Abstract
During exercise, tidal volume initially contributes to ventilatory responses more than respiratory frequency, and respiratory frequency then increases rapidly while tidal volume stabilizes. Dyspnea intensity is also known to increase in a threshold-like manner. We tested the possibility that the threshold of tachypneic breathing is equal to that of dyspnea perception during cycle ergometer exercise (n = 27). Dyspnea intensity was scored by a visual analog scale. Thresholds were expressed as values of pulmonary O2 uptake at each breakpoint. Dyspnea intensity and respiratory frequency started increasing rapidly once the intensity of stimuli exceeded a threshold level. The thresholds for dyspnea intensity and for occurrence of tachypnea were significantly correlated. An intraclass correlation coefficient of 0.71 and narrow limits of agreement on the Bland–Altman plot indicated a good agreement between these thresholds. These results suggest that the start of tachypneic breathing coincides with the threshold for dyspnea intensity during cycle ergometer exercise.
Keywords: Dyspnea, Exercise hyperpnea, Pulmonary O2 uptake, Respiratory frequency, Tachypnea
Introduction
Respiratory output is primarily regulated by an autonomic metabolic control system in the brainstem to maintain blood gas homeostasis via reflex feedback pathways from chemoreceptors. The ventilatory responses to experimental challenges such as hypercapnia and exercise have been investigated to evaluate the capability of metabolic control to cope with an increased demand for pulmonary gas exchange. As a result, participants’ overall ventilation increases, depending on an individual’s unique responsiveness to the demand [1–3].
The mechanisms that control ventilation seem more complex during exercise than during hypercapnia. Although the primary driver of exercise hyperpnea is still controversial [4, 5], a feed-forward control mechanism [6] and reflex responses to afferent feedback from the periphery to the respiratory centers [7] are likely involved. Nevertheless, the depth and rhythm of breathing show a similar and stereotypical pattern in response to both increases in the level of hypercapnia and exercise intensity [3, 8, 9]. During both hypercapnia and exercise, breathing patterns comprise an initial increase in tidal volume (Vt) and a subsequent rapid increase in respiratory frequency (fr) at the Vt plateau. This tachypneic breathing occurs once the intensity of stimuli exceed a threshold level [8, 10]. Duffin et al. [3] referred to this as the breathing patterning threshold. This tachypneic pattern may result from reflexes mediated by pulmonary stretch receptors and chest wall receptors [11]. However, particularly in humans, mechanisms underlying this tachypneic pattern are still unclear [3, 12].
Exercise and hypercapnia increase overall ventilation with changes in breathing patterns, accompanied by differing levels of dyspnea [13, 14]. Dyspnea increases in a threshold-like manner against pulmonary O2 uptake (VO2) during exercise and against the end-tidal CO2 fraction during hypercapnia [15–18]. We have previously reported that the thresholds for dyspnea and fr are significantly correlated, and show a good agreement during gradual hypercapnia in healthy human participants [19]. In the previous paper, the intensity of dyspnea and fr both showed rapid increases that were virtually simultaneous at a similar level of CO2. That is, changes in the subjective measure of dyspnea coincide with those in the objective measure of respiration, particularly the onset of tachypneic breathing during hypercapnia. Therefore, to extend our previous investigation [19], here we ask: does this subjective–objective coupling of respiratory variables reside in exercise hyperpnea?
In a series of recent human and animal research under conscious conditions, we have attempted to show that nonmetabolic factors play a part in alteration in the rhythm of breathing [19–21]. The fr, rather than the Vt, is susceptible to the influence of nonmetabolic factors such as negative emotions [22]. Because dyspnea causes negative emotions [23], the rhythm of breathing is likely to be affected by dyspnea. Therefore, in the present study, we tested the possibility that the threshold of tachypneic breathing is equal to that of dyspnea perception during cycle ergometer exercise, as previously shown by hypercapnic stimuli [19]. We measured the ventilatory and dyspnea responses to increases in exercise intensity and compared the thresholds for dyspnea and objective respiratory variables. This straightforward approach succeeded in demonstrating a subjective–objective coupling of respiratory variables during exercise, by seeing the results from a new perspective concerning whether or not nonmetabolic factors contribute to alteration in the rhythm of breathing.
Methods
Participants
A total of 27 young adult males (mean age, 21.9 years) participated in this study. Participants gave written informed consent before participating in the experiments, which were approved by the Showa University Committee for Human Experimentation. The study conformed to the principles of the Declaration of Helsinki.
Of the 27 participants, 22 exercised at least once a week. We checked before the start of exercise to see whether the participant was healthy enough to pedal a cycle ergometer.
Measurement of pulmonary gas exchange and ventilation
The participants each wore a facemask. Ventilation and pulmonary gas exchange was measured with a respiratory monitor (Aeromonitor AE300, Minato Medical Science, Osaka, Japan). This monitor measured flow rate at the mouth with a hot-wire flow meter, and continuously calculated minute ventilation (Ve, l/min), Vt (l), and fr (breaths/min). The monitor also measured (breath-by-breath) the fractions of O2 and CO2 in expired gas to determine VO2 and pulmonary CO2 output (VCO2). The monitor was calibrated before each test.
Measurement of dyspnea intensity
The intensity of dyspnea was measured using a 100-mm horizontal visual analog scale (VAS) with the maximum feeling of dyspnea on the right side and the feeling of no dyspnea on the left side of the scale [19]. A piece of paper displaying the VAS was presented to the participants every 2 min (Fig. 1). This means that dyspnea intensity was measured immediately before the start of exercise (3 min), in the middle of each level of exercise (5, 9, and 13 min), immediately before the time of change in exercise intensity (7 and 11 min), and immediately before the cessation of exercise (15 min) (Fig. 1). The participants made a mark on the scale at each time point using a pen, and were not allowed to see their previous scores during each test.
Fig. 1.
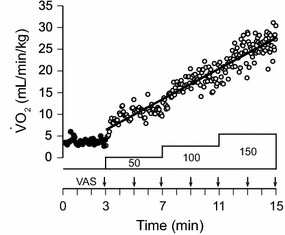
Pulmonary O2 uptake (VO2) during exercise in one participant. Exercise testing was performed on an electrically braked bicycle ergometer. After a 3-min rest (filled circles), he started exercise at an initial work rate of 50 W, which was increased in a stepwise manner by 50 W every 4 min to 150 W. The VO2 values at each time during exercise (open circles) were predicted by regression analysis between time and VO2. The intensity of dyspnea was measured using a 100-mm horizontal visual analog scale (VAS) at 2-min intervals
Exercise
The intensity of dyspnea and the breathing pattern were measured during cycle ergometer exercise (Fig. 1). Exercise testing was performed on an electrically braked bicycle ergometer. The participants sat on the seat of the ergometer and kept their feet on the pedals for a 3-min rest period, during which we measured baseline values of the respiratory parameters. After the 3-min rest period, the participants started exercise at an initial work rate of 50 W, which was increased in a stepwise manner by 50 W every 4 min to 150 W. All participants completed the testing.
Breath-by-breath VCO2, Ve, fr, and Vt were plotted against VO2 during offline analysis. However, dyspnea VAS was not measured in a breath-by-breath way because it was scored at 2-min intervals. Therefore, individual VAS scores were plotted against VO2 at the corresponding time, which was predicted by regression analysis between time and VO2 in each participant (Fig. 1). The baseline VAS score was plotted against mean VO2 at rest for offline analysis.
Thresholds for dyspnea and respiratory variables
Commercial software was used for analyses (SigmaPlot 12, SYSTAT, Chicago, IL, USA). Each plot was fitted with a segmented linear regression model composed of two segments connected by a breakpoint to predict the thresholds for dyspnea (T VAS), VCO2 (T VCO2), Ve (T VE), fr (T fR), and Vt (T VT) [19]. Dyspnea VAS, VCO2, Ve, and fr began to increase rapidly at the breakpoint. However, T VT was determined separately where the regression slope started to decrease. The thresholds were taken as VO2 values at the breakpoint.
Assessment of agreement
We used intraclass correlation coefficients (ICC) and Bland–Altman plots to measure the agreement between the thresholds of the subjective (T VAS) and objective variables (T VCO2, T VE, T fR, and T VT). The agreement between the thresholds was tested by the ICC using two-way random single measures with absolute agreement [24] (IBM SPSS Statistics 23; IBM Corp., Armonk, NY, USA). The level of clinical significance is considered poor agreement, fair agreement, good agreement, and excellent agreement when ICC < 0.40, 0.40 < ICC < 0.59, 0.60 < ICC < 0.74, and 0.75 < ICC < 1.00, respectively [25]. In addition to the agreement, Pearson correlation analyses were performed (Prism 6; GraphPad software Inc., La Jolla, CA, USA). The Bland–Altman plot is typically used to assess the level of agreement between measurements derived from two different methods [26]. In this plot, the within-participant differences between the two methods (Y-axis) are plotted against the mean of both methods (X-axis). This plot provides “95 % limits of agreement” as the mean value for the within-participant difference ±2 standard deviations. The 95 % limits of agreement indicate graphically how well the two methods agree. The 95 % confidence interval (CI) of the mean within-participant difference is calculated to detect systematic bias, and linear regression analysis between the within-participant differences and the mean of both methods is used to show proportional bias (Prism 6; GraphPad software Inc.). A study exploring the relationship between the threshold for dyspnea and the ventilatory threshold during exercise used this plot and showed similarities between these thresholds [17].
Statistics
The mean values of the threshold were compared with one-way repeated measures analysis of variance. Post hoc comparisons were performed using Dunnett’s test (Prism 6; GraphPad software Inc.). For all analyses, significance was recorded at P < 0.05. All values are presented as mean ± SE.
Results
Predicted VO2 at the time of dyspnea measurement
Participants performed incremental exercise starting at a work rate of 50 W, which was increased by 50 W every 4 min to 150 W. Breath-by-breath VO2 values before and during exercise in a representative participant are shown in Fig. 1. After the onset of exercise, VO2 increased stepwise at the beginning and then almost linearly with time, even though the work rate increased in a stepwise manner. In the present study, we planned to present the thresholds as the VO2. However, we measured dyspnea intensity in a time-based manner. Linear trends of VO2 with time, as shown in this representative participant, were confirmed in all participants. Therefore, VO2 at each time predicted by the regression analysis between time and breath-by-breath VO2 was sufficient to estimate T VAS, which was taken as the VO2.
Excluded participants
This experiment was performed in 27 participants, but five participants were excluded from the final analysis because their responses differed from the typical breathing patterns, which comprise an initial increase in Vt and a subsequent increase in fr. One participant was excluded because fr started to increase immediately after the onset of exercise. In four participants, the segmented linear regression could not detect a breakpoint in any of the five variables, primarily because of data scattering. Accordingly, data from 22 participants were included in the statistical analyses.
Single participant
Typical examples from one participant are shown in Fig. 2a. The participant is the same person for whom results are shown in Fig. 1. In this participant, the intensity of dyspnea started to increase rapidly at a VO2 of 13.4 ml/min/kg (T VAS), as estimated by the segmental linear regression model. T VCO2, which is comparable to the ventilatory threshold, determined by plotting VO2 and VCO2, was 12.0 ml/min/kg. For T VE, the slope was steeper after than before the threshold in all of the 22 participants eligible for the statistical analyses. The T VE of this participant was 14.7 ml/min/kg. The fr increased biphasically, similar to the intensity of dyspnea. During the initial phase, fr increased gradually and then rapidly after reaching the threshold. T fR was a VO2 of 16.9 ml/min/kg in this participant. Although Vt changed in a biphasic manner, the response showed an initial rapid increase and subsequently reached a plateau. T VT, where the slope became less steep, was estimated to be 17.9 ml/min/kg.
Fig. 2.
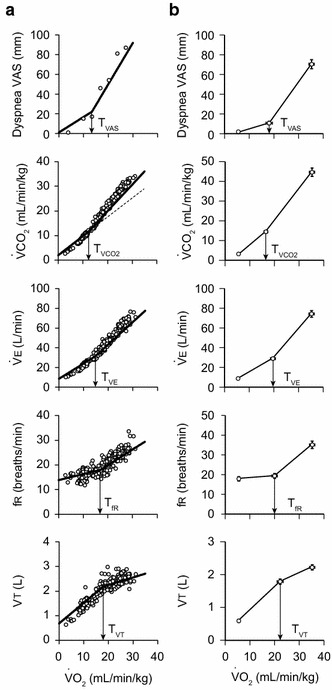
Typical examples from one participant and pooled data from 22 participants. a The participant is the same person for whom results are shown in Fig. 1. Changes in dyspnea VAS, pulmonary CO2 output (VCO2), minute ventilation (Ve), respiratory frequency (fr), and tidal volume (Vt) during exercise are shown. Each plot is fitted with a segmental linear regression model composed of two segments connected by a breakpoint to predict the threshold VO2 value (T VAS, T VCO2, T VE, T fR, T VT). b Pooled data from 22 participants. Values are mean ± SE (n = 22)
Comparison of mean values of the thresholds
The mean regression lines and breakpoints for the 22 participants showed a similar trend to the results for a typical participant (Fig. 2b). Two regression lines were constructed for each variable in each participant, and they lay on either side of the threshold. For dyspnea VAS, fr, Ve, and VCO2, the regression slope was smaller before the threshold than after the threshold in all 22 participants. For Vt, the regression slope was larger before T VT than after T VT in 21 of the 22 participants. The remaining participant showed a small increase in the slope after T VT (before: 0.037 l/VO2, after: 0.040 l/VO2), but his dyspnea VAS, fr, Ve, and VCO2 showed common responses to those found in the other 21 participants. Therefore, we included this participant in the analysis.
For the mean regression lines, we averaged the slope and intercept of 22 (<threshold) and another 22 (>threshold) regression lines for each variable. The mean threshold values, with the threshold values of each individual (open circles), are shown in Fig. 3. Statistical analysis showed that the thresholds were significantly different between the five parameters (P < 0.0001, one-way analysis of variance). Dunnett’s multiple-comparison post hoc tests, where T VAS served as a control, showed that T VT (22.3 ± 1.2 ml/min/kg) was higher than T VAS (18.1 ± 1.2 ml/min/kg, P < 0.01). There were no significant differences between T VAS and T VCO2, T VE, or T fR.
Fig. 3.
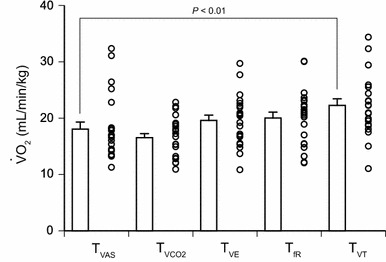
Mean threshold values of 22 participants, with the threshold values of each individual (open circles). The threshold for Vt (T VT) was significantly higher than that for dyspnea VAS (T VAS) (P < 0.01, Dunnett’s multiple-comparison post hoc test with T VAS serving as a control). There were no significant differences between T VAS and T VCO2, T VAS and T VE, or T VAS and T fR. Values are mean ± SE (n = 22)
Agreement between the thresholds assessed by ICC
We analyzed the agreement between T VAS and any of the other thresholds using the ICC and the Bland–Altman plot (Fig. 4). T VAS and all other thresholds were significantly correlated (Fig. 4, left panels). The calculated ICC values between T VAS and any of the other thresholds were also significant. The data plots are close to the identical line (dotted line) in Fig. 4b, c, and a good agreement of ICC values was found between T VAS and T VE (0.65) and between T VAS and T fR (0.71).
Fig. 4.
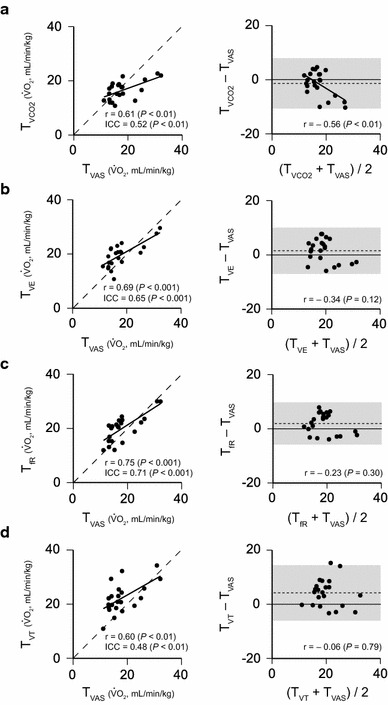
Agreement between the thresholds assessed by the intraclass correlation coefficients (ICC) and the Bland–Altman plot. We analyzed the agreement between T VAS and any of the other thresholds using ICC and the Bland–Altman plot. T VAS and all other thresholds were significantly correlated (left panels; a–d). The calculated ICC values between T VAS and any of the other thresholds were also significant. A good agreement of ICC values was given between T VAS and T VE (0.65) (b) and between T VAS and T fR (0.71) (c). The Bland–Altman plots (right panels; a–d) show that the widths of the 95 % limits of agreement (gray color area) are 18.5 ml/min/kg of VO2 for T VCO2 and T VAS (a), 17.0 ml/min/kg for T VE and T VAS (b), 15.5 ml/min/kg for T fR and T VAS (c), and 20.4 ml/min/kg for T VT and T VAS (d). The Bland–Altman plot suggests better agreement between T VAS and T fR than other combinations
Agreement between the thresholds assessed by the Bland–Altman plot
We further assessed the level of agreement between T VAS and any of the other thresholds (Fig. 4, right panels) using Bland–Altman plots. The widths of the 95 % limits of agreement (gray color area) were 18.5 ml/min/kg of VO2 for T VCO2 and T VAS (Fig. 4a, right panel), 17.0 ml/min/kg for T VE and T VAS (Fig. 4b, right panel), 15.5 ml/min/kg for T fR and T VAS (Fig. 4c, right panel), and 20.4 ml/min/kg for T VT and T VAS (Fig. 4d, right panel). Therefore, the smaller width of the 95 % limits of agreement suggests better agreement between T fR and T VAS than other combinations. A negative correlation was found between the mean value and the difference value (Fig. 4a, right panel), suggestive of proportional bias between T VCO2 and T VAS. This implies that the difference between T VCO2 and T VAS (i.e., T VCO2 − T VAS) varies depending on their mean.
Discussion
In the present study, we tested the possibility that the threshold of tachypneic breathing is equal to that of dyspnea perception during cycle ergometer exercise. This possibility was supported by the results of this study. We found that the mean value of T VAS was similar to those of T VCO2, T VE, and T fR, while it was significantly lower than that of T VT. Correlation analyses showed significant correlations between T VAS and any of the other thresholds (T VCO2, T VE, T fR, and T VT), indicating that a subjective respiratory parameter (dyspnea) is linked with objective respiratory parameters. Furthermore, a good agreement between T VAS and T fR, supported by both the ICC and the Bland–Altman plot, suggests that T VAS coincides most with T fR in exercise hyperpnea. This meant that dyspnea VAS and fr both showed rapid increases that were virtually simultaneous during incremental exercise.
We found that changes in the subjective measure of dyspnea coincided with those in the objective measure of respiration, particularly the onset of tachypneic breathing. Dyspnea VAS increased gradually before reaching its threshold, and then increased more rapidly thereafter. Thus, we detected a breakpoint in dyspnea. Our results showed that T VAS, T VCO2, T VE, and T fR occurred nearly simultaneously during incremental exercise. Evidence supporting this finding was reported by Martin and Weil [10], who showed that T VE and T fR occur simultaneously at the ventilatory threshold. Similarly, Scheuermann and Kowalchuk [27] reported that fr showed a sudden increase at the ventilatory threshold. Furthermore, Amiard et al. [17] reported that T VAS and T VCO2 were significantly correlated and showed a good agreement. We confirmed these previously reported relationships, and we further demonstrated that T VAS agreed most with T fR, which is consistent with our previous paper showing a good agreement between T VAS and T fR during increasing hypercapnia [19]. Therefore, we can say that tachypneic breathing is associated with the perception of dyspnea during exposure to the two leading physical ventilatory stimuli, exercise and hypercapnia.
We found that T fR is more agreeable to T VAS than T VE is, and also that T fR is more agreeable to T VAS than T VT is. This is consistent with our previous observation during hypercapnia [19]. Amiard et al. attributed the similarity between the threshold for dyspnea and the ventilatory threshold to a nonlinear increase in overall ventilation resulting from an increase in VCO2 caused by the accumulation of blood lactate. This may be true, but we need to consider overall ventilation as a product of fr and Vt when discussing mechanisms that control ventilation. Dejours [1] stressed the importance of considering the combination of fr and Vt when studying ventilation. Otherwise, one may neglect the other features of ventilatory control.
T VT occurred in a delayed fashion, and it showed less coincidence with T VAS than T fR did. The interpretation of this result needs some considerations of measuring dyspnea intensity. It is generally accepted that the quality of dyspnea is grouped into three principal types: air hunger, respiratory work/effort, and chest tightness [28]. Smith et al. [14] reported the dominance of air hunger at the end of exercise in healthy participants. Air hunger is derived from increased central respiratory drive that is not matched by adequate ventilatory responses, while respiratory work/effort derives from corollary discharge [29]. In fact, air hunger has been shown experimentally to be caused by reduced Vt combined with chemical respiratory stimuli such as hypercapnia or hypoxia [23, 30, 31]. In a pathological state of the lung, lung expansion is mechanically restricted during exercise by lung hyperinflation in patients with chronic obstructive pulmonary disease, and the restriction of lung expansion is linked with air hunger [32, 33]. If the trend toward the leveling off of Vt were a cause of dyspnea in the present study, T VT should precede or coincide with T VAS. However, T VAS significantly preceded T VT. Our possible explanation for the preceding appearance of T VAS is that the dyspnea we measured included both air hunger and respiratory work/effort. In patients with stable asthma, Laveneziana et al. [34] reported the dominance of respiratory work/effort among dyspnea descriptors during exercise before the inflection of Vt, and they also reported that difficult/unsatisfied inspiration, which is similar to air hunger [32], was markedly increased at peak exercise. Thus, in the present study, the dyspnea of respiratory work/effort may have increased as ventilation increased before exercise intensity reached T VT, which could explain the preceding appearance of T VAS. T VT may be associated with the threshold for air hunger, but we did not specifically measure the threshold for air hunger. We should have distinguished between air hunger and respiratory work/effort in the present study. In this regard, there is still some ambiguity in the meaning of T VAS. We measured dyspnea VAS at 2-min intervals, but the thresholds for air hunger and respiratory work/effort may be determined by measuring dyspnea VAS more frequently if these thresholds occur separately.
We also have a somewhat new perspective about the close relationship between T VAS and T fR. Behavioral respiratory control that relies on inputs from the higher centers [22, 35] would be involved in the alteration of the respiratory rhythm during exercise. Gallagher et al. [12] proposed that tachypneic breathing during exercise is not based on the mechanical limitations of lung expansion in healthy humans. That is, whether the restriction of lung expansion plays a role in healthy humans is unclear. The similarity between T VAS and T fR we found could explain the tachypneic breathing pattern that emerges with a strong respiratory stimulus in terms of emotional respiratory behavior. Dyspnea incorporates affective dimensions that include unpleasantness [29]. During laboratory dyspnea challenges, unpleasantness was accompanied by both respiratory work/effort and air hunger [23]. Masaoka et al. [36] reported that unpleasantness at rest does not affect the metabolic rate, but it increases fr with a slight decrease in Vt. Therefore, one possible explanation for the onset of tachypneic breathing during exercise is that unpleasantness accompanied by dyspnea reaches a level that induces emotional respiratory reactions to induce a tachypneic breathing pattern.
T VAS may be a clinical predictor of exercise tolerance. Akaishi et al. [37] reported that in heart disease patients, the more rapid and shallower that breathing patterns are during cardiopulmonary exercise testing, the lower exercise tolerance is. It is speculated that patients beginning to show rapid and shallow breathing patterns at low exercise intensities have an early onset of dyspnea. Amiard et al. [17] stated that in children with congenital heart impairments, particularly those who cannot perform up to maximal exercise levels, dyspnea thresholds may be a good alternative to ventilatory gas exchange thresholds because these two thresholds occur concomitantly with each other. Thus, presumably, exercise tolerance is approximated by the inflection points of fr, Vt, and dyspnea intensity.
We excluded five participants. Data scattering prevented the segmented linear regression analysis with SigmaPlot software in four participants. We acknowledge that the reason for the data scattering is unclear. It may be better to increase exercise intensity more slowly over time to increase the number of data points. The final participant was excluded because fr started to increase immediately after the onset of exercise. In our previous study, there were different types of breathing pattern responses during increasing hypercapnia. The most common response during increasing hypercapnia was similar to that found during exercise in the present study [19]; however, in two of the 21 participants in the previous study, fr started to increase at the beginning of hypercapnia and reached a plateau [19]. We succeeded in finding a good agreement between T VAS and T fR in most of the participants of the present study, but we have to remember that breathing pattern responses were not always uniform during physical stimuli.
A major shortcoming of this work is that we could not demonstrate a causal relationship between dyspnea perception and tachypneic breathing during exercise. Our straightforward approach showed a good agreement between T VAS and T fR during exercise, but our results do not give evidence that dyspnea-induced negative emotions change the respiratory rhythm. At least, we should have discerned the quality of dyspnea and scored affective components of dyspnea such as distress, simultaneously with the level of dyspnea [29, 33]. We have tried to identify where the rhythm of emotion-related respiration is generated in conscious humans using brain-imaging techniques such as functional magnetic resonance imaging and electroencephalogram dipole modeling [36, 38, 39]. These techniques may enable us to demonstrate whether or not there is a cause-and-effect relationship between dyspnea and tachypneic breathing. We also have to mention here that we increased exercise intensity by 50 W every 4 min to 150 W. Using a gradient increase might have been a better option to determine the thresholds. In particular, for T VAS, dyspnea VAS should have been measured more frequently, with exercise intensity more gradually and slowly increased.
In conclusion, these results suggest that the start of tachypneic breathing coincides with the threshold for dyspnea perception during cycle ergometer exercise. Dyspnea seems to be a candidate for contributing to the sudden change in breathing patterns during exercise, but whether dyspnea is a cause of tachypneic breathing is a subject for further investigation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Dejours P. Neurogenic factors in the control of ventilation during exercise. Circ Res. 1967;20(Suppl 1):146–153. [Google Scholar]
- 2.Bechbache RR, Chow HH, Duffin J, Orsini EC. The effects of hypercapnia, hypoxia, exercise and anxiety on the pattern of breathing in man. J Physiol. 1979;293:285–300. doi: 10.1113/jphysiol.1979.sp012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respir Physiol. 2000;120:13–26. doi: 10.1016/S0034-5687(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 4.Haouzi P (2006) Counterpoint: supraspinal locomotor centers do not contribute significantly to the hyperpnea of dynamic exercise. J Appl Physiol 100: 1079–1082; (discussion 1082–1073) [DOI] [PubMed]
- 5.Waldrop TG, Iwamoto GA. Point: supraspinal locomotor centers do contribute significantly to the hyperpnea of dynamic exercise. J Appl Physiol. 2006;100:1077–1079. doi: 10.1152/japplphysiol.01528.2005. [DOI] [PubMed] [Google Scholar]
- 6.Waldrop TG, Eldridge FL, Iwamoto GA, Mitchel J. Central neural control of respiration and circulation during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of physiology, exercise: regulation and integration of multiple systems. Oxford: The American Physiological Society, Oxford University Press; 1996. pp. 333–380. [Google Scholar]
- 7.Haouzi P, Chenuel B, Huszczuk A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: neurophysiological basis and implication for respiratory control. J Appl Physiol. 2004;96:407–418. doi: 10.1152/japplphysiol.00597.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hey EN, Lloyd BB, Cunningham DJ, Jukes MG, Bolton DP. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir Physiol. 1966;1:193–205. doi: 10.1016/0034-5687(66)90016-8. [DOI] [PubMed] [Google Scholar]
- 9.Clark JM, Hagerman FC, Gelfand R. Breathing patterns during submaximal and maximal exercise in elite oarsmen. J Appl Physiol. 1983;55:440–446. doi: 10.1152/jappl.1983.55.2.440. [DOI] [PubMed] [Google Scholar]
- 10.Martin BJ, Weil JV. CO2 and exercise tidal volume. J Appl Physiol. 1979;46:322–325. doi: 10.1152/jappl.1979.46.2.322. [DOI] [PubMed] [Google Scholar]
- 11.Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher CG, Brown E, Younes M. Breathing pattern during maximal exercise and during submaximal exercise with hypercapnia. J Appl Physiol. 1987;63:238–244. doi: 10.1152/jappl.1987.63.1.238. [DOI] [PubMed] [Google Scholar]
- 13.Simon PM, Schwartzstein RM, Weiss JW, Lahive K, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis. 1989;140:1021–1027. doi: 10.1164/ajrccm/140.4.1021. [DOI] [PubMed] [Google Scholar]
- 14.Smith J, Albert P, Bertella E, Lester J, Jack S, Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax. 2009;64:713–718. doi: 10.1136/thx.2008.104869. [DOI] [PubMed] [Google Scholar]
- 15.Lane R, Adams L, Guz A. The effects of hypoxia and hypercapnia on perceived breathlessness during exercise in humans. J Physiol. 1990;428:579–593. doi: 10.1113/jphysiol.1990.sp018229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane R, Adams L. Metabolic acidosis and breathlessness during exercise and hypercapnia in man. J Physiol. 1993;461:47–61. doi: 10.1113/jphysiol.1993.sp019500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amiard V, Jullien H, Nassif D, Maingourd Y, Ahmaidi S. Relationship between dyspnea increase and ventilatory gas exchange thresholds during exercise in children with surgically corrected heart impairment. Int J Sports Med. 2007;28:333–339. doi: 10.1055/s-2006-924396. [DOI] [PubMed] [Google Scholar]
- 18.Wan L, Van Diest I, De Peuter S, Bogaerts K, Oyen N, Hombroux N, Van de Woestijne K, Gallego J, Van den Bergh O. Repeated experiences of air hunger and ventilatory behavior in response to hypercapnia in the standardized rebreathing test: effects of anxiety. Biol Psychol. 2008;77:223–232. doi: 10.1016/j.biopsycho.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Izumizaki M, Masaoka Y, Homma I. Coupling of dyspnea perception and tachypneic breathing during hypercapnia. Respir Physiol Neurobiol. 2011;179:276–286. doi: 10.1016/j.resp.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi S, Izumizaki M, Atsumi T, Homma I. CO2 homeostasis is maintained in conscious humans by regulation of tidal volume, but not of respiratory rhythm. Respir Physiol Neurobiol. 2013;186:155–163. doi: 10.1016/j.resp.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Izumizaki M, Iwase M, Tsuchiya N, Homma I. Hyperpnoeic response independent of limb movements at exercise onset in mice. Respir Physiol Neurobiol. 2013;185:319–331. doi: 10.1016/j.resp.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Homma I, Masaoka Y. Breathing rhythms and emotions. Exp Physiol. 2008;93:1011–1021. doi: 10.1113/expphysiol.2008.042424. [DOI] [PubMed] [Google Scholar]
- 23.Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med. 2008;177:1384–1390. doi: 10.1164/rccm.200711-1675OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Zeng L, Lin ZJ, Cazzell M, Liu H. Tutorial on use of intraclass correlation coefficients for assessing intertest reliability and its application in functional near-infrared spectroscopy-based brain imaging. J Biomed Opt. 2015;20:50801. doi: 10.1117/1.JBO.20.5.050801. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 27.Scheuermann BW, Kowalchuk JM. Breathing patterns during slow and fast ramp exercise in man. Exp Physiol. 1999;84:109–120. doi: 10.1111/j.1469-445X.1999.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishino T. Dyspnoea: underlying mechanisms and treatment. Br J Anaesth. 2011;106:463–474. doi: 10.1093/bja/aer040. [DOI] [PubMed] [Google Scholar]
- 29.Laviolette L, Laveneziana P, Faculty ERSRS. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J. 2014;43:1750–1762. doi: 10.1183/09031936.00092613. [DOI] [PubMed] [Google Scholar]
- 30.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB. Reduced tidal volume increases ‘air hunger’ at fixed PCO2 in ventilated quadriplegics. Respir Physiol. 1992;90:19–30. doi: 10.1016/0034-5687(92)90131-F. [DOI] [PubMed] [Google Scholar]
- 31.Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol. 2003;94:141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, Gelb AF, Mahler DA, Webb KA. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007;4:145–168. doi: 10.1513/pats.200611-159CC. [DOI] [PubMed] [Google Scholar]
- 33.Mahler DA, O’Donnell DE. Recent advances in dyspnea. Chest. 2015;147:232–241. doi: 10.1378/chest.14-0800. [DOI] [PubMed] [Google Scholar]
- 34.Laveneziana P, Bruni GI, Presi I, Stendardi L, Duranti R, Scano G. Tidal volume inflection and its sensory consequences during exercise in patients with stable asthma. Respir Physiol Neurobiol. 2013;185:374–379. doi: 10.1016/j.resp.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Shea SA. Behavioural and arousal-related influences on breathing in humans. Exp Physiol. 1996;81:1–26. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- 36.Masaoka Y, Koiwa N, Homma I. Inspiratory phase-locked alpha oscillation in human olfaction: source generators estimated by a dipole tracing method. J Physiol. 2005;566:979–997. doi: 10.1113/jphysiol.2005.086124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akaishi S, Adachi H, Oshima S, Taniguchi K, Hasegawa A, Kurabayashi M. Relationship between exercise tolerance and TV vs. RR relationship in patients with heart disease. J Cardiol. 2008;52:195–201. doi: 10.1016/j.jjcc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Masaoka Y, Izumizaki M, Homma I. Where is the rhythm generator for emotional breathing? Prog Brain Res. 2014;209:367–377. doi: 10.1016/B978-0-444-63274-6.00019-9. [DOI] [PubMed] [Google Scholar]
- 39.Masaoka Y, Harding IH, Koiwa N, Yoshida M, Harrison BJ, Lorenzetti V, Ida M, Izumizaki M, Pantelis C, Homma I. The neural cascade of olfactory processing: a combined fMRI-EEG study. Respir Physiol Neurobiol. 2014;204:71–77. doi: 10.1016/j.resp.2014.06.008. [DOI] [PubMed] [Google Scholar]


