebroventricular injection of hypertonic saline which stimulated central osmo- or (Na+-) receptors caused distinct differentiation of sympathetic innervation controlling two functionally different organs, the heart and the kidney. The right-hand diagram of Fig. 19 illustrates the putative involvement of the brain-intrinsic ANGII system, an assumption derived from the close similarity between the observed differential sympathetic responses and those documented before as attributable to centrally acting ANGII.
Fig. 19.
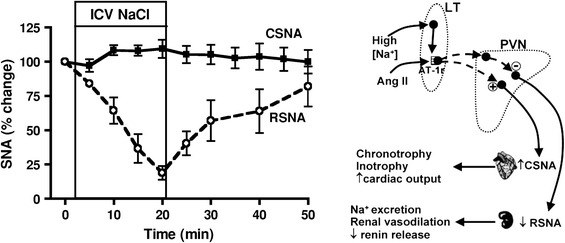
Left-hand diagram differential percent changes in activity of cardiac (CSNA) and renal (RSNA) sympathetic innervation (means with standard errors) induced in conscious sheep by intracerebroventricular infusion of 0.6 M NaCl for 20 min (ICV NaCl). CSNA and RSNA were recorded separately in two groups of animals. Right-hand diagram indicated are hypothalamic lamina terminalis (LT, with partial absence of a blood–brain barrier) as common target for systemic AngII and Na+ and paraventricular nucleus (PVN) as common site of signal integration. Mediation of preoptic/anterior hypothalamic Na+-(or osmo-) receptor signals by the central angiotensinergic system would explain similarities of the central effects of elevated Na+ and of ANGII (Fig. 18) on efferent sympathetic control and their mutual reinforcement. Source of diagrams: May et al. [50]
Role of baroreflex modulation in regional sympathetic differentiation
Baroreceptor signals provide the afferent input of the cardiovascular negative feedback control system. Their main function is to stabilize arterial pressure. The extent to which different vascular beds become involved if the baroreceptor input is changed may differ quantitatively. Analysis of regional sympathetic vasoconstrictor activity showed similar degrees of responsiveness in some [60], but quantitative differences of responsiveness in other, vascular beds [59]. In particular, sympathetic efferents to skin vessels are barely affected by changes of baroreceptor input.
Role of baroreceptors in challenges to homeostasis
With respect to thermoregulatory adjustments of cardiovascular sympathetic innervation, the differential response pattern induced by changing the deep-body thermosensory input from the spinal cord was preserved in principle, after baroreceptor denervation and vagotomy in rabbits, although partially distorted [76], and arterial pressure balance became impaired as shown by blood pressure increases during both spinal cord cooling and heating. With peripheral thermal stimulation, the antagonism between changes of cutaneous and renal sympathetic activity was fully preserved [58] in cats with interrupted baroreflex control (Fig. 20). The assumption that baroreflex control does not contribute essentially to the autonomic nervous thermoregulatory response pattern is supported by hemodynamic studies (Fig. 21). Baroreceptor-denervated and vagotomized dogs responded with antagonistic regional blood flow changes in the vascular beds of the paw skin and the spleen when the hypothalamus as a deep-body sensor was locally warmed or cooled, and arterial pressure was little affected [11].
Fig. 20.
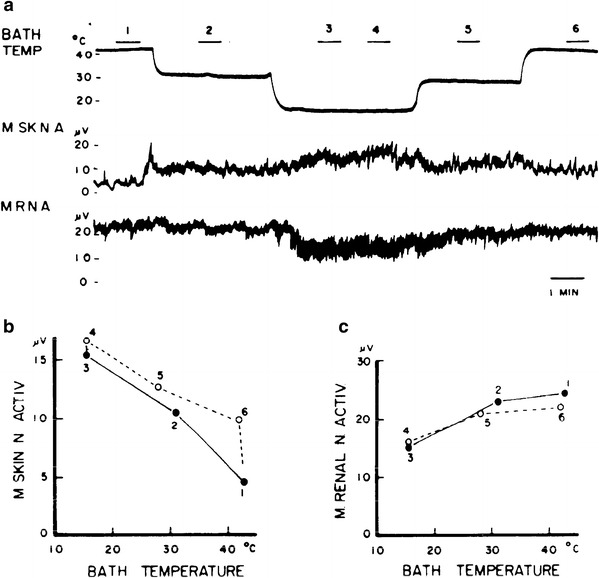
Integrated discharge activities in two sympathetic filaments to the skin (M SK N A) and kidney (M R N A) recorded in anesthetized artificially ventilated cats under neuromuscular blockade. Baroreceptor control loop is surgically interrupted. More than half of the body was immersed into a temperature-controlled water bath (BATH TEMP). a Decreasing bath temperature stepwise enhanced cutaneous and reduced renal sympathetic activity, and stepwise re-increase of temperature reduced cutaneous and increased renal sympathetic activity. The two sympathetic filaments respond oppositely as it may be expected for a thermoregulatory vasomotor response. During six intervals in the course of the experiment (bars above bath temperature curve numbered 1–6) relationships between bath temperature and mean sympathetic activities were determined. b Shows some hysteresis between downward and upward stepwise changes of bath temperature and mean cutaneous sympathetic activity (M SKIN N ACTIV). c Shows that no hysteresis develops for mean renal sympathetic activity (M RENAL N ACTIV). From Ninomiya and Fujita [58]
Fig. 21.
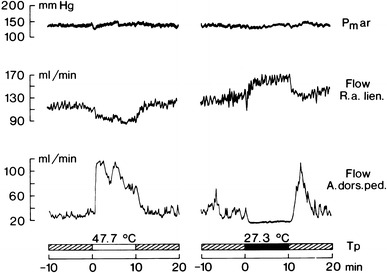
Mean arterial pressure (Pm ar), blood flow (electromagnetic flow probe) in a hindfoot arteria (flow A. dors. ped.) and in an arterial branch to the spleen (flow R. a. lien) measured in an anesthetized artificially ventilated dog under neuromuscular blockade with sino-aortic denervation and vagotomy. Shown are effects induced by heating (white bar) and cooling (black bar) the hypothalamus as a deep-body thermosensor by means of a water-perfused, stereotaxically inserted thermode (T p water temperature at the entrance of the thermode). Enhanced skin blood flow during heating and reduced skin blood flow during cooling are approximately matched by opposing visceral blood flow changes. Note that arterial pressure changes little despite the absence of baroreflex control. From Göbel et al. [11]
With respect to challenges to blood gas homeostasis, complete sino-aortic denervation will remove the afferent inputs of baroreceptors and peripheral chemoreceptors, and hence the differential response pattern induced by hypoxia should be lost. The same denervation procedure should, however, not abolish medullary inputs from CO2- (and/or pH-) sensitive nervous structures. Indeed, after abolishing peripheral baro- and chemoreceptor control by cutting the respective afferent nerves, hypercapnia induced the same differential response pattern (see Fig. 7) with decreasing cutaneous and cardiac and increasing visceral sympathetic efferent activities as in intact animals, and arterial pressure increased only moderately [76]. Since intact animals display virtually identical differential sympathetic response patterns during exposure to hypoxia as well as hypercapnia, pattern generation seems to be induced as a stereotyped response, if blood gas homeostasis is challenged, irrespective of whether the specific input signal is provided by peripheral and/or medullary chemoreceptors. As a conclusion, if both thermal or blood gas homeostasis are challenged, the typical differential regional cardio/vasomotor responses are preserved, in principle, irrespective of whether or not baroreceptor-mediated blood pressure balance is maintained. Changes of baroreceptor feedback signals induced by changes of systemic arterial pressure may modulate or even distort the homeostatic differential response patterns but are not essential for their generation.
Is baroreflex resetting involved in qualitative sympathetic differentiation?
The roles of baroreceptor signals in the control of different sections of the cardiovascular system have, on the one hand, been frequently assessed by determining relationships between the strength of the afferent baroreceptor signal, and, on the other hand, regional hemodynamic and heart rate responses or, respectively, activity changes in regional vascular and cardiac sympathetic efferent activities. From the usually sigmoid response curves, the operation point and the gain of the stimulus response relationship may be defined. The operating point of the baroreceptor feedback loop and/or its gain may be “reset” by central modulation of the afferent baroreceptor input under a variety of stimuli being transmitted to, or acting within, the central nervous system.
As shown before, the qualitatively differential response pattern induced by challenges to temperature homeostasis is virtually independent of baroreceptor control. For this reason, little resetting of the baroreflex response curve may be expected, especially in view of the hemodynamic results after sinoaortic denervation [11].
As reported in detail, arterial hypoxia as a challenge to blood gas homeostasis induces a well-defined qualitatively differential sympathetic response pattern. The response was re-evaluated (Fig. 22) to look for the potential contributions of baroreflex resetting [25]. Similar to rabbits with intact vagi, the baroreflex gain of the renal sympathetic response curve increased under hypoxia in vagotomized animals, whereas the baroreflex gain of the cardiac sympathetic response curve was reduced. When carotid and aortic nerves were additionally cut, the relationship between arterial pressure and renal sympathetic activity was completely lost, and there was no difference between the states of normoxia and hypoxia. In this condition hypoxia rather elevated than reduced the level of cardiac sympathetic activity. Operating points differed greatly between normoxic and hypoxic rabbits and gains were absent or very low. Taken together, sino-aortic denervation completely abolished the differential response of renal and cardiac innervation to hypoxia, probably due to the complete loss of the peripheral chemoreceptor input. If baroreceptor control is intact, some degree of modulation by the baroreceptor input is indicated by the opposing changes of gain in the relationships between arterial pressure and renal and cardiac sympathetic efferent activities in response to hypoxia. The observed changes were minor, however, and only quantitative, and, therefore, do not indicate an essential role of the baroreceptor signal in the generation of differential sympathetic responses. In line with this conclusion, observations in human subjects exposed to hypoxia or hypercapnia suggested “that hypercapnia and hypoxia exert differential effects on cardiovagal, but not sympathetic, baroreflex gain and set point in a manner not related to ventilatory chemoreflex sensitivity” [77].
Fig. 22.
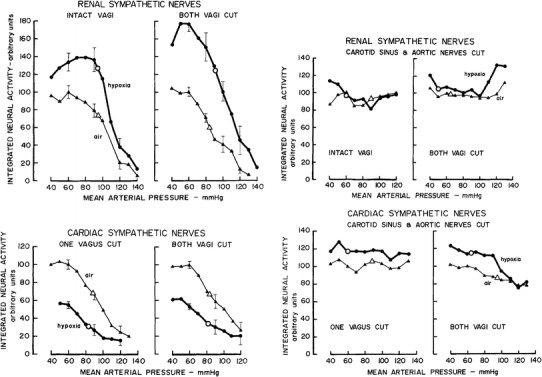
Baroreflex response curves of integrated renal (upper diagrams) and cardiac (lower diagrams) sympathetic activities during normoxia (triangles, thin lines) and hypoxia (≈10 % O2 in N2, circles, thick lines). Left-hand diagrams irrespective of whether vagus nerves are intact or severed, hypoxia increases the gain of the renal but reduces the gain of the cardiac sympathetic baroreceptor response curve without major shifts of pressure operating points. Right-hand diagrams sino-aortic denervation, i.e., elimination of baroreceptor and peripheral chemoreceptor inputs, is followed in each sympathetic branch by complete loss of gain, but pressure operation points differ between states of normoxia and hypoxia. For details, see text. Data from Iriki et al. [25]
Volume expansion of the circulatory system of rabbits was shown to exert quantitatively different effects on renal and lumbar sympathetic activities. Arterial pressure did not change significantly. Heart rate increased slightly. Renal sympathetic activity decreased, but lumbar sympathetic activity did not change significantly. Abolishing the vasodilator effect of endogenous nitric oxide (NO) by administration of L-NAME increased arterial pressure and reduced heart rate. Renal sympathetic activity was distinctly reduced but lumbar sympathetic activity was less responsive as indicated by its very moderate decrease. There is no clear indication as to which extent changes in baroreceptor input contributed to the observed quantitatively differential regional sympathetic responses of the two sympathetic branches, because gains and operating points of their baroreflex response curves were closely similar [64].
Baroreflex resetting by centrally acting angiotensin II or of its endogenous analogs (hepta- or hexapeptides) has been analyzed frequently as a putative mechanism bearing significance for arterial hypertension. Several studies have revealed strong effects on functionally different sections of the cardiovascular system and, respectively, their sympathetic nervous control. There is evidence for quantitatively different degrees of resetting [10, 16, 23, 68, 69]. Investigations in conscious rabbits indicate that the gains of the baroreflex response curve of heart rate as an indicator for cardiac sympathetic innervation and the baroreflex response curve of renal sympathetic activity do not change in response to acute ANGII injection into the 4th cerebral ventricle, but their operating points shift to higher blood pressure levels [16]. At first sight, results would suggest equidirectional effects of ANGII on cardiac and renal sympathetic baroreflex response curves. There is, at least, no indication for qualitative sympathetic differentiation due to baroreflex resetting.
Perspectives
The data reviewed here have amply confirmed the capability of the sympathetic nervous system to exert its control of bodily functions in a highly differential manner. First evidence for differential control of sympathetic efferent activity was disclosed by challenging homeostasis, i.e., with an approach of regulatory physiology proceeding from the concept of negative feedback input–output relationships. Autonomic reflex analysis as the more orthodox neurophysiological approach has subsequently contributed predominantly to the disclosure of differential cardiovascular sympathetic reflex response patterns, and, moreover, has proven successful in identifying non-cardiovascular and non-catecholaminergic sympathetic efferents serving specific functions. However, for some of the reported differential response patterns, it seems difficult to distinguish between reflex and regulatory responses. A problem inherent in the reflex analysis approach is the wide scope of phenomena to which the term “reflex” is attributed. Its conventional use covers the range from complex learned behavioral reactions, e.g., the “pawlow reflex”, to the well-defined monosynaptic somatomotor “tendon jerk reflex”. Reflexes involving autonomic nervous efferents are polysynaptic, as a rule, and often require supraspinal processing of the input signal. Knowledge of the supraspinal components of an autonomic neuronal reflex arc currently is, however, still limited. For homeostatic regulatory feedback systems, supraspinal signal integration and processing is as a rule, considered essential. Homeostatic effector responses to disturbances of the controlled variable are usually graded and sustained. Biological feedback control is often redundant with multiple inputs and outputs. Moreover, neuronal networks at different levels of the central nervous system sometimes contribute significantly to signal integration and processing. Exemplary in this respect is temperature regulation. Here, (1) somatomotor, behavioral and autonomic thermoregulatory effectors may replace each other to some extent in their responses to thermosensory inputs, depending on their functions as effectors of other homeostatic control systems [74]. Further, (2) in addition to the hypothalamus as the ultimate controller in thermoregulation, subsidiary controller functions are established along the central nervous axis [72]. It explains why typical thermoregulatory response patterns of sympathetic cardiovascular innervation were preserved in decerebrated and even in chronically spinalized animals. Especially in the latter case, it would seem tenable to categorize the differential thermoregulatory response pattern simply as a spinal reflex. Taken together, there are conditions in which (1) the results of analyzing a particular autonomic stimulus–response relationship may vary with changing experimental conditions, and (2) distinguishing between regulatory and reflex responses of sympathetic efferent activities may become meaningless.
Sustained or extreme challenges to homeostasis are receiving increasing attention as potential causes of persistent pathophysiological alterations of controlled variables such as arterial pressure, blood glucose level, and blood lipids, to mention a few clinically important parameters. However, the concept of homeostasis may become equivocal, once pathological states such as diabetes, excess fat deposition, arterial hypertension, and heart failure have become established. To cover the range extending from temporary physiological to persistent pathological disturbances of homeostatic parameters, the concept of “allostasis” has been developed to “discuss the overused term ‘stress’, and how, in its place, the concept of allostasis may allow us to consider the life cycle in general as a continuum from daily routines to allostatic overload and the accompanying pathologies” [52]. Allostasis was conceived to apply specifically to human health and comprises a wide range of external challenges to physiological as well as psychological well being. Its suitability as an analytical concept has recently been discussed [67]. Allostasis includes homeostasis as a special, well-regulated state. A particular set of challenges to homeostasis may be defined as Type 1 allostatic overload. It comprises diurnal, seasonal, or other temporary adjustments of controlled variables defined in regulatory physiology as adaptation or acclimatization. Adjustments of this type may be extreme, as in the case of hibernation or of long periods of food deprivation, but they remain reversible. Type 2 allostatic overload defines challenges which exceed the adaptive capacity of homeostatic control and eventually may result in persisting pathological disturbances. Especially, altered activity of the hypothalamic–pituitary–adrenocortical axis has been in the focus of interest as a contributor to pathological states of allostatic overload. On the other hand, the sympathetic nervous system may be similarly relevant because of its all-pervading influence on bodily functions.
With special respect to differential sympathetic control, examples have been presented in this review for its involvement in the generation of pathophysiological disturbances such as experimental fever, altered immune responses, and experimental hypertension. Also mentioned here is the comparative analysis of effects of blood volume changes in animals with normal cardiac function and with experimentally induced heart failure. Results are drawing attention to a particular mode of sympathetic differentiation, namely, “that the number of fibers recruited and their firing frequency are controlled independently” [65].
Future research on animal models to elucidate the involvement of altered sympathetic efferent control in the generation of pathological states should include, not least, its interaction with endocrine control. Virtually all endocrine glands, as well as organs and tissues producing hormones, are under sympathetic control and, in part, also under parasympathetic control. It is suggestive to speculate about sympathetically mediated disturbances of endocrine balance as initiating steps for persistent hormonal dysfunctions.
References
- 1.Abrahams VC, Hilton SM, Zbrozyna A. Active muscle vasodilatation produced by stimulation of the brain stem: its significance in the defence reaction. J Physiol (Lond) 1960;154:491–513. doi: 10.1113/jphysiol.1960.sp006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcroft H. Sympathetic control of vessels in the hand and forearm skin. Physiol Rev. 1960;40(Suppl 4):81–92. [PubMed] [Google Scholar]
- 3.Bell C, Jänig W, Kümmel H, Xu H. Differentiation of vasodilator and sudomotor responses in the cat paw pad to preganglionic sympathetic stimulation. J Physiol (Lond) 1985;364:93–104. doi: 10.1113/jphysiol.1985.sp015732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bligh J. Temperature regulation in mammals and other vertebrates. Amsterdam: North Holland; 1973. [Google Scholar]
- 5.Calaresu FR, Thomas MR. Electrophysiological connections in the brain stem involved in cardiovascular regulation. Brain Res. 1975;87:335–338. doi: 10.1016/0006-8993(75)90430-8. [DOI] [PubMed] [Google Scholar]
- 6.Cannon WB. Bodily changes in pain, hunger, fear and rage. 2. Boston: Branford ; 1929. [Google Scholar]
- 7.Duan Y-F, Winters RW, McCabe PM, Green EJ, Huang Y, Schneiderman N. Modulation of the baroreceptor reflex by stimulation of the hypothalamic defense and vigilance areas. Physiol Behav. 1996;59:1093–1098. doi: 10.1016/0031-9384(95)02145-0. [DOI] [PubMed] [Google Scholar]
- 8.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 9.Folkow B, Heymans C, Neil E (1965) Integrated aspects of cardiovascular regulation. In: Hamilton WF (ed) Handbook of physiology, sect 2, vol III. Am Physiol Soc, Washington, DC, pp 1787–1824
- 10.Gaudet E, Godwin SJ, Head GA. Effect of central infusion of ANG II and losartan on the cardiac baroreflex in rabbits. Am J Physiol Heart Circ Physiol. 2000;278:H558–H566. doi: 10.1152/ajpheart.2000.278.2.H558. [DOI] [PubMed] [Google Scholar]
- 11.Göbel D, Martin H, Simon E. Primary cardiac responses to stimulation of hypothalamic and spinal cord temperature sensors evaluated in anaesthetized paralyzed dogs. J Therm Biol. 1977;2:41–47. doi: 10.1016/0306-4565(77)90010-9. [DOI] [Google Scholar]
- 12.Gregor M, Jänig W, Riedel W. Response pattern of cutaneous postganglionic neurones to the hindlimb on spinal cord heating and cooling in the cat. Pflügers Arch Eur J Physiol. 1976;363:135–140. doi: 10.1007/BF01062281. [DOI] [PubMed] [Google Scholar]
- 13.Grewe W, Jänig W, Kümmel H. Effects of hypothalamic thermal stimuli on sympathetic neurones innervating skin and skeletal muscle. J Physiol (Lond) 1995;488:139–152. doi: 10.1113/jphysiol.1995.sp020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosse M, Jänig W. Vasoconstrictor and pilomotor fibres in skin nerves to the cat’s tail. Pflügers Arch Eur J Physiol. 1976;361:221–229. doi: 10.1007/BF00587286. [DOI] [PubMed] [Google Scholar]
- 15.Häbler H-J, Hilbers K, Jänig W, Koltzenburg M, Kümmel H, Lobenberg-Khosravi N, Michaelis M. Viscero-sympathetic reflex responses to mechanical stimulation of pelvic viscera in the cat. J Auton Nerv Syst. 1992;38:147–158. doi: 10.1016/0165-1838(92)90234-8. [DOI] [PubMed] [Google Scholar]
- 16.Head GA, Saigusa T, Mayorov DN. Angiotensin and baroreflex control of the circulation. Brazil J Med Biol Res. 2002;35:1947–1959. doi: 10.1590/s0100-879x2002000900005. [DOI] [PubMed] [Google Scholar]
- 17.Head GA, Williams NS. Hemodynamic effects of central angiotensin I, II, and III in conscious rabbits. Am J Physiol Regul Integr Comp Physiol. 1992;263:R845–R851. doi: 10.1152/ajpregu.1992.263.4.R845. [DOI] [PubMed] [Google Scholar]
- 18.Hellmann K. The effect of temperature changes on the isolated pilomotor muscles. J Physiol (Lond) 1963;169:621–629. doi: 10.1113/jphysiol.1963.sp007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horeyseck G, Jänig W. Reflexes in postganglionic fibres within skin and muscle nerves after noxious stimulation of skin. Exp Brain Res. 1974;20:125–134. doi: 10.1007/BF00234007. [DOI] [PubMed] [Google Scholar]
- 20.Horeyseck G, Jänig W. Reflex activity in postganglionic fibres within skin and muscle nerves elicited by somatic stimuli in chronic spinal cats. Exp Brain Res. 1974;21:155–168. doi: 10.1007/BF00234387. [DOI] [PubMed] [Google Scholar]
- 21.Horeyseck G, Jänig W, Kirchner F, Thämer V. Activation and inhibition of muscle and cutaneous postganglionic neurones to hindlimb during hypothalamically induced vasoconstriction and atropine-sensitive vasodilation. Pflügers Arch Eur J Physiol. 1976;361:231–240. doi: 10.1007/BF00587287. [DOI] [PubMed] [Google Scholar]
- 22.Hori T, Katafuchi T, Take S, Shimizu N. Neuroimmunomodulatory actions of hypothalamic interferon-alpha. Neuroimmunomodulation. 1998;5:172–177. doi: 10.1159/000026334. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Yoshimoto M, Miki K, Johns EJ. The contribution of brain angiotensin II to the baroreflex regulation of renal sympathetic nerve activity in conscious normotensive and hypertensive rats. J Physiol (Lond) 2006;574:597–604. doi: 10.1113/jphysiol.2006.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iriki M. Fever and fever syndrome—current problems. Jpn J Physiol. 1988;38:233–250. doi: 10.2170/jjphysiol.38.233. [DOI] [PubMed] [Google Scholar]
- 25.Iriki M, Dorward P, Korner PI. Baroreflex “resetting” by arterial hypoxia in the renal and cardiac sympathetic nerves of the rabbit. Pflügers Arch Eur J Physiol. 1977;370:1–7. doi: 10.1007/BF00707938. [DOI] [PubMed] [Google Scholar]
- 26.Iriki M, Kozawa E. Factors controlling the regional differentiation of sympathetic outflow—influence of the chemoreceptor reflex. Brain Res. 1975;87:281–291. doi: 10.1016/0006-8993(75)90425-4. [DOI] [PubMed] [Google Scholar]
- 27.Iriki M, Kozawa E. Patterns of differentiation in various efferents induced by hypoxic and by central thermal stimulation in decerebrated rabbits. Pflügers Arch Eur J Physiol. 1976;362:101–108. doi: 10.1007/BF00583634. [DOI] [PubMed] [Google Scholar]
- 28.Iriki M, Saigusa T. Regional differentiation of sympathetic efferents during fever. Progr Brain Res. 1998;115:477–497. doi: 10.1016/S0079-6123(08)62048-8. [DOI] [PubMed] [Google Scholar]
- 29.Iriki M, Simon E. Differential autonomic control of regional circulatory reflexes evoked by thermal stimulation and by hypoxia. Aust J Exp Biol Med. 1973;5:283–293. doi: 10.1038/icb.1973.28. [DOI] [PubMed] [Google Scholar]
- 30.Iriki M, Riedel W, Simon E. Patterns of differentiation in various sympathetic efferents induced by changes of blood gas composition and by central thermal stimulation in anesthetized rabbits. Jpn J Physiol. 1972;22:585–602. doi: 10.2170/jjphysiol.22.585. [DOI] [PubMed] [Google Scholar]
- 31.Iriki M, Walther O-E, Pleschka K, Simon E. Regional cutaneous and visceral sympathetic activity during asphyxia in the anesthetized rabbit. Pflügers Arch Eur J Physiol. 1971;322:167–182. doi: 10.1007/BF00592296. [DOI] [PubMed] [Google Scholar]
- 32.Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- 33.Jänig W. Spinal cord integration of visceral sensory systems and sympathetic nervous system reflexes. Progr Brain Res. 1986;67:255–277. doi: 10.1016/S0079-6123(08)62767-3. [DOI] [PubMed] [Google Scholar]
- 34.Jänig W. Spinal cord reflex organization of sympathetic systems. Progr Brain Res. 1996;107:43–77. doi: 10.1016/S0079-6123(08)61858-0. [DOI] [PubMed] [Google Scholar]
- 35.Jänig W. The integrative action of the autonomic nervous system—neurobiology of homeostasis. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 36.Jänig W, Häbler H-J. Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic functions. Progr Brain Res. 2000;122:351–367. doi: 10.1016/S0079-6123(08)62150-0. [DOI] [PubMed] [Google Scholar]
- 37.Jänig W, Häbler H-J. Neurophysiological analysis of target-related sympathetic pathways—from animal to human: similarities and differences. Acta Physiol Scand. 2003;177:255–274. doi: 10.1046/j.1365-201X.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 38.Jänig W, Kümmel H. Functional discrimination of postganglionic neurones to the cat’s hindpaw with respect to the skin potentials recorded from the hairless skin. Pflügers Arch Eur J Physiol. 1977;371:217–225. doi: 10.1007/BF00586261. [DOI] [PubMed] [Google Scholar]
- 39.Jänig W, Kümmel H. Organization of the sympathetic innervation supplying the hairless skin of the cat’s paw. J Auton Nerv Syst. 1981;3:215–230. doi: 10.1016/0165-1838(81)90064-3. [DOI] [PubMed] [Google Scholar]
- 40.Katafuchi T, Take S, Hori T. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol (Lond) 1993;465:343–357. doi: 10.1113/jphysiol.1993.sp019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leucoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 42.King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension. 2006;48:927–933. doi: 10.1161/01.HYP.0000243799.84573.f8. [DOI] [PubMed] [Google Scholar]
- 43.Korner PI. Integrative neural cardiovascular control. Physiol Rev. 1971;51:312–367. doi: 10.1152/physrev.1971.51.2.312. [DOI] [PubMed] [Google Scholar]
- 44.Kosaka M, Simon E, Thauer R. Shivering in intact and spinal rabbits during spinal cord cooling. Experientia. 1966;23:385. doi: 10.1007/BF02144533. [DOI] [PubMed] [Google Scholar]
- 45.Kullmann R, Schönung W, Simon E. Antagonistic changes of blood flow and sympathetic activity in different vascular beds following central thermal stimulation. I. Blood flow in skin, muscle and intestine during spinal cord heating and cooling in anesthetized dogs. Pflügers Arch Eur J Physiol. 1970;319:146–1971. doi: 10.1007/BF00592493. [DOI] [PubMed] [Google Scholar]
- 46.Kümmel H. Activity in sympathetic neurons supplying skin and skeletal muscle in spinal cats. J Auton Nerv Syst. 1983;7:319–327. doi: 10.1016/0165-1838(83)90085-1. [DOI] [PubMed] [Google Scholar]
- 47.Lynn B, Schütterle S, Pierau F-K. The vasodilator component of neurogenic inflammation is caused by a special class of heat-sensitive nociceptors in the skin of the pig. J Physiol (Lond) 1996;494:587–593. doi: 10.1113/jphysiol.1996.sp021516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 49.Matsukawa K, Shirai M, Murata J, Tsuchimochi H, Komine H, Ninomiya I, Shimizu K. Sympathetic cholinergic vasodilation of skeletal muscle small arteries. Jpn J Pharmacol. 2002;88:14–18. doi: 10.1254/jjp.88.14. [DOI] [PubMed] [Google Scholar]
- 50.May CN, Frithiof R, Hood GS, McAllen RM, McKinley MJ, Ramchandra R. Specific control of sympathetic nerve activity to the mammalian heart and kidney. Exp Physiol. 2009;95:34–40. doi: 10.1113/expphysiol.2008.046342. [DOI] [PubMed] [Google Scholar]
- 51.McDowall LM, Horiuchi J, Killinger S, Dampney RAI. Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1020–R1026. doi: 10.1152/ajpregu.00541.2005. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS, Wingfield JC. The concept of allostasis in biology and medicine. Horm Behav. 2003;43:2–15. doi: 10.1016/S0018-506X(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 53.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol. 2004;16:340–347. doi: 10.1111/j.0953-8194.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- 54.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–R698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 55.Morrison SF, Cao W-H. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1763–R1775. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
- 56.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niijima A, Hori T, Oomura Y. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst. 1991;36:183–192. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 58.Ninomiya I, Fujita S. Reflex effects of thermal stimulation on sympathetic nerve activity to skin and kidney. Am J Physiol. 1976;230:271–278. doi: 10.1152/ajplegacy.1976.230.2.271. [DOI] [PubMed] [Google Scholar]
- 59.Ninomiya I, Irisawa H, Nisimaru N. Nonuniformity of sympathetic nerve activity to the skin and kidney. Am J Physiol. 1973;224:256–264. doi: 10.1152/ajplegacy.1973.224.2.256. [DOI] [PubMed] [Google Scholar]
- 60.Ninomiya I, Nisimaru N, Irisawa I. Sympathetic nerve activity to the spleen, kidney and heart in response to baroreceptor input. Am J Physiol. 1971;221:1346–1351. doi: 10.1152/ajplegacy.1971.221.5.1346. [DOI] [PubMed] [Google Scholar]
- 61.Ohashi K, Saigusa T. Sympathetic nervous responses during cytokine-induced fever. Pflügers Arch Eur J Physiol. 1997;433:691–698. doi: 10.1007/s004240050333. [DOI] [PubMed] [Google Scholar]
- 62.Peter W, Riedel W. Neurogenic non-adrenergic cutaneous vasodilatation elicited by hypothalamic thermal stimulation in dogs. Pflügers Arch Eur J Physiol. 1982;395:115–120. doi: 10.1007/BF00584723. [DOI] [PubMed] [Google Scholar]
- 63.Pierau F-K, Sann H, Müller S. Regulation of efferent functions of C-fiber nociceptors. In: Kosaka M, Sugahara T, Schmidt KL, Simon E, editors. Thermotherapy for neoplasia, inflammation, and pain. Tokyo: Springer; 2001. pp. 514–522. [Google Scholar]
- 64.Ramchandra R, Barret CJ, Guild S-J, Malpus SM. Evidence of differential control of renal and lumbar sympathetic nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol. 2006;290:R701–R708. doi: 10.1152/ajpregu.00504.2005. [DOI] [PubMed] [Google Scholar]
- 65.Ramchandra R, Hood SG, Frithiof R, May CN. Discharge properties of cardiac and renal sympathetic nerves and their impaired responses to changes in blood volume in heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R665–R674. doi: 10.1152/ajpregu.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramchandra R, Wan L, Hood SG, Frithiof R, Bellomo R, May CN. Septic shock induces distinct changes in sympathetic nerve activity to the heart and kidney in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1247–R1253. doi: 10.1152/ajpregu.00437.2009. [DOI] [PubMed] [Google Scholar]
- 67.Romero LM, Dickens MJ, Cyr NE. The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 68.Saigusa T, Granger NS, Godwin SJ, Head GA. The rostral ventrolateral medulla mediates sympathetic baroreflex responses to intraventricular angiotensin II in rabbits. Auton Neurosci Basic Clin. 2003;107:20–31. doi: 10.1016/S1566-0702(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 69.Saigusa T, Iriki M, Arita J. Brain angiotensin II tonically modulates baroreflex in rabbit. Am J Physiol (Heart Circ Physiol) 1996;271:H1015–H1021. doi: 10.1152/ajpheart.1996.271.3.H1015. [DOI] [PubMed] [Google Scholar]
- 70.Schönung W, Wagner H, Simon E. Neurogenic vasodilatatory component in the thermoregulatory skin blood flow response of the dog. Naunyn Schmiedeberg’s Arch Pharmacol. 1972;273:230–241. doi: 10.1007/BF00501415. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu N, Hori T, Nakane H. An interleukin-1-beta-induced noradrenaline release in the spleen is mediated by brain corticotropin releasing factor: an in vivo microdialysis study in conscious rats. Brain Behav Immun. 1994;7:14–23. doi: 10.1006/brbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- 72.Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol. 1974;71:1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]
- 73.Simon E. Effect of CNS temperature on generation and transmission of temperature signals in homeotherms. A common concept for mammalian and avian thermoregulation. Pflügers Arch Eur J Physiol. 1981;392:79–88. doi: 10.1007/BF00584586. [DOI] [PubMed] [Google Scholar]
- 74.Simon E. Thermoregulation as a switchboard of autonomic nervous and endocrine control. Jpn J Physiol. 1999;49:297–323. doi: 10.2170/jjphysiol.49.297. [DOI] [PubMed] [Google Scholar]
- 75.Simon E, Pierau FK, Taylor DCM. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986;66:235–300. doi: 10.1016/0034-5687(86)90076-9. [DOI] [PubMed] [Google Scholar]
- 76.Simon E, Riedel W. Diversity of regional sympathetic outflow in integrative cardiovascular control: patterns and mechanisms. Brain Res. 1975;87:322–333. doi: 10.1016/0006-8993(75)90429-1. [DOI] [PubMed] [Google Scholar]
- 77.Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J, Shoemaker JK. Hypercapnic vs. hypoxic control of cardiovascular cardiovagal and sympathetic function. Am J Physiol Regul Integr Comp Physiol. 2009;296:R402–R410. doi: 10.1152/ajpregu.90772.2008. [DOI] [PubMed] [Google Scholar]
- 78.Straub RH. Autoimmune diseases and innervation. Brain Behav Immun. 2007;21:528–534. doi: 10.1016/j.bbi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Take S, Uchimura D, Kanemitsu Y, Katafuchi T, Hori T. Interferon-alpha acts at the preoptic hypothalamus to reduce natural killer cytotoxicity in rats. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1406–R1410. doi: 10.1152/ajpregu.1995.268.6.R1406. [DOI] [PubMed] [Google Scholar]
- 80.Uther JB, Hunyor SN, Shaw J, Korner PI. Bulbar and suprabulbar control of the cardiovascular autonomic effects during arterial hypoxia in the rabbit. Circ Res. 1970;26:491–506. doi: 10.1161/01.RES.26.4.491. [DOI] [PubMed] [Google Scholar]
- 81.Walther O-E, Iriki M, Simon E. Antagonistic changes of blood flow and sympathetic activity in different vascular beds following central thermal stimulation. II. Cutaneous and visceral sympathetic activity during spinal cord heating and cooling in anesthetized rabbits and cats. Pflügers Arch Eur J Physiol. 1970;319:162–184. doi: 10.1007/BF00592494. [DOI] [PubMed] [Google Scholar]
- 82.Walther O-E, Riedel W, Iriki M, Simon E. Differentiation of sympathetic activity at the spinal level in response to central cold stimulation. Pflügers Arch Eur J Physiol. 1971;329:220–230. doi: 10.1007/BF00586616. [DOI] [PubMed] [Google Scholar]
- 83.Walther O-E, Simon E, Jessen C. Thermoregulatory adjustments of skin blood flow in chronically spinalized dogs. Pflügers Arch Eur J Physiol. 1971;322:323–335. doi: 10.1007/BF00587750. [DOI] [PubMed] [Google Scholar]
- 84.Yardley CP, Hilton SM. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst. 1987;19:127–136. doi: 10.1016/0165-1838(87)90006-3. [DOI] [PubMed] [Google Scholar]
- 85.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic activity in rats. Hypertension. 2010;55:644–651. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]


