Abstract
In mammals, circadian rhythms are associated with multiple physiological events. The aim of the present study was to examine the effect of lipopolysaccharide (LPS) on circadian systems in the ovary. Immature female mice were received an intra-peritoneal injection of equine chorionic gonadotropin (eCG) and LPS. Total RNA was collected from the ovary at 6-h intervals throughout a 48 h of experimental period. The expression of the circadian genes period 2 (Per2) and brain and muscle ARNT-like 1 (Bmal1) such as circadian genes was measured by quantitative PCR. Although expression of Per2 and Bmal1 in the ovary did not display clear diurnal oscillation, LPS suppressed the amplitude of Per2 expression. Additionally, LPS inhibited the expression of cytochrome P450 aromatase (CYP19) and luteinizing hormone receptor (LHr) genes in the ovary of eCG-treated mice. Our data suggest that Per2 may be associated with the inhibition of CYP19 and LHr expression by LPS in the ovaries of immature mice.
Keywords: Lipopolysaccharide, Circadian rhythm, Ovary, Liver
Introduction
In mammals, circadian rhythms are associated with multiple physiological events and are regulated by “central clock” located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus [1]. Circadian rhythms have also been observed in peripheral tissues includeing liver, kidney [2, 3], uterus and ovary [4], which are referred to as “peripheral clocks”. The peripheral clocks synchronize with central clocks through neuronal and hormonal systems [5]. Circadian oscillations are generated by a set of clock genes forming a transcriptional autoregulatory feedback loop. In mammals, Clock, Bmal1, Per1, Per2, Cry1, and Cry2 are associated with this transcriptional feedback loop.
The mammalian ovary is an organ in which follicular development, ovulation and the formation of corpus luteum can occur periodically. These physiological events in the ovary are referred to as the estrous cycle (animal) or menstrual cycle (human). The functions of granulosa and theca cells that are the major components of ovarian follicles is associated with the estrous cycle (or menstrual cycle) in mammals. In mammals, follicle-stimulating hormone (FSH) from the pituitary enhances follicular development by promoting granulosa cell function, including estradiol production and cell proliferation. Estradiol is synthesized by the enzyme CYP19 (P450aromatase) in the granulosa cells of growing follicles and subsequently induces the gene expression of luteinizing hormone receptor (LHr) in granulosa cells. Follicle-derived estradiol exerts positive feedback on both the hypothalamus and the pituitary to trigger a luteinizing hormone (LH) surge that precedes ovulation.
Recent studies support a role of circadian clock genes on granulosa and theca cell function in the ovary. The rhythmic expression of clock genes was observed in both granulosa and theca cells in rodent ovary [6, 7]. Recent evidence indicates that FSH can induce the expression of circadian clock genes in the granulosa cells [8]. It has also been reported that clock genes expressed in granulosa cells are involved in the steroidogenesis. Bmal1 is associated with the production of progesterone (P4) and prostaglandin (PGE2) in rat granulosa cells [9]. Moreover, Clock is associated with estradiol (E2) production by enhancing mRNA expression of LHr and CYP19 in granulosa cells [10]. Thus, circadian clock genes play an important role for ovarian cellular functions in mammals.
Ascending infection of the upper female genital tract with gram-negative bacteria can lead to the development of pelvic inflammatory disease (PID) in women [11] or endometritis in dairy cattle [12]. Both are pathophysiological conditions that have been associated with infertility. Lipopolysaccharide (LPS), the major component of the outer membrane of gram-negative bacteria, can disturb normal ovarian function. Injection of intravenous LPS can inhibit in peripheral plasma estradiol concentrations despite normal plasma luteinizing hormone (LH) levels in the rhesus monkey [13]. Moreover, LPS can delay ovulation by attenuating the increased preovulatory estradiol in heifer [14]. LPS can also been shown to disturb estradiol production in granulosa cells [15] and progesterone production in theca cells [16] in vitro. Thus, LPS can induce ovarian dysfunction by affecting the functions of follicular cells such as granulosa and theca cells.
Although circadian clock genes are associated with ovarian function, it is still unknown whether LPS affects the circadian rhythm of clock genes during follicular development in the ovary. We hypothesized that LPS affects follicular development by disturbing the circadian rhythm of clock genes. To test this hypothesis, we examined the effect of exogenous LPS treatment on the circadian rhythm of Per2 and Bmal1 during ovarian follicular development that is induced by exogenous hormone treatment (equine chorionic gonadotropin).
Materials and methods
Animals and sample collection
ICR female mice (4-week-old; 22–25 g) were purchased from SANKYO LABO SERVICE Co. Inc. The animals were housed with free access to food and water at all times and were maintained on a 12-h light (AM 8:00, zeitgeber time 0, ZT 0): 12-h dark (PM 8:00, ZT 12) cycle at a controlled temperature (22–24 °C). The animals were divided into two randomly assigned groups. In the control group, the mice received an intra-peritoneal (i.p.) injection of equine chorionic gonadotropin (eCG, 5 IU, ASKA Animal Health Co., Ltd. Tokyo, Japan) and saline at ZT 0 on day 1 and saline alone at ZT0 on day 2. In the LPS group, the mice received an i.p. injection of eCG (5 IU) and LPS (1.0 μg/g body weight) at ZT 0 on day 1, and LPS alone at ZT 0 on day 2. In both groups, the mice were sacrificed by decapitation every 6 h: ZT 0, 6, 12, 18, and 24 on day 1 and day 2, and the livers and ovaries were removed rapidly. The removed tissues were placed in Trizol (Life Technologies, Inc., DriveRockville, MD, USA) and were stored −80 °C until RNA extraction.
RNA extraction, reverse transcription (RT), and quantitative polymerase chain reaction (PCR)
Collected liver and ovary samples were homogenized in Trizol reagent and total RNA was extracted from each ovary and liver according to the manufacturer’s instructions and then frozen at −80 °C. Before the RT reaction, samples were treated with DNase and single-strand cDNA was then reverse transcribed from total RNA using a commercial kit (PrimeScript™ RT Reagent Kit with gDNA Eraser; TAKARA BIO INC., Shiga, Japan). The RT reaction conditions were as follows: 15 min of cDNA synthesis at 37 °C and 5 s of inactivation at 85 °C. The mRNA levels of Per2 and Bmal1, steroid-synthesis related genes such as LHr and CYP19 were quantified by real-time PCR using an iQcycler (Bio-Rad Laboratories, Inc., Tokyo, Japan) and a commercial kit (QuantiTectTM SYBR® Green PCR; QIAGEN GmbH, Hilden, Germany).Each primer used is showed in Table 1. The amplification program included 10 min of activation at 95 °C followed by 50 cycles of PCR (95 °C for 10 s, specific temperature of each primer’s annealing for 30 s and 72 °C for 20 s). Values were normalized using β-actin as the internal standard.
Table 1.
Primer pairs used for detection of mRNAs
| Genes | Primer sequence | Size (bp) | GeneBank accession no. |
|---|---|---|---|
| Per2 |
Forward: 5′-GGCACATCTCGGGATCG-3′ Reverse: 5′-GAGCAGAGGTCCTCGCC-3′ |
112 | NM_011066 |
| Bmal 1 |
Forward: 5′-GGAGAAGGTGGCCCAAA-3′ Reverse: 5′-AGGCGATGACCCTCTTA-3′ |
135 | NM_001243048 |
| CYP19 |
Forward: 5′-CATGGTCCCGGAAACTGTGA-3′ Reverse: 5′-CTAGTAGTTGCAGGCACTTC-3′ |
186 | NM_007810.3 |
| LHr |
Forward: 5-TGAGTCCATCACGCTGAAAC-3′ Reverse: 5′-AGATTAGCGTCGTCCCATTG-3′ |
80 | NM_013582.2 |
| β-Actin |
Forward: 5′-CACACCTTCTACAATGAGCTGC-3′ Reverse: 5′-CATGATCTGGGTCATCTTTTCA-3′ |
108 | NM_007393.5 |
Statistical analysis
All data are presented as mean ± SEM. A one-way ANOVA was used to test for any diurnal variations in Per2 and Bmal1 expression in each tissue type. Student’s t test was used to investigate any gene expression differences between control and LPS-treated mice for expression of multiple genes. Analyses were considered to be statistically significant at P < 0.05.
Results
Effect of LPS treatment on circadian expression of Per2 and Bmal1 in the liver
The expressions of Per2 and Bmal1 mRNA in the liver of the control group displayed diurnal rhythms and anti-phase (Figs. 1a, 2a). However, in the LPS treatment group, whilst the expression of Per2 mRNA showed a diurnal rhythm, levels during the experimental period were suppressed (Fig. 1b). Additionally, LPS inhibited the mean level of Per2 mRNA at day 1 and day 2 (Fig. 1c, d). In contrast with the expression pattern of Per2, LPS treatment did not affect the diurnal rhythms of Bmal1 (Fig. 2b), or the mean level of Bmal1 at day 1 or day 2 (Fig. 2c, d).
Fig. 1.
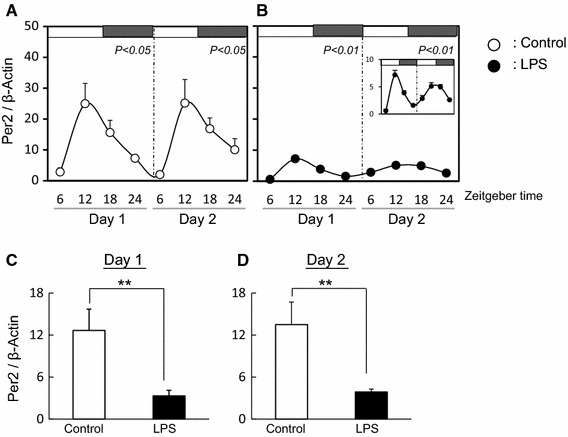
Effect of LPS on circadian rhythm of Per2 in mouse liver The quantified data of Per2 at day 1 and day 2 in liver from mice with (b) or without (a) LPS treatment. White and black bars at the top of the figure indicate the time of lights-on and lights-off, respectively. Data are presented as mean ± SEM, n = 3–4, at each time point and analyzed by one-way ANOVA to determine diurnal variations in clock gene expression. Mean expression level of Per2 is shown during day 1 (c) and day 2 (d) in liver from mice with or without LPS treatment. Data are presented as mean ± SEM and analyzed by t test to investigate any differences between control and LPS-treated mice (*P < 0.05, **P < 0.01)
Fig. 2.
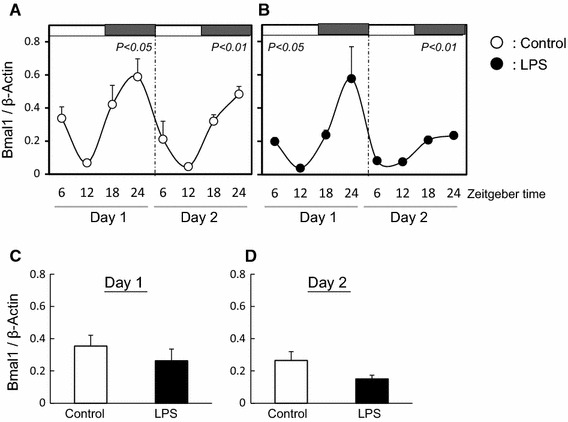
Effect of LPS on circadian rhythm of Bmal1 in mouse liver The quantified data of Bmal1 at day 1 and day 2 in liver from mice with (b) or without (a) LPS treatment. White and black bars at the top of the figure indicate the time of lights-on and lights-off, respectively. Data are presented as mean ± SEM, n = 3–4, at each time point and analyzed by one-way ANOVA to investigate diurnal variations in clock gene expression. Mean expression level of Bmal1 is shown during day 1 (c) and day 2 (d) in liver from mice with or without LPS treatment. Expression data are presented as mean ± SEM
Effect of LPS treatment on circadian expression of Per2 and Bmal1 and the expression of CYP19 and LHr genes in the ovary
In control ovary tissues, the diurnal rhythm of Per2 expression was observed at day 2 but not at day 1 (Fig. 3a). LPS treatment disturbed the diurnal rhythm of Per2 expression at day 2 (Fig. 3b) and the average level of Per2 expression decreased at day 2 (Fig. 3d). The diurnal rhythms of Bmal1 expression in the ovary with or without LPS treatment was not observed at day 1 and day 2 (Fig. 4a, b). Additionally, the average levels of Bmal1 expression was the same between both groups (Fig. 4c, d). The expression of CYP19 and LHr in LPS treated ovary was suppressed at day 2 (Fig. 5).
Fig. 3.
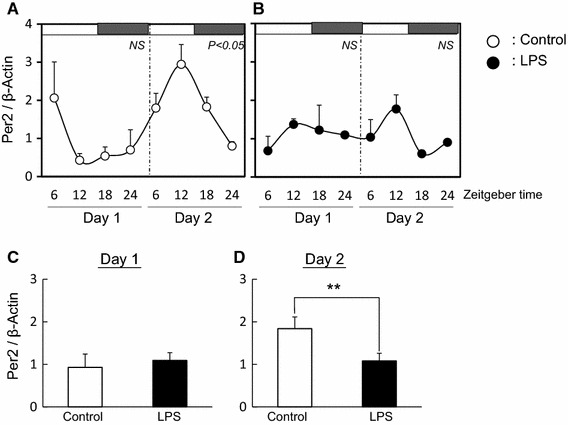
Effect of LPS on circadian rhythm of Per2 in mouse ovary The quantified data of Per2 at day 1 and day 2 in ovary from mice with (b) or without (a) LPS treatment. White and black bars at the top of the figure indicate the time of lights-on and lights-off, respectively. Data are presented as mean ± SEM, n = 3–4, at each time point and analyzed by one-way ANOVA to examine diurnal variations in clock gene expression. Mean expression level of Per2 is shown during day 1 (c) and day 2 (d) in ovary from mice with or without LPS treatment. Data are presented as mean ± SEM analyzed by t test to determine any difference between control and LPS-treated mice (**P < 0.01)
Fig. 4.
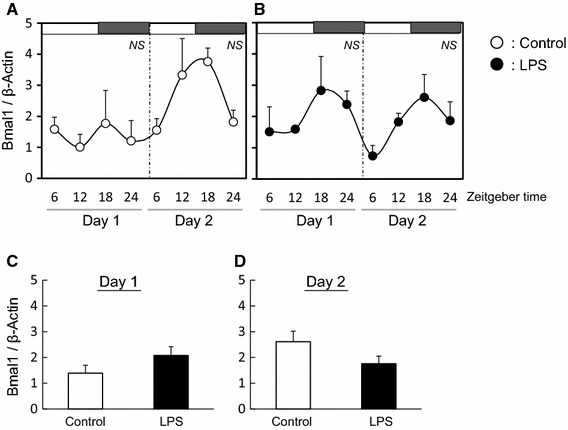
Effect of LPS on circadian rhythm of Bmal1 in mouse ovary The quantified data of Bmal1 at day 1 and day 2 in ovary from mice with (b) or without (a) LPS treatment. White and black bars at the top of the figure indicate the time of lights-on and lights-off, respectively. Data are presented as mean ± SEM, n = 3–4, at each time point and analyzed by one-way ANOVA to determine diurnal variations in clock gene expression. Mean expression level of Bmal1 is shown during day 1 (c) and day 2 (d) in ovary from mice with or without LPS treatment. Data are presented as mean ± SEM
Fig. 5.
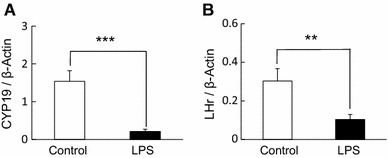
Effect of LPS on CYP 19 and LHr in the mouse ovary The quantified data of CYP19 (a) and LHr (b) expression in ovary (ZT 24 on day 2) from mice with (n = 4) or without (n = 4) LPS treatment 48 h after eCG injection (**P < 0.01, ***P < 0.001). Data are presented as mean ± SEM
Discussion
This study examined the effect of LPS treatment on liver and ovarian circadian rhythm in mice. In the present study, we used exogenous hormone-treated immature mice to induce ovarian follicular development. Although administration of LPS did not affect the circadian rhythm of Per2 and Bmal1 in the ovary, change to the circadian rhythm of these genes were observed in the liver. The liver is a well-established peripheral oscillator that displays robust circadian rhythms of clock gene expression. To verify the correct analysis of circadian rhythm in ovary, mRNA expression of Per2 and Bmal1 in the liver was measured. Periodic rhythm and antiphase form of Per2 and Bmal1 was observed in the liver. However, injection of LPS suppressed Per2 expression in the liver at day 1 and day 2. This observation is consistent with the published literature that indicates that a single dose of LPS (1 mg/kg) significantly suppressed the expression levels of Per2 in the liver at day 1 [17]. Several clock-controlled elements are present in the Per2 promoter region [18]. A noncanonical E-box enhancer (CATGTG, −497 in human and −163 in mouse, and CACGTT, −356 in human and −23 in mouse) drives circadian expression of Per2 through Clock/Bmal1-mediated transcriptional activation [19, 20]. Another important regulatory element is the DBP/E4BP4-binding element (D-box). This element influences Per2 expression by a repressor-antiphasic-to-activator mechanism, which generates high-amplitude transcriptional activity [18]. Thus, LPS may inhibit transcription of Per2 by inhibiting transcription factor binding to E-box or D-box in mouse liver.
Rhythmic expression of clock genes in the ovary has been observed in mature rat [6, 7, 21] and mouse [22]. Rhythmic expression of Per2 was induced within 24 h after FSH treatment in immature rat granulosa cells within in vitro culture [7, 23]. We observed that the circadian rhythms of Per2 and Bmal1 developed in mouse ovary at day 2 after eCG treatment without LPS. These results suggest that the generation of clock gene rhythmicity by gonadotropin in immature granulosa cells may occur prior to rhythmicity in the ovarian tissue. Although LPS did not affect the rhythmic expression of Per2 and Bmal1, LPS suppressed the mean level of Per2 at day 2 in the ovary. As in the liver, LPS may inhibit the transcription of Per2 by inhibiting transcription factor binding to E-box or D-box in the ovary.
In the present study, we demonstrated that expression of CYP19 and LHr genes was inhibited in the mouse ovary treated with LPS 48 h after eCG injection. CYP19 is a critical enzyme in the production of estradiol in granulosa cells of the growing follicles in the mammalian ovary. Intraperitoneal injection of LPS in mice can lead to increased levels of inflammatory cytokines such as interleukin (IL)-10, IL-6 and tumor necrosis factor alpha (TNF-α) in blood [24, 25]. TNF-α and IL-6 inhibit the expression of CYP19 (P450aromatase) and LHr in cultured granulosa cells [26–28]. Therefore, IL-6 and TNF-α activation by LPS may be associated with the observed decrease in the expression of CYP19 and LHr in the mouse ovary. Interestingly, we observed that the mean level of Per2 expression at day 2 decreased in the ovaries treated with LPS compared to those in the control group. Our previous study reported that siRNA knock-down of Per2 decreased the expression of LHr gene in bovine granulosa cells [10]. Together, these results suggest that LPS or LPS-induced cytokines may suppress LHr expression by inhibiting Per2 in the mouse ovary.
In conclusion, our data indicate that the amplitude of Per2, but not of Bmal1, in the liver is influenced by LPS and that Per2 may be associated with the inhibition of CYP19 and LHr expression by LPS in the ovary. This study suggests that Per2 is a target factor of LPS in peripheral tissues. Our results contribute to the understanding of ovarian pathophysiological functions in mammals.
Compliance with ethical standards
Conflict of interest
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of this manuscript.
References
- 1.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA. 2003;100:6795–6800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huisman SA, Oklejewicz M, Ahmadi AR, Tamanini F, Ijzermans JN, van der Horst GT, de Bruin RW. Colorectal liver metastases with a disrupted circadian rhythm phase shift the peripheral clock in liver and kidney. Int J Cancer. 2015;136:1024–1032. doi: 10.1002/ijc.29089. [DOI] [PubMed] [Google Scholar]
- 4.Sellix MT. Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology. Front Endocrinol (Lausanne) 2013;23:4–91. doi: 10.3389/fendo.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohawk JA, Green CB, Joseph S, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- 7.He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem. 2007;302:111–118. doi: 10.1007/s11010-007-9432-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Zhao L, Chu G, Kito G, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA. FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway. Am J Physiol Endocrinol Metab. 2013;304:E566–E575. doi: 10.1152/ajpendo.00432.2012. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Zhao L, Kumazawa M, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA. Downregulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol. 2013;304:C1131–C1140. doi: 10.1152/ajpcell.00008.2013. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Hirai Y, Murayama C, Miyamoto A, Miyazaki H, Miyazaki K. Circadian Clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochem Biophys Res Commun. 2011;412:132–135. doi: 10.1016/j.bbrc.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Ross JDC. An update on pelvic inflammatory disease. Sex Transm Infect. 2002;78:18–19. doi: 10.1136/sti.78.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheldon IM, Williams EJ, Miller AN, Nash DM, Herath S. Uterine diseases in cattle after parturition. Vet J. 2008;176:115–121. doi: 10.1016/j.tvjl.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao E, Xia-Zhang L, Ferin M. Stress and the menstrual cycle: short- and long-term response to a 5-day endotoxin challenge during the luteal phase in the rhesus monkey. J Clin Endocrinol Metab. 1999;84:623–626. doi: 10.1210/jcem.84.2.5448. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki C, Yoshioka K, Iwamura S, Hirose H. Endotoxin induces delayed ovulation following endocrine aberration during the proestrous phase in Holstein heifers. Domst Anim Endocrinol. 2001;20:267–278. doi: 10.1016/S0739-7240(01)00098-4. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu T, Miyauchi K, Shirasuna K, Bollwein H, Magata F, Murayama C, Miyamoto A. Effects of lipopolysaccharide (LPS) and peptidoglycan (PGN) on estradiol production in bovine granulosa cells from small and large follicles. Toxicol In Vitro. 2012;26:1134–1142. doi: 10.1016/j.tiv.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Magata F, Horiuchi M, Miyamoto A, Shimizu T. Lipopolysaccharide (LPS) inhibits steroid production in theca cells of bovine follicles in vitro: distinct effect of LPS on theca cell function in pre- and post-selection follicles. J Reprod Dev. 2014;60:280–287. doi: 10.1262/jrd.2013-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, Nakayama M, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M. Injection of LPS causes transient suppression of biological clock genes in rats. J Surg Res. 2008;145:5–12. doi: 10.1016/j.jss.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, Yoo OJ, Yamazaki S, Lee C, Takahashi JS. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci. 2005;102:2608–2613. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akashi M, Ichise T, Mamine T, Takumi T. Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock. Mol Biol Cell. 2006;17:555–565. doi: 10.1091/mbc.E05-05-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gräs S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–3776. doi: 10.1210/en.2006-0305. [DOI] [PubMed] [Google Scholar]
- 22.Tischkau SA, Jeager CD, Krager SL. Circadian clock disruption in the mouse ovary in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Lett. 2011;201:116–122. doi: 10.1016/j.toxlet.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu G, Misawa I, Chen H, Yamauchi N, Shigeyoshi Y, Hashimoto S, Hattori MA. Contribution of FSH and triiodothyronine to the development of circadian clocks during granulosa cell maturation. Am J Physiol Endocrinol Metab. 2012;302:E645–E653. doi: 10.1152/ajpendo.00470.2011. [DOI] [PubMed] [Google Scholar]
- 24.Dong C, Zhang JC, Yao W, Ren Q, Yang C, Ma M, Han M, Saito R, Hashimoto K. Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2016;144:7–12. doi: 10.1016/j.pbb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Wei T, Gao J, Chang X, He H, Miao M, Yan T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci Lett. 2015;606:1–6. doi: 10.1016/j.neulet.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Ghersevich S, Isomaa V, Vihko P. Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol Cell Endocrinol. 2001;172:21–30. doi: 10.1016/S0303-7207(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Kawaguchi T, Hara T, Takatoshi S, Tohei A, Miyajima A, Seishi T, Kogo H. Interleukin-6 decreases estrogen production and messenger ribonucleic acid expression encoding aromatase during in vitro cytodifferentiation of rat granulosa cell. Mol Cell Endocrinol. 2000;170:103–111. doi: 10.1016/S0303-7207(00)00334-8. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Kawaguchi T, Kogo H. Interleukin-6 inhibits the expression of luteinizing hormone receptor mRNA during the maturation of cultured rat granulosa cells. J Endocrinol. 2001;170:121–127. doi: 10.1677/joe.0.1700121. [DOI] [PubMed] [Google Scholar]


