Abstract
Near-infrared spectroscopy (NIRS) has become an increasingly valuable tool to monitor tissue oxygenation (Toxy) in vivo. Observations of changes in the absorption of light with Toxy have been recognized as early as 1876, leading to a milestone NIRS paper by Jöbsis in 1977. Changes in the absorption and scatting of light in the 700–850-nm range has been successfully used to evaluate Toxy. The most practical devices use continuous-wave light providing relative values of Toxy. Phase-modulated or pulsed light can monitor both absorption and scattering providing more accurate signals. NIRS provides excellent time resolution (~ 10 Hz), and multiple source–detector pairs can be used to provide low-resolution imaging. NIRS has been applied to a wide range of populations. Continued development of NIRS devices in terms of lower cost, better detection of both absorption and scattering, and smaller size will lead to a promising future for NIRS studies.
Keywords: Muscle, Oximetry, Tissue oxygenation, Oxidative metabolism, Exercise
Introduction
This article addresses the use of in vivo near-infrared spectroscopy (NIRS) for evaluating muscle oxygenation and oxidative metabolism, with a focus on the historical development of the method. There are a series of excellent review papers on the use of NIRS to study skeletal muscle [1–8]. There is also a recent article on principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy [9] with corresponding commentaries [10]. NIRS has also been applied to study other tissues such as the brain [11, 12], but this topic will not be addressed in this review. NIRS has been used to study muscle oxygen levels in athletes, control subjects and patients with chronic illnesses or injuries [13, 14]. Given the increasing use of NIRS to study skeletal muscle, the purpose of this review is to guide future studies by providing: (1) descriptions of early studies that developed the instrumentation and methodologies that has led to current NIRS measurements, (2) The basic principles behind the NIRS measurements along with key limitations of the various NIRS based methodologies, and (3) a summary with selected examples of current the NIRS approaches used to understand the biochemistry and physiology of skeletal muscle oxidative metabolism.
Early studies with in vitro and in vivo oximetry leading to the use of NIR for the evaluation of skeletal muscle
In 1876, Karl von Vierordt observed by eye (visible light) spectral changes of hemoglobin (Hb) in trans-illuminated human fingers as well as in solutions containing Hb [15]. When circulation to the finger was occluded, the oxygenated Hb (oxy-Hb) bands disappeared and the deoxygenated Hb (deoxy-Hb) bands appeared, demonstrating the potential for qualitative oximetry, defined as a noninvasive method for monitoring tissue oxygenation. This was followed up a half century later with the building of reliable devices to perform in vitro spectrophotometry utilizing visible light (400–650 nm) by Drabkin and Austin in 1932 [16], and Millikan in 1933 [17]. Millikan invented a colorimeter, which had been conventionally operated by the eye of the observer requiring subjective judgements and the potential for eye strain. The device objectively measured the degree of oxygenation of dilute Hb solutions using a differential copper copper-oxide photoelectric cell and two color filters. Later, it is known that Millikan developed the first portable ear oximeter using red and NIR light for monitoring black-outs of the pilots at extreme high altitude, which provided a prototype of a clinically useful oximeter [18]. In 1970s, Aoyagi at Nihon Kohden, a Japanese company, attempted to measure cardiac output using dye dilution methods along with a commercially available ear oximeter [18]. Initially, he found that light transmitted through the earlobe exhibited pulsatile variations that interfered with measurements of cardiac output. By balancing the red and infrared signals to cancel the pulsatile signal variations, he was able to accurately measure cardiac output using the kinetics of the dye washout. In addition, he successfully monitored the pulsating changes in the light transmission through the ear to measure arterial oxygen saturation. At the same time, a researcher with Minolta, another Japanese company, was conducting similar experiments and applied for a patent soon after. These experiments led to the marketing of a successful pulse oximeter by Minolta around 1978 [18].
Professor Frans F. Jöbsis of Duke University is regarded as the pioneer of medical applications of NIRS [19]. Before his 1977 milestone article, reflectance spectrophotometry and surface fluorescence using visible light were primarily used for investigating large solid organs such as the brain. Jöbsis extended NIRS research to other tissues, including skeletal muscle. He learned about optical monitoring techniques as a postdoctoral fellow in Britton Chance’s laboratory at the University of Pennsylvania. Using ultraviolet and visible regions, he attempted to study the redox state of the cytochrome c oxidase (cyt c) to understand the behavior of the mitochondrial respiration [20]. As the photons in the ultraviolet and visible regions do not travel deep into the tissue, surgical exposure of tissue was needed. In open-skull animal setups, he had attempted to study the heme a component, the absorption peak of which appears in the orange-colored region at about 605 nm and the heme a3, in the violet region at about 445 nm. Jöbsis was puzzled by the observation that cyt c in skeletal muscle was less reduced than other tissues, and less reduced than cyt c obtained from isolated mitochondria. The disparity in the oxidation/reduction (redox) states of the respiratory chain between skeletal muscle and other tissues motivated him to study the in vivo behavior of cyt c in skeletal muscle. Using NIRS on skeletal muscle, he found that the copper atom associated with heme a3 did not respond to anoxia, and therefore may be reduced under normoxic conditions; whereas, the heme-a copper was at least partially reducible [19].
Unfortunately, the observation that Hb absorbs more intensely than cytochromes in the violet region made these measurements more difficult in intact organs under normal circulation conditions. In this sense, Jöbsis noticed that the NIR region was more appropriate to study the relative absorption strength of Hb and cyt c. He has presented his rationale for deciding to adopt NIR light for tissue monitoring as follows [21]. Briefly, he enjoyed a dinner with his family, the menu of which featured a grilled chuck roast on December 28, 1976. The very American cut of beef still contains part of the shoulder blade of the steer; a flat piece of bone perhaps 3- or 4-mm thick, about the same as the human skull. He held the pink object up against the light and noticed that the shadow of a finger could easily be noted in the diffuse red light coming through the bone. If the red light could, then certainly NIR light at the longer wavelengths would penetrate the human skull and provide access to the brain. In addition, it was possible that other tissues could also be monitored in a minimally invasive way [21].
In 1977, in Science [19], Jobsis reported Hb oxygenation, total-Hb, and cyt c under hypoxic conditions in the exposed heart and in the brain without surgical intervention. He demonstrated using animal preparations that oxygen sufficiency for cyt c can be recorded using NIR effectively. He also extended the experiment to the human head and successfully monitored the decrease in total-Hb in the brain by the hypocapnea induced by voluntary hyperventilation. Later, simultaneous measurements were made of both tissue oxygen tension (PtO2) and the redox ratio of cyt c from rat cerebral cortex, in situ. These studies showed that decreased PtO2 was accompanied by cyt c reduction [22]. Hoshi et al. developed a new approach for measuring the redox state of cyt c in the brain under normal blood-circulation conditions in rats [23]. When fractional inspired oxygen was decreased in a stepwise manner from 100 to < 10%, at which point the concentration of oxy-Hb [oxy-Hb] decreased by approximately 60%, cyt c started to reduce. Increases in arterial PO2 (PaO2) under hyperoxic conditions caused an increase in [oxy-Hb], whereas further oxidation of cyt c was not observed. The dissociation of the responses of Hb and cyt c was also clearly observed after the injection of epinephrine under severely hypoxic conditions; that is, cyt c was re-oxidized with increasing blood pressure, whereas Hb oxygenation was not changed. Using newly developed time-resolved NIR (NIRTRS), Chance reported that the intensity profile of photon migration in tissues permits determination of the path length and thus concentration changes in oxy-Hb/myoglobin (Mb) in the resting and ischemic muscle model [24]. NIRTRS emits short light pulses (100 ps) and counts photons, which are scattered and absorbed in the tissue. The distribution of the photons migrated follows the photon-diffusion equation and the mean light path length and tissue optical properties can be determined by solving the equation [24].
Basic principles for and operation of in vivo NIRS
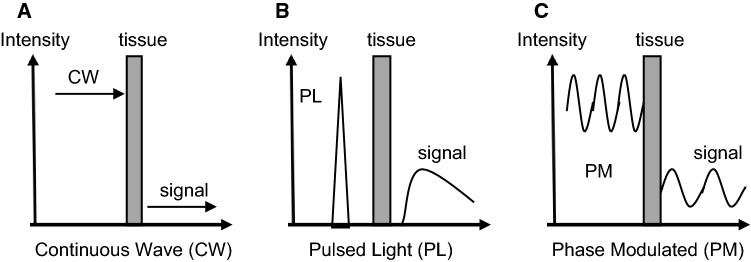
The NIR (700–2000 nm) shows much less scattering, and thus better penetration into biological tissue than visible light (360–700 nm). However, light absorption by water progressively increases at wavelengths above 900 nm, which limits the light penetration into tissue above these wavelengths in larger water- and lipid-containing biological tissue (a peak at around 976 nm and 927 nm, respectively). Thus, NIRS measurements usually adopt wavelengths in the range of 700–850 nm [19]. The major absorbing compounds of this wavelength region are intravascular Hb, intramuscular Mb, and mitochondrial cyt c. NIRS measurements (for example, 760 nm and 850 nm in this case) rely on O2-dependent absorption changes that occur in the heme in the brain, muscle, liver, etc. (Fig. 1). There are several types of NIRS devices: NIR single-distance continuous wave spectroscopy (NIRSDCWS), multi-distance continuous wave spectroscopy (NIRMDCWS), NIRTRS, phase modulation spectroscopy (NIRPMS) (Fig. 2), diffuse correlation spectroscopy (NIRDCS), and diffuse reflectance spectroscopy (NIRDRS) [25]. NIRDCS uses coherent NIR light to penetrate deep tissues and measures speckle fluctuations of the diffuse light, which are sensitive to the motions of red blood cells in tissues [8]. NIRDCS, a new rapidly developing technique, can continuously measure blood flow in the superficial muscles. NIRDRS methodology uses the unique approach of monitoring muscle blood flow (mBF) by measuring the optical phase shift caused by moving blood cells. While NIRTRS and NIRPMS are more accurate due to their ability to monitor changes in both absorption and scattering, they are limited in practicality by higher costs and complexity. This leaves the continuous wavelength devices (NIRSDCWS and NIRMDCWS) as affordable alternatives for measuring changes in oxygen levels in tissues, even if these devices cannot account for changes in scattering.
Fig. 1.
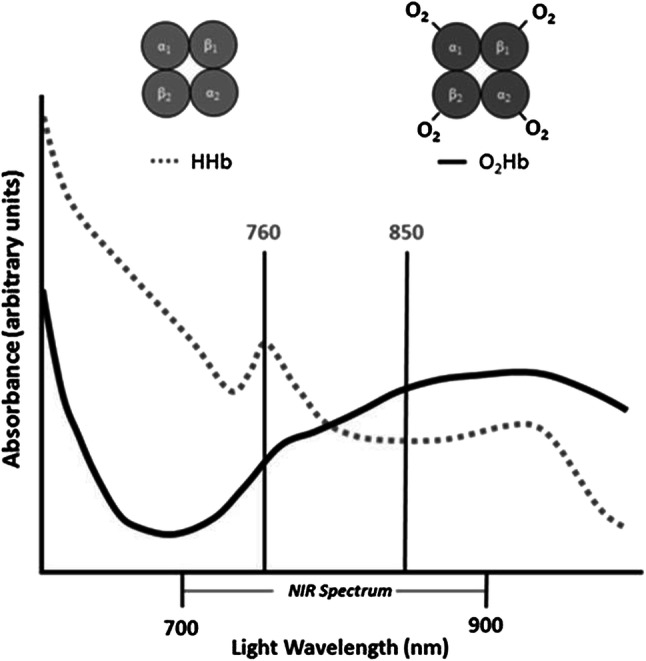
Changes in absorbance (optical density) in accordance to a wavelength. The optical density (absorption) increases at 760 nm when the oxygenated hemoglobin (O2Hb) is deoxygenated (HHb). When blood volume increases, the line shifts to the upper (increase in absorbance)
Copyright © 2017 Willingham and McCully from Ref. [14]
Fig. 2.
Schematic illustration of main types of near-infrared (NIR) spectroscopy instrumentation. a NIR continuous wave spectroscopy with single-distance (NIRSDCWS) or multi-distance (NIRMDCWS). b NIR time-resolved spectroscopy (NIRTRS). c NIR phase modulation spectroscopy (NIRPMS)
To measure wider areas of the limb and image regional differences in skeletal muscle oxygenation, continuous wavelength multi-channel or imaging devices were built and applied to exercise physiology [5, 26–30]. NIRDCS would be suitable to low-cost, user-friendly wearable/wireless 2D imagers in different pathophysiological conditions and in sports sciences [13]. NIRDRS can be used to reconstruct images of the internal distribution of optical absorption and scattering coefficients. However, NIRDRS requires physically accurate model, which is parameterized by the spatial distribution of scattering and absorption properties in the media [25]. The properties of the model need to be adjusted iteratively until the predicted measurements from the forward model match the physical measurements from the device [25].
To continuously monitor muscle oxygenation during untethered human locomotion in the field or a clinical setup, small portable NIRS devices have been built [31]. For example, a wireless NIRMDCWS system which is the size of a cell phone was developed by Artinis Medical Systems (the Netherlands) [32], which is one of the first commercially available wireless and portable devices using 2-channel and 6-laser diodes as a light source. A portable NIRMDCWS device has been developed by Astem Inc. (Japan), which consists of an LED light source, two photodiodes, microprocessors, and a Bluetooth networking module (less than 100 g) [33]. An American company has developed a small, inexpensive NIRS device (Moxy) for sports applications [34], which adopts Monte Carlo modeling and specifically measures Hb oxygen saturation (SO2) with 1-channel and 4-LEDs as a light source. Another American company has developed an even less expensive NIRS device for the athletic market using portable NIRMDCWS [35].
The pathlength of the light varies due to optical characteristics of tissue such as muscle, adipose tissue, blood volume, and motion artifact created by muscle contractions. NIRSDCWS can only provide the relative values of tissue oxygenation due to undeterminable optical path and its length, but is commercially available because of inexpensive and portable instrumentation. To calculate the relative changes in oxy-Hb/Mb, deoxy-Hb/Mb, and total-Hb/Mb, the equation of a 2-, or multiple-wavelength method can be applied according to the modified Beer–Lambert law [36]. Because of the difficulty in quantifying NIRSDCWS, muscle oxy-Hb/Mb, deoxy-Hb/Mb, and total-Hb/Mb are usually expressed in the following way:
Arbitrary units [OD],
μM × cm, or
μM (DPF × source-detector spacing),
where OD, optical density; DPF, differential pathlength factor defined by pathlength in terms of fold of the light source to detector distance.
It has been reported in a few studies that changes in pathlength were less than 10% during and after the end of arterial occlusion and during exercise [37–39]. DPF is determined in the thigh muscle and decreased slightly (around 5%), but significantly from baseline to peak cycle exercise [38]. There is still not an enough data available on whether and how much optical pathlength changes during varying interventions, such as arterial occlusion, muscular contractions, and recovery from hyperemia. The absolute optical path length can be measured by NIRTRS and NIRPMS [40]. NIRDCS and NIRDRS have been developed for measuring changes in muscle oxygenation and mBF, and are able to compute muscle oxygen consumption (mVO2) [41].
The similar absorption spectra of Hb and Mb make it difficult to differentiate the two by optical properties alone. What we know of the differences between the two molecules is based on 1proton-magnetic resonance spectroscopy (1H-MRS) using the water suppression to separate their deoxy forms [42]. Studies using 1H-MRS have suggested < 10% Hb and 90% Mb contribution to the overall NIR signals [43]. Computer modeling has quantified the Mb contribution to the NIRS signal in human muscle to be 85–95% of the total signal [44]. Attempts have been made to use wavelength shift analysis of the NIR signal from muscle tissue to determine relative concentrations of Hb and Mb [45]. Wavelength shift analysis found that Hb accounts for only 20% of the overall signal in human muscle, and the position of the NIR deoxy-heme peak at 760 nm is linear with Hb/Mb concentration ratio. A recent study has examined the question of the NIRS signal origin by measuring simultaneously the 1H-MRS, 31phosphorus (31P)-MRS, and NIRS signals in finger flexor muscles during the transition from rest to contraction, recovery, ischemia, and reperfusion. The experiment results support a predominant Mb contribution to the NIRS signal from muscle [46]. Most experimental analysis often referred a study by Seiyama et al. [47], which reported on the comparative NIRS observation with and without a blood substitute. With a blood substitute, the NIRS signal decreases precipitously, which leads to the supposition that Hb contributes predominantly to the NIR signal. Yet, recent studies with buffer perfused hindlimb and with a proper physiologically monitored conditions show that NIRS can still detect a robust signal [48, 49]. Additional studies will be needed to clarify not only the contribution of Mb/Hb to the NIR signal, but also the relative kinetics of Mb and Hb deoxygenation during a variety of interventions under different conditions.
When using reflected light, that is the path of light from source and detector pair which is sent orthogonally into the tissue, the pattern of the continuous light path follows a “banana shaped” curve. The penetration depth into the tissue is approximately equal to half the distance between the light source and the detector [50]. In a study using NIRTRS [51], the mean depth of the light penetration was found to be greater (approximately 20 mm at a 30-mm optode separation) than half of the emitter–detector separation reported in many previous studies [48], mainly due to the time gating for photon counting designed for selectively collecting photons in a deeper tissue. The volume of tissue being evaluated using continuous light and 3-cm separation distance was estimated to be ~ 4 cm3 in the milk model by Chance et al. [52], when it is assumed that the volume of interest be a rotation body of the ellipse revolved across 180° or a hemisphere (Fig. 3a). If a banana-like pattern is assumed rather than a hemisphere [53], this volume might be overestimated (Fig. 3b). On the other hand, the volume of interest would be greater when measured by NIRTRS [51].
Fig. 3.
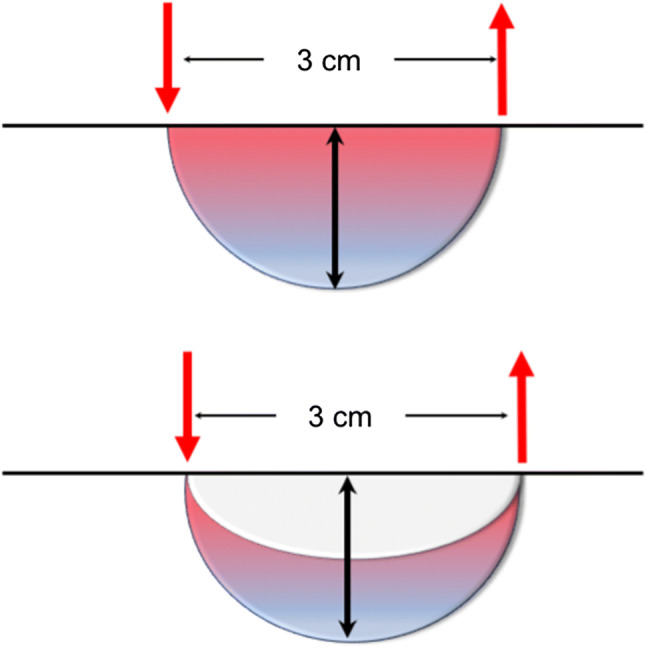
Schematic illustration of the volume of tissue being measured. a The volume of tissue being evaluated using continuous light and 3-cm separation distance was estimated to be ~ 4 cm3, when it is assumed that the volume of interest be a rotation body of the ellipse revolved across 180° or a hemisphere. b If a banana-like pattern is assumed rather than a hemisphere, the volume might be smaller
Relative versus absolute values from NIRS measurements
The reason why NIRCWS cannot determine the absolute value of oxygenation using reflected light is the unknown path length of NIR light in the tissue, which is generally assumed to be a banana shape as discussed above. The presence of skin and adipose tissue (subcutaneous adipose tissue thickness: SATT) above the muscle will increase penetration depth, as a result of the reduced absorption of light due to the reduced concentrations of heme in the SATT. Conversely, increased blood volume in the skeletal muscle will increase light absorption and reduce penetration depth. Melanin in the skin also absorbs light in the NIR region, such that darker skin can reduce reflected light intensity and penetration depth [54].
Absolute values from NIRS can be measured using NIRTRS [24, 25, 39] and NIRPMS [55–58]. NIRTRS emits short light pulses (100 ps) and counts photons in a several cm apart from the light emission on the skin surface. The emitted photons are scattered and absorbed in the tissue and arrive at the detectors with a varying timing, which depends on the path of each photon travels. The photons that travel shallower arrive earlier, while those that travel deeper arrive later. The distribution of the photons migrated follows the photon-diffusion equation and the light path length, absorption coefficient, and reduced scattering coefficient can be computed by solving the equation. NIRPMS, a frequency-domain method, usually uses intensity-modulated light at a radio frequency from 50 MHz to 1 GHz and monitors the migrated light intensity (DC), amplitude (AC), and phase shift, which comprises the time of the light travelled in the tissue. Intensity-modulated light propagates through tissue with a coherent front, forming photon-density wave. The detected photon-density wave is delayed because of the phase velocity of the wave being altered by the optical characteristics of the tissue. In a study, it is suggested that the absolute scattering and absorption coefficients can be accurately determined by the combination of the phase shift and the DC with relationships provided by diffusion theory [58].
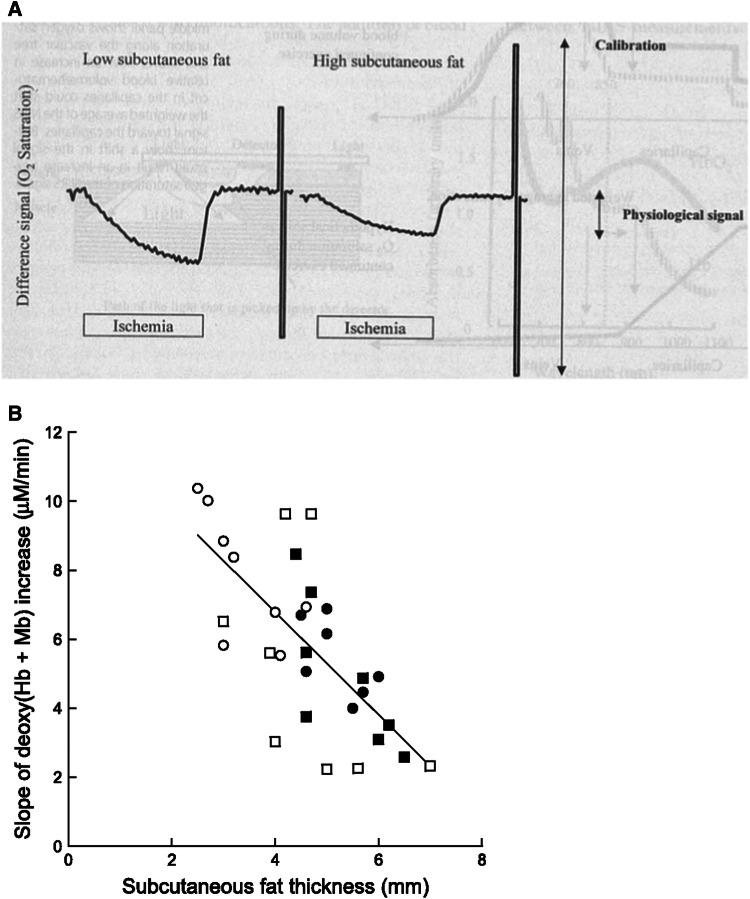
There are several studies which used NIRTRS for monitoring muscle deoxygenation during resting arterial occlusion and exercise [38, 59–61]. Although NIRTRS can quantitatively measure muscle oxygenation, the values are still influenced by the SATT and tissue heterogeneity. The reason is that the measured oxygenation/deoxygenation values would be diluted by the interference of the lower basal Hb/Mb concentration and/or lower metabolic rates in the SATT [1] (Fig. 4a). The relationship between the slope (S) of change in [deoxy(Hb/Mb)] and SATT (mm) was examined and attempted to be expressed the following equation: S = 12.73–1.49·SATT, which might be case sensitive for each experiment (Fig. 4b) [62]. However, this technique does not always overcome the accurate quantification of tissue oxygenation [62]. Thus, a further research needs to be warranted to quantify tissue oxygenation using NIRTRS.
Fig. 4.
The influence of subcutaneous adipose tissue thickness on the near-infrared signals (a). b Relationship between slope (S) of increase in muscle deoxy-[hemoglobin/myoglobin (Hb/Mb)] during the ischemia occlusion test and the subcutaneous adipose tissue thickness (ATT) for all subjects: S = 12.73–1.49·ATT (r = 0.71, P < 0.01). Note that the greater the ATT, the more the slope was attenuated. Open and closed circles denote the deoxygenation responses of the vastus lateralis (VL) and rectus femoris (RF) muscles at the distal sites, respectively. Open and closed squares denote the deoxygenation responses of the VL and RF muscles at the proximal sites, respectively
a Copyright © 2000 The Amercian College of Sports Medicine. Adopted from Ref. [1]. b Copyright © The American Physiological Society. Reproduced by permission of the publisher. Adopted from Ref. [61]
Needs for noninvasive measurements of muscle oxidative metabolism
Skeletal muscle possesses two major biochemical processes for synthesizing ATP, namely oxidative phosphorylation and anaerobic ATP production (PCr and glycolytic pathways). During a low-to-moderate intensity of exercise at which we normally perform in a daily activity, skeletal muscle primarily relies on oxidative metabolism. During exercise, skeletal mVO2 can raise 50-fold with abrupt increases in O2 delivery (DO2) of up to tenfold [63, 64]. Due to the strong dependence of skeletal muscle on oxidative metabolism, improvements in the oxidative system of the body lead to higher performance in an athletic event. On the other hand, impairments of VO2 and/or DO2 will limit exercise performance leading to a functional deterioration. Invasive methods have been applied to evaluate muscle oxidative metabolism, including peripheral and cardiorespiratory measurements. Peripheral measurements include tissue O2 microelectrodes, Mb O2 saturation by spectrophotometric analysis [65], and NADH analysis from exposed tissue surfaces [66]. In an alternative approach to evaluating muscle oxidative metabolism, MRS has been developed to measure in vivo active forms of high-energy phosphorus metabolites and intramuscular pH [67, 68]. Since these studies, MRS has evolved into the “gold standard” for noninvasive detection of skeletal muscle bioenergetics. The limitation to 31P-MRS is that it is relatively expensive option to standard 1H-MRS systems and is not readily available. In addition, changes in high-energy phosphorus metabolites are influenced by both the delivery of oxygen as well as oxidative capacity, making the interpretation of the results somewhat imprecise [69]. In comparison to MRS methodology, the strength of NIRS for measuring skeletal muscle oxidative metabolism is that it is relatively inexpensive and portable, making it far more assessable. NIRS devices can make biochemical measurements at frequent intervals on even frail or vulnerable populations [70], and can be employed in both laboratory- and field-based studies. The ability to collect data during human locomotion is a major reason NIRS lends itself to the study of exercise and athletic performance.
Acceptable approaches for the in vivo muscle NIRS evaluation
A number of studies have reported the validity of NIRS-measured oxy-Hb/Mb and deoxy-Hb/Mb signals in animals and humans under steady-state conditions by comparing these signals with venous blood [31, 39, 71–73]. Thus, it is generally accepted that NIRS-oxygenation/deoxygenation signal has a considerable agreement with the changes in venous saturation under varying oxygenation status of the human muscles. However, there have been a few studies that have failed to validate NIRS measurements especially under non-steady-state conditions [74, 75]. A possible explanation for the discrepancies found in these studies is that the NIRS signal contains information from arterioles, capillaries, venules, and intracellular Mb. The O2 gradient from an arteriole to a venule is large in normoxic conditions such that variations in blood volume from arteriole to venule could alter the NIRS signal without change in venous oxygen signals [1]. The lower oxygen levels during hypoxic conditions would reduce this effect and produce a good agreement with values determined by NIRS and blood samples [1]. However, as it is suggested < 10% Hb and 90% Mb contribution to the overall NIR signals [44, 46], we have to carefully consider the above-mentioned interpretation.
One of the challenges of measuring muscle oxygenation levels during exercise concerns comparing oxygen levels between individuals with different SATT values [76]. People with higher SATT values will have lower oxygen levels and lower metabolic rates. Appropriate and acceptable approaches for accounting for the effect of SATT values on muscle oxygenation are described as the following: (1) directly measure the absolute value in the absorption and scattering coefficient computing the optical path length and absolute Hb/Mb parameters using NIRTRS and NIRPMS, (2) normalize signal using a physiological calibration by determining muscle O2 store (0–100% level of oxygenation) and oxidative metabolic rate during arterial occlusion, (3) adjust oxygen levels using a calibration equation that includes measurements of SATT, and (4) perform kinetics measurements at the onset of or following muscle contractions or muscle contractions either voluntarily or by electrical stimulation. The kinetic measurements are acceptable because only the muscle tissue responds with increased metabolic activity and the kinetic measurements are reported with rate constants in units of time (or 1/time).
Physiological calibration and oxygen consumption measurements by arterial occlusion
In vivo NIRSDCWS provides relative changes in oxygen levels due to the inability to correct for the effects of scattering related to varying amounts of SATT and unknown path lengths of light [1]. A simple and common method for calibrating NIRSDCWS signals is “physiological calibration” to use the range of muscle deoxygenation caused by arterial occlusion followed by reactive hyperemia [50]. The arterial occlusion method is based on the assumptions that a period of ischemia will result in the complete disappearance of oxy-Hb/Mb in a measurement area, and that the reactive hyperemia after occlusion will almost completely eliminate deoxy-Hb/Mb. While oxy-Hb/Mb and deoxy-Hb/Mb in arbitrary units may vary between measurement sites and individuals, the arterial occlusion calibration adjusts for these changes making inter-individual or inter-occasion comparisons possible. The units of measurement for the NIRS device now become %Hb/MbO2, and the values vary from 0 to 100%. This is in contrast to the units provided by the NIRSDCWS devices provided by Artinis, LTDs that report units in tissue oxygen index (TSI) values. TSI values are also presented in units of %, but because the TSI is a percentage of ratios of absorbance, the values typically have a maximal range of ~ 35 to ~ 75% as absorption at the two separate wavelengths can never reach zero even if the concentration of oxygenated or deoxygenated heme is zero. An important consideration when performing a physiological calibration is the duration of the ischemic period. Starting from resting conditions, 5–6 min of ischemia is sufficient to reach functionally zero oxygen levels in most humans [50, 77] because phosphocreatine (PCr) begins to decline after this amount of time. The use of a short duration of exercise prior to inflation of the cuff increases metabolic rate, and reduces the time to reach zero oxygen levels to 3–4 min [78]. Leaving the ischemic period long enough to confirm zero oxygen levels will also result in some depletion of PCr. The result of this is that there will be some post ischemia oxygen consumption. This oxygen consumption will slow the rate of return of oxygen levels compared to reactive hyperemia without an oxygen debt. If the investigator wants to use an ischemic period to induce and measure reactive hyperemia, then a balance must be considered between the need to confirm zero oxygen levels to obtain the physiological calibration, and the need to avoid phosphocreatine depletion and post ischemic oxygen consumption delaying the recovery of oxygen levels. Quantitative calibration of NIRSDCWS signal is possible in a combination with MRS measurement by applying a 15-min period of ischemia to the muscles (Fig. 5) [77]. The rate of decline of muscle oxy-Hb/Mb during ischemia can be compared with that of muscle phosphocreatine (PCr) in mM per second or a conversion to mM O2 per second. Interestingly, muscle metabolic rate appears to remain constant in the presence or absence of oxygen. As a result, this method provides quantitative values of both muscle oxygen stores and mVO2.
Fig. 5.
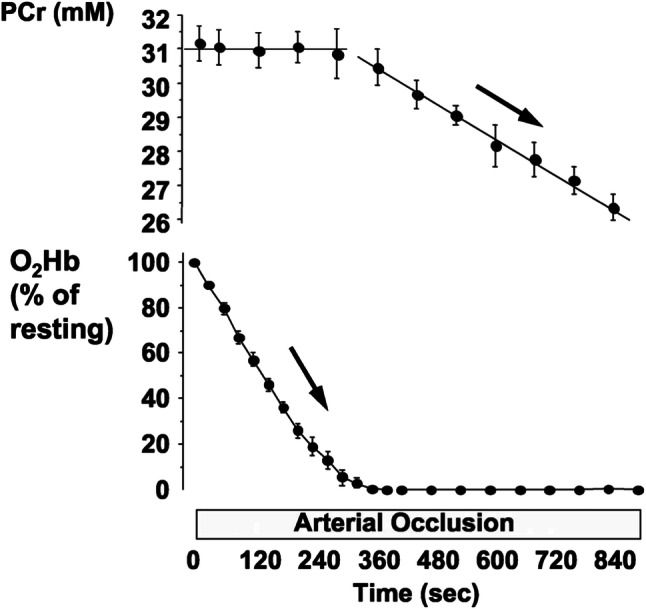
Changes in phosphocreatine (PCr) and oxygenation in human forearms during 15 min of arterial occlusion measured by near-infrared and 31P-magnetic resonance spectroscopy. No significant changes were found in pH or ATP throughout arterial occlusion
Copyright © The American Physiological Society. Reproduced by permission of the publisher. Adopted from Ref. [77]
Evaluation of muscle energy metabolism using NIRS is difficult because the measured oxygenation levels do not specifically reflect mVO2, rather they reflect the balance between muscle DO2 and mVO2. To distinguish mVO2 from DO2 using NIRS, two approaches have been used; the transient arterial occlusion method and the venous occlusion method. The transient occlusion uses 10–30 s of arterial occlusion provided by a pneumatic tourniquet. With no blood entering or leaving the tissue of interest, changes in NIRS-measured oxygen levels now reflect mVO2 [76, 77, 79–82]. Resting mVO2 of young health males determined by NIRSDCW was found to be a small amount of variability (23.0 ± 1.2%/min) [70], and to be consistent between studies by different investigators [83]. NIRTRS has also been used to measure resting mVO2, providing results in absolute units (0.82 μM s−1) [39]. In the former study, a significant correlation was found between NIRSDCW measured mVO2 and MRS measured PCr (r2 = 0.99, p < 0.01), and ADP (r2 = 0.98, p < 0.01) concentrations. The linear relationships between the NIRS and MRS measured indicators supports both the thermodynamic [84, 85] and the kinetic [86] regulation models of muscle mitochondrial respiration. There is an intimate relationship between NIRS-measured mVO2 using the transient arterial occlusion method and the rate of PCr recovery, a biochemical process of ATP resynthesis via oxidative phosphorylation after muscle contractions [82]. These studies suggest that the initial rate of muscle deoxygenation during transient arterial occlusion is a direct measure of mVO2. Venous occlusion has also been used with NIRS [87]. The change in NIRS signals with patent arteries and venous occlusion is similar to venous plethysmography [88, 89]. In this approach, venous occlusion can provide measurements of muscle blood flow.
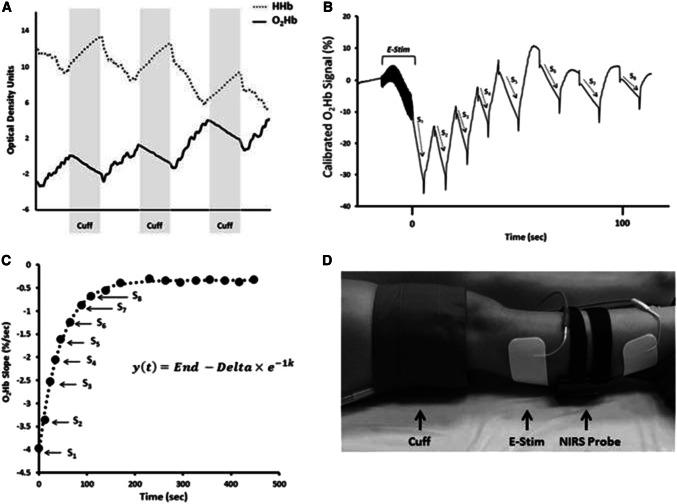
Repeated transient arterial occlusions after exercise can provide successive mVO2 values, and the recovery of mVO2 values after exercise provides information that is basically same as that determined from the kinetics of PCr levels after exercise [90, 91]. The recovery kinetics of PCr has been developed as a method of measuring mitochondrial capacity [69, 84, 92–94]. Thus, time constant (Tc) or rate constant k (1/Tc) for mVO2 recovery is an indicator for evaluating muscle oxidative capacity (Fig. 6) [14, 95]. The advantages of the NIRS method of monitoring the kinetics of mVO2 compared to the 31P-MRS measuring the kinetics of PCr are that in addition to the lower cost, greater availability, and greater ease of use, recovery of mVO2 can be made independent of blood flow limitations while recovery of PCr is not. The measurements of mVO2 are made during transient ischemia and thus do not depend on oxygen delivery. However, caution must be taken to make sure that oxygen levels are high enough in the tissue to assure that mVO2 is not oxygen limited [96].
Fig. 6.
a Changes in oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) during periods of cuff-induced ischemia. b NIRS O2Hb kinetics during a series of arterial occlusions following 15-s electrical stimulation (E-Stim). c Slope values from NIRS O2Hb recovery kinetics plotted over time and fit to exponential equation. In this equation, y is the relative rate of oxygen metabolism, End is the rate of oxygen metabolism at the end of exercise (S1), and Δ is the difference between the rates of resting oxygen metabolism and End. The rate constant, k, is used an index of muscle mitochondrial capacity. d NIRS set up for assessment of mitochondrial capacity in the gastrocnemius
Copyright © 2017 Willingham and McCully from Ref. [14]
Sensitivity adjustment by measuring SATT
Muscle NIR signal intensity is greatly influenced by overlying SATT [1, 97, 98]. SATT values can vary from 3 mm over the forearm muscles of lean subjects, to greater than 20 mm over the vastus lateralis muscles of obese subjects. In addition to increasing penetration depth, increasing SATT also reduces the signal intensity coming from the deeper tissues, assumed to be skeletal muscle. For example, a SATT thickness of 5 mm reduces the signal intensity by approximately 20% with a light source–detector separation being 30–40 mm. The use of shorter separation distances, 15 or 20 mm with a 5-mm fat thickness, attenuates the signal intensity by 30 and 60%, respectively [26]. Perhaps of more importance, SATT values greater than 10 mm attenuate the signal from the deeper muscle by over 90% [98, 99], making the study of people with obesity very difficult. A correction equation based on optical properties has been used to adjust values of absolute StO2 based on SATT values [100]. SATT independently confounds NIRMDCWS-derived StO2 by overestimating actual skeletal muscle oxygenation and by decreasing the magnitude of exercise-induced changes in oxygen levels. Several available NIRS units use the multiple source–detector pairs to separate out signals primarily from skeletal muscle from signals coming from SATT [39, 101, 102].
Kinetics parameter measurements
There has been a lot of research interest in the study of onset and offset kinetics of muscle oxidative metabolism during exercise [103, 104]. Muscle oxygenation determined by NIRS has been used to provide relevant information on onset and offset kinetics [46]. Early studies measured changes deoxy-Hb/Mb signals during ramp cycling exercise to differentiate trained cyclists from physically inactive subjects [105]. The rate of deoxygenation at the onset of exercise [72, 106] recovery time of muscle reoxygenation after submaximal to maximal exercise [41, 107–112] and the rate of reoxygenation after brief high-intensity exercise [112] are among indicators for evaluating muscle oxidative capacity. These studies have reported a good agreement between faster PCr recovery kinetics and faster oxygenation kinetics measured with NIRS [113]. A different outcome was obtained after maximal short-duration isometric exercise, where higher oxidative capacity muscle (faster PCr kinetics) was inversely related to the rate of muscle reoxygenation after the exercise. [114]. The result of this study was attributed to the hypothesis that muscle reoxygenation rate after this type of short high-intensity exercise may be influenced more by VO2 than by DO2, when O2 demand is still high and O2 supply is not fully activated. In a study, a new method was proposed to noninvasively approximate muscle capillary blood flow kinetics from the kinetics of the primary component of pulmonary O2 uptake and deoxy-Hb/Mb in humans during exercise [101].
Muscle blood flow has been measured using the kinetic changes in oxygen levels after reactive hyperemia [115]. As mentioned earlier, if muscle oxygen levels are reduced to near zero by 5–6 min of ischemia, without significantly lowering PCr levels, then the return of oxygen levels with reactive hyperemia reflects the indicator wash-in methods used to measure muscle blood flow. Several studies have used these approaches by measuring the rate of recovery of oxygen saturation with NIRS to evaluate blood flow in the calf muscles of people with peripheral arterial disease [114, 115].
Conclusions and perspectives
Observations of changes in the absorption of light with oxygen levels have been recognized as early as 1876. Gradual advances lead to a milestone paper by Jöbsis in 1977 illuminating the potential of NIRS to study tissue oxygen levels. Subsequent studies have shown NIRS to be useful for the in vivo evaluation of changes in muscle oxygenation and oxidative metabolism during these 40 years. The most practical and inexpensive devices use continuous wave light, which requires assumptions related to changes in scattering to work successfully. More expensive devices using phase-modulated or pulsed light can monitor both absorption and scattering, and can provide more accurate signals under a wider range of conditions. Compared to other imaging methods, NIRS provides excellent time resolution, and multiple source–detector pairs can be used to provide low-resolution imaging. To image whole body activity, we might invent NIRS device embedded in exercise clothing, which can be also used outside the laboratory with the help of energy harvesting technologies (solar batteries, light source with sunlight and band pass filters). NIRS device will be used in combination with magnetic resonance imaging (MRI), MRS, electromyogram (EMG), respiratory gas analysis, etc. However, along with applied clinical studies, basic research is still needed on topics such as the origin of the NIR signals, the NIR penetration depth or measurement area in tissue including the effect of non-muscular tissue, changes in optical properties during wide range of oxygenation status, varying subjects, and exercise modality. Continued development of NIRS devices in terms of lower cost, better detection of both absorption and scattering, and smaller size will lead to a promising future for NIRS studies.
Author contributions
TH wrote about early development of near-infrared spectroscopy and methodological section and organized throughout the manuscript. KKMC wrote about application of near-infrared spectroscopy to sports and clinical science.
Funding
This study was supported by JSPS KAKENHI Grant Number 15H03100, Japan.
Compliance with ethical standards
Conflict of interest
Takafumi Hamaoka declares that he has no conflict of interest. Kevin K. McCully is the President of Infrared Rx, Inc, and NIRS software company.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCully KK, Hamaoka T. Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev. 2000;28(3):123–127. [PubMed] [Google Scholar]
- 2.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bülow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sport. 2001;11(4):213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 3.Quaresima V, Lepanto R, Ferrari M. The use of near infrared spectroscopy in sports medicine. J Sport Med Phys Fit. 2003;43(1):1–13. [PubMed] [Google Scholar]
- 4.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 5.Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;2:62105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci. 2011;369(1955):4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 7.Hamaoka T, McCully KK, Niwayama M, Chance B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments. Philos Trans A Math Phys Eng Sci. 2011;369(1955):4591–4604. doi: 10.1098/rsta.2011.0298. [DOI] [PubMed] [Google Scholar]
- 8.Grassi B, Quaresima V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt. 2016;21(9):091313. doi: 10.1117/1.JBO.21.9.091313. [DOI] [PubMed] [Google Scholar]
- 9.Adami A, Rossiter HB. Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy. J Appl Physiol. 2018;124(1):245–248. doi: 10.1152/japplphysiol.00445.2017. [DOI] [PubMed] [Google Scholar]
- 10.Chung S, Nelson MD, Hamaoka T, Jacobs RA, Pearson J, Subudhi AW, Jenkins NT, Bartlett MF, Fitzgerald LF, Miehm JD, Kent JA, Lucero AA, Rowlands DS, Stoner L, McCully KK, Call J, Rodriguez-Miguelez P, Harris RA, Porcelli S, Rasica L, Marzorati M, Quaresima V, Ryan TE, Vernillo G, Millet GP, Malatesta D, Millet GY, Zuo L, Chuang CC. Commentaries on viewpoint: principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy. J Appl Physiol. 2018;124(1):249–255. doi: 10.1152/japplphysiol.00857.2017. [DOI] [PubMed] [Google Scholar]
- 11.Wolf M, Ferrari M, Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt. 2007;12(6):062104. doi: 10.1117/1.2804899. [DOI] [PubMed] [Google Scholar]
- 12.Rooks CR, Thom NJ, McCully KK, Dishman RK. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol. 2010;92(2):134–150. doi: 10.1016/j.pneurobio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Perrey S, Ferrari M. Muscle oximetry in sports science: a systematic review. Sport Med. 2018;48(3):597–616. doi: 10.1007/s40279-017-0820-1. [DOI] [PubMed] [Google Scholar]
- 14.Willingham TB, McCully KK. In vivo assessment of mitochondrial dysfunction in clinical populations using near-infrared spectroscopy. Front Physiol. 2017;8:689. doi: 10.3389/fphys.2017.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vierordt K. Die quantitative Spektralanalyse in ihrer Anwendung auf Physiologie, Physik, Chemie und Technologie. Tübingen: H Lauppsche Buchhandlung; 1876. [Google Scholar]
- 16.Drabkin DL, Austin JH. Spectrophotometric studies: I. Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J Biol Chem. 1932;98:719–733. [Google Scholar]
- 17.Millikan GA. A simple photoelectric colorimeter. J Physiol. 1933;79(2):152–157. doi: 10.1113/jphysiol.1933.sp003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Severinghaus JW. Takuo Aoyagi: discovery of pulse oximetry. Anesth Analg. 2007;105(6):S1–S4. doi: 10.1213/01.ane.0000269514.31660.09. [DOI] [PubMed] [Google Scholar]
- 19.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 20.Mills E, Jöbsis FF. Simultaneous measurement of cytochrome a3 reduction and chemoreceptor afferent activity in the carotid body. Nature. 1970;225(5238):1147–1149. doi: 10.1038/2251147a0. [DOI] [PubMed] [Google Scholar]
- 21.Jöbsis FF. Discovery of the near-infrared window into the body and the early development of near-infrared spectroscopy. J Biomed Opt. 1999;4(4):392–396. doi: 10.1117/1.429952. [DOI] [PubMed] [Google Scholar]
- 22.Kreisman NR, Sick TJ, LaManna JC, Rosenthal M. Local tissue oxygen tension-cytochrome a, a3 redox relationships in rat cerebral cortex in vivo. Brain Res. 1981;218(1–2):161–174. doi: 10.1016/0006-8993(81)91298-1. [DOI] [PubMed] [Google Scholar]
- 23.Hoshi Y, Hazeki O, Kakihana Y, Tamura M. Redox behavior of cytochrome oxidase in the rat brain measured by near-infrared spectroscopy. J Appl Physiol. 1997;83(6):1842–1848. doi: 10.1152/jappl.1997.83.6.1842. [DOI] [PubMed] [Google Scholar]
- 24.Chance B, Nioka S, Kent J, McCully K, Fountain M, Greenfeld R, Holtom G. Time-resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Anal Biochem. 1988;174(2):698–707. doi: 10.1016/0003-2697(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage. 2012;63(2):921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Niwayama M, Yamamoto K, Kohata D, Hirai K, Kudo N, Hamaoka T, Kime R, Katsumura T. A 200-channel imaging system of muscle oxygenation using CW near-infrared spectroscopy. IEICE Trans Inf Syst. 2002;E85-D:115–123. [Google Scholar]
- 27.Yamamoto K, Niwayama M, Kohata D, Kudo N, Hamaoka T, Kime R, Katsumura T. Functional imaging of muscle oxygenation using 200-channel CW-NIRS system. Proc SPIE. 2001;4250:142–152. doi: 10.1117/12.434486. [DOI] [Google Scholar]
- 28.Quaresima V, Colier WN, van der Sluijs M, Ferrari M. Nonuniform quadriceps O2 consumption revealed by near infrared multipoint measurements. Biochem Biophys Res Commun. 2001;285(4):1034–1039. doi: 10.1006/bbrc.2001.5292. [DOI] [PubMed] [Google Scholar]
- 29.Miura H, McCully K, Nioka S, Chance B. Relationship between muscle architectural features and oxygenation status determined by near infrared device. Eur J Appl Physiol. 2004;91(2–3):273–278. doi: 10.1007/s00421-003-0964-6. [DOI] [PubMed] [Google Scholar]
- 30.McCully KK. The influence of passive stretch on muscle oxygen saturation. Adv Exp Med Biol. 2010;662:317–322. doi: 10.1007/978-1-4419-1241-1_45. [DOI] [PubMed] [Google Scholar]
- 31.Shiga T, Tanabe K, Nakase Y, Shida T, Chance B. Development of a portable tissue oximeter using near infra-red spectroscopy. Med Biol Eng Comput. 1995;33(4):622–626. doi: 10.1007/BF02522525. [DOI] [PubMed] [Google Scholar]
- 32.Buchheit M, Laursen PB, Ahmaidi S. Effect of prior exercise on pulmonary O2 uptake and estimated muscle capillary blood flow kinetics during moderate-intensity field running in men. J Appl Physiol. 2009;107(2):460–470. doi: 10.1152/japplphysiol.91625.2008. [DOI] [PubMed] [Google Scholar]
- 33.Niwayama M, Sone S, Murata H, Yoshida H, Shinohara S. Errors in muscle oxygenation measurement using spatially-resolved NIRS and its correction. J Jpn Coll Angiol. 2007;47:17–20. [Google Scholar]
- 34.Crum EM, O’Connor WJ, Van Loo L, Valckx M, Stannard SR. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur J Sport Sci. 2017;17(8):1037–1043. doi: 10.1080/17461391.2017.1330899. [DOI] [PubMed] [Google Scholar]
- 35.Farzam P, Starkweather Z, Franceschini MA. Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle. Physiol Rep. 2018;6(7):e13664. doi: 10.14814/phy2.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seiyama A, Hazeki O, Tamura M. Simultaneous measurement of haemoglobin oxygenation of brain and skeletal muscle of rat in vivo by near-infrared spectrophotometry. Adv Exp Med Biol. 1987;215:291–295. doi: 10.1007/978-1-4684-7433-6_32. [DOI] [PubMed] [Google Scholar]
- 37.Ferrari M, Wei Q, Carraresi L, De Blasi RA, Zaccanti G. Time-resolved spectroscopy of the human forearm. J Photochem Photobiol B. 1992;16(2):141–153. doi: 10.1016/1011-1344(92)80005-G. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira LF, Hueber DM, Barstow TJ. Effects of assuming constant optical scattering on measurements of muscle oxygenation by near-infrared spectroscopy during exercise. J Appl Physiol. 2007;102(1):358–367. doi: 10.1152/japplphysiol.00920.2005. [DOI] [PubMed] [Google Scholar]
- 39.Hamaoka T, Katsumura T, Murase N, Nishio S, Osada T, Sako T, Higuchi H, Kurosawa Y, Shimomitsu T, Miwa M, Chance B. Quantification of ischemic muscle deoxygenation by near infrared time-resolved spectroscopy. J Biomed Opt. 2000;5(1):102–105. doi: 10.1117/1.429975. [DOI] [PubMed] [Google Scholar]
- 40.Quaresima V, Ferrari M, Franceschini MA, Hoimes ML, Fantini S. Spatial distribution of vastus lateralis blood flow and oxyhemoglobin saturation measured at the end of isometric quadriceps contraction by multichannel near-infrared spectroscopy. J Biomed Opt. 2004;9(2):413–420. doi: 10.1117/1.1646417. [DOI] [PubMed] [Google Scholar]
- 41.Yu G, Durduran T, Lech G, Zhou C, Chance B, Mohler ER, 3rd, Yodh AG. Time-dependent blood flow and oxygenation in human skeletal muscles measured with noninvasive near-infrared diffuse optical spectroscopies. J Biomed Opt. 2005;10(2):024027. doi: 10.1117/1.1884603. [DOI] [PubMed] [Google Scholar]
- 42.Wang DJ, Nioka S, Wang Z, Leigh JS, Chance B. NMR visibility studies of N-delta proton of proximal histidine in deoxyhemoglobin in lysed and intact red cells. Magn Reson Med. 1993;30(6):759–763. doi: 10.1002/mrm.1910300616. [DOI] [PubMed] [Google Scholar]
- 43.Tran TK, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole P, Kuno S, Jue T. Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am J Physiol. 1999;276(6 Pt 2):R1682–R1690. doi: 10.1152/ajpregu.1999.276.6.R1682. [DOI] [PubMed] [Google Scholar]
- 44.Davis ML, Barstow TJ. Estimated contribution of hemoglobin and myoglobin to near infrared spectroscopy. Respir Physiol Neurobiol. 2013;186(2):180–187. doi: 10.1016/j.resp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Marcinek DJ, Amara CE, Matz K, Conley KE, Schenkman KA. Wavelength shift analysis: a simple method to determine the contribution of hemoglobin and myoglobin to in vivo optical spectra. Appl Spectrosc. 2007;61(6):665–669. doi: 10.1366/000370207781269819. [DOI] [PubMed] [Google Scholar]
- 46.Bendahan D, Chatel B, Jue T. Comparative NMR and NIRS analysis of oxygen-dependent metabolism in exercising finger flexor muscles. Am J Physiol Regul Integr Comp Physiol. 2017;313(6):R740–R753. doi: 10.1152/ajpregu.00203.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seiyama A, Hazeki O, Tamura M. Noninvasive quantitative analysis of blood oxygenation in rat skeletal muscle. J Biochem. 1988;103(3):419–424. doi: 10.1093/oxfordjournals.jbchem.a122285. [DOI] [PubMed] [Google Scholar]
- 48.Masuda K, Takakura H, Furuichi Y, Iwase S, Jue T. NIRS measurement of O2 dynamics in contracting blood and buffer perfused hindlimb muscle. Adv Exp Med Biol. 2010;662:323–328. doi: 10.1007/978-1-4419-1241-1_46. [DOI] [PubMed] [Google Scholar]
- 49.Takakura H, Masuda K, Hashimoto T, Iwase S, Jue T. Quantification of myoglobin deoxygenation and intracellular partial pressure of O2 during muscle contraction during haemoglobin-free medium perfusion. Exp Physiol. 2010;95(5):630–640. doi: 10.1113/expphysiol.2009.050344. [DOI] [PubMed] [Google Scholar]
- 50.Chance B, Dait MT, Zhang C, Hamaoka T, Hagerman F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol. 1992;262:C766–C775. doi: 10.1152/ajpcell.1992.262.3.C766. [DOI] [PubMed] [Google Scholar]
- 51.Gunadi S, Leung TS, Elwell CE, Tachtsidis I. Spatial sensitivity and penetration depth of three cerebral oxygenation monitors. Biomed Opt Express. 2014;5(9):2896–2912. doi: 10.1364/BOE.5.002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chance B, Leigh JS, Miyake H, Smith DS, Nioka S, Greenfeld R, Finander M, Kaufmann K, Levy W, Young M, Cohen P, Yoshida H, Boretsky R. Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proc Natl Acad Sci USA. 1988;85(14):4971–4975. doi: 10.1073/pnas.85.14.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui W, Kumar C, Chance B. Experimental study of migration depth for the photons measured at sample surface. I. Time resolved spectroscopy and imaging. Proc SPIE Int Soc Opt Eng. 1991;1431:180–191. [Google Scholar]
- 54.Wassenaar EB, Van den Brand JG. Reliability of near-infrared spectroscopy in people with dark skin pigmentation. J Clin Monit Comput. 2005;19(3):195–199. doi: 10.1007/s10877-005-1655-0. [DOI] [PubMed] [Google Scholar]
- 55.Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol. 1995;40(2):295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- 56.Franceschini MA, Boas DA, Zourabian A, Diamond SG, Nadgir S, Lin DW, Moore JB, Fantini S. Near-infrared spiroximetry: noninvasive measurements of venous saturation in piglets and human subjects. J Appl Physiol. 2002;92(1):372–384. doi: 10.1152/jappl.2002.92.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf M, Wolf U, Choi JH, Gupta R, Safonova LP, Paunescu LA, Michalos A, Gratton E. Functional frequency-domain near-infrared spectroscopy detects fast neuronal signal in the motor cortex. Neuroimage. 2002;17(4):1868–1875. doi: 10.1006/nimg.2002.1261. [DOI] [PubMed] [Google Scholar]
- 58.Fishkin JB, So PT, Cerussi AE, Fantini S, Franceschini MA, Gratton E. Frequency-domain method for measuring spectral properties in multiple-scattering media: methemoglobin absorption spectrum in a tissuelike phantom. Appl Opt. 1995;34(7):1143–1155. doi: 10.1364/AO.34.001143. [DOI] [PubMed] [Google Scholar]
- 59.Endo T, Kime R, Fuse S, Watanabe T, Murase N, Kurosawa Y, Hamaoka T. Evaluation of functional hyperemia using NIRTRS without the influence of fat layer thickness. Adv Exp Med Biol. 2018;1072:97–101. doi: 10.1007/978-3-319-91287-5_16. [DOI] [PubMed] [Google Scholar]
- 60.Ohmae E, Nishio S, Oda M, Suzuki H, Suzuki T, Ohashi K, Koga S, Yamashita Y, Watanabe H. Sensitivity correction for the influence of the fat layer on muscle oxygenation and estimation of fat thickness by time-resolved spectroscopy. J Biomed Opt. 2014;19(6):067005. doi: 10.1117/1.JBO.19.6.067005. [DOI] [PubMed] [Google Scholar]
- 61.Okushima Dai, Poole David C., Rossiter Harry B., Barstow Thomas J., Kondo Narihiko, Ohmae Etsuko, Koga Shunsaku. Muscle deoxygenation in the quadriceps during ramp incremental cycling: Deep vs. superficial heterogeneity. Journal of Applied Physiology. 2015;119(11):1313–1319. doi: 10.1152/japplphysiol.00574.2015. [DOI] [PubMed] [Google Scholar]
- 62.Koga S, Poole DC, Fukuoka Y, Ferreira LF, Kondo N, Ohmae E, Barstow TJ. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R534–R541. doi: 10.1152/ajpregu.00101.2011. [DOI] [PubMed] [Google Scholar]
- 63.Burtscher M, Nachbauer W, Wilber R. The upper limit of aerobic power in humans. Eur J Appl Physiol. 2011;111(10):2625–2628. doi: 10.1007/s00421-011-1885-4. [DOI] [PubMed] [Google Scholar]
- 64.Hellsten Y, Maclean D, Rådegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98(1):6–8. doi: 10.1161/01.CIR.98.1.6. [DOI] [PubMed] [Google Scholar]
- 65.Gayeski TE, Honig CR. Direct measurement of intracellular O2 gradients; role of convection and myoglobin. Adv Exp Med Biol. 1983;159:613–621. doi: 10.1007/978-1-4684-7790-0_54. [DOI] [PubMed] [Google Scholar]
- 66.Guezennec CY, Lienhard F, Louisy F, Renault G, Tusseau MH, Portero P. In situ NADH laser fluorimetry during muscle contraction in humans. Eur J Appl Physiol Occup Physiol. 1991;63(1):36–42. doi: 10.1007/BF00760798. [DOI] [PubMed] [Google Scholar]
- 67.Gadian DG, Hoult DI, Radda GK, Seeley PJ, Chance B, Barlow C. Phosphorus nuclear magnetic resonance studies on normoxic and ischemic cardiac tissue. Proc Natl Acad Sci USA. 1976;73(12):4446–4448. doi: 10.1073/pnas.73.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chance B, Eleff S, Leigh JS., Jr Noninvasive, nondestructive approaches to cell bioenergetics. Proc Natl Acad Sci USA. 1980;77(12):7430–7434. doi: 10.1073/pnas.77.12.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Jr, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol. 1994;77(1):5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- 70.Southern WM, Ryan TE, Kepple K, Murrow JR, Nilsson KR, McCully KK. Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiol Rep. 2015;3(4):e12353. doi: 10.14814/phy2.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esaki K, Hamaoka T, Rådegran G, Boushel R, Hansen J, Katsumura T, Haga S, Mizuno M. Association between regional quadriceps oxygenation and blood oxygen saturation during normoxic one-legged dynamic knee extension. Eur J Appl Physiol. 2005;95(4):361–370. doi: 10.1007/s00421-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 72.Wilson JR, Mancini DM, McCully K, Ferraro N, Lanoce V, Chance B. Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation. 1989;80(6):1668–1674. doi: 10.1161/01.CIR.80.6.1668. [DOI] [PubMed] [Google Scholar]
- 73.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol. 1994;77(6):2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 74.Costes F, Barthélémy JC, Féasson L, Busso T, Geyssant A, Denis C. Comparison of muscle near-infrared spectroscopy and femoral blood gases during steady-state exercise in humans. J Appl Physiol. 1996;80(4):1345–1350. doi: 10.1152/jappl.1996.80.4.1345. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald MJ, Tarnopolsky MA, Green HJ, Hughson RL. Comparison of femoral blood gases and muscle near-infrared spectroscopy at exercise onset in humans. J Appl Physiol. 1999;86(2):687–693. doi: 10.1152/jappl.1999.86.2.687. [DOI] [PubMed] [Google Scholar]
- 76.Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol. 2001;90(2):511–519. doi: 10.1152/jappl.2001.90.2.511. [DOI] [PubMed] [Google Scholar]
- 77.Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol. 1996;81(3):1410–1417. doi: 10.1152/jappl.1996.81.3.1410. [DOI] [PubMed] [Google Scholar]
- 78.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol. 2013;115(12):1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheatle TR, Potter LA, Cope M, Delpy DT, Coleridge Smith PD, Scurr JH. Near-infrared spectroscopy in peripheral vascular disease. Br J Surg. 1991;78(4):405–408. doi: 10.1002/bjs.1800780408. [DOI] [PubMed] [Google Scholar]
- 80.De Blasi RA, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J Appl Physiol. 1994;76(3):1388–1393. doi: 10.1152/jappl.1994.76.3.1388. [DOI] [PubMed] [Google Scholar]
- 81.Boushel R, Pott F, Madsen P, Rådegran G, Nowak M, Quistorff B, Secher N. Muscle metabolism from near infrared spectroscopy during rhythmic handgrip in humans. Eur J Appl Physiol Occup Physiol. 1998;79(1):41–48. doi: 10.1007/s004210050471. [DOI] [PubMed] [Google Scholar]
- 82.Sako T, Hamaoka T, Higuchi H, Kurosawa Y, Katsumura T. Validity of NIR spectroscopy for quantitatively measuring muscle oxidative metabolic rate in exercise. J Appl Physiol. 2001;90(1):338–344. doi: 10.1152/jappl.2001.90.1.338. [DOI] [PubMed] [Google Scholar]
- 83.Sahlin K. Non-invasive measurements of O2 availability in human skeletal muscle with near-infrared spectroscopy. Int J Sport Med Suppl. 1992;1:S157–S160. doi: 10.1055/s-2007-1024625. [DOI] [PubMed] [Google Scholar]
- 84.Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988;254(4 Pt 1):C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- 85.Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol. 1994;77(4):1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- 86.Chance B, Leigh JS, Jr, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci USA. 1985;82(24):8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Binzoni T, Quaresima V, Ferrari M, Hiltbrand E, Cerretelli P. Human calf microvascular compliance measured by near-infrared spectroscopy. J Appl Physiol. 2000;88(2):369–372. doi: 10.1152/jappl.2000.88.2.369. [DOI] [PubMed] [Google Scholar]
- 88.Hiatt WR, Huang SY, Regensteiner JG, Micco AJ, Ishimoto G, Manco-Johnson M, Drose J, Reeves JT. Venous occlusion plethysmography reduces arterial diameter and flow velocity. J Appl Physiol. 1989;66(5):2239–2244. doi: 10.1152/jappl.1989.66.5.2239. [DOI] [PubMed] [Google Scholar]
- 89.Irace CR, Ceravolo L, Notarangelo A, Crescenzo G, Ventura O, Tamburrini F, Perticone Gnasso A. Comparison of endothelial function evaluated by strain gauge plethysmography and brachial artery ultrasound. Atherosclerosis. 2001;158(1):53–59. doi: 10.1016/S0021-9150(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 90.Nagasawa T, Hamaoka T, Sako T, Murakami M, Kime R, Homma T, Ueda C, Ichimura S, Katsumura T. A practical indicator of muscle oxidative capacity determined by recovery of muscle O2 consumption using NIR spectroscopy. Eur J Sport Sci. 2003;3:1–10. doi: 10.1080/17461390300073207. [DOI] [Google Scholar]
- 91.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol. 2013;115:1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. The Journal of General Physiology. 1985;86(1):135–165. doi: 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barbiroli B, Montagna P, Cortelli P, Martinelli P, Sacquegna T, Zaniol P, Lugaresi E. Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia. 1990;10(5):263–272. doi: 10.1046/j.1468-2982.1990.1005263.x. [DOI] [PubMed] [Google Scholar]
- 94.McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75(2):813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- 95.Motobe M, Murase N, Osada T, Homma T, Ueda C, Nagasawa T, Kitahara A, Ichimura S, Kurosawa Y, Katsumura T, Hoshika A, Hamaoka T. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med. 2004;3(1):2. doi: 10.1186/1476-5918-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murrow JR, Brizendine JT, Djire B, Young HJ, Rathbun S, Nilsson KR, Jr, McCully KK. Near infrared spectroscopy-guided exercise training for claudication in peripheral arterial disease. Eur J Prev Cardiol. 2018;28:2047487318795192. doi: 10.1177/2047487318795192. [DOI] [PubMed] [Google Scholar]
- 97.Komiyama T, Quaresima V, Shigematsu H, Ferrari M. Comparison of two spatially resolved near-infrared photometers in the detection of tissue oxygen saturation: poor reliability at very low oxygen saturation. Clin Sci (Lond) 2001;101(6):715–718. doi: 10.1042/cs1010715. [DOI] [PubMed] [Google Scholar]
- 98.Craig JC, Broxterman RM, Wilcox SL, Chen C, Barstow TJ. Effect of adipose tissue thickness, muscle site, and sex on near-infrared spectroscopy derived total-[hemoglobin + myoglobin] J Appl Physiol. 2017;123(6):1571–1578. doi: 10.1152/japplphysiol.00207.2017. [DOI] [PubMed] [Google Scholar]
- 99.Miura H, McCully K, Hong L, Nioka S, Chance B. Regional difference of muscle oxygen saturation and blood volume during exercise determined by near infrared imaging device. Jpn J Physiol. 2001;51(5):599–606. doi: 10.2170/jjphysiol.51.599. [DOI] [PubMed] [Google Scholar]
- 100.Niemeijer VM, Jansen JP, van Dijk T, Spee RF, Meijer EJ, Kemps HM, Wijn PF. The influence of adipose tissue on spatially resolved near-infrared spectroscopy derived skeletal muscle oxygenation: the extent of the problem. Physiol Meas. 2017;38(3):539–554. doi: 10.1088/1361-6579/aa5dd5. [DOI] [PubMed] [Google Scholar]
- 101.Rittweger J, Moss AD, Colier W, Stewart C, Degens H. Muscle tissue oxygenation and VEGF in VO-matched vibration and squatting exercise. Clin Physiol Funct Imaging. 2010;30(4):269–278. doi: 10.1111/j.1475-097X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 102.Ferreira LF, Townsend DK, Lutjemeier BJ, Barstow TJ. Muscle capillary blood flow kinetics estimated from pulmonary O2 uptake and near-infrared spectroscopy. J Appl Physiol. 2005;98(5):1820–1828. doi: 10.1152/japplphysiol.00907.2004. [DOI] [PubMed] [Google Scholar]
- 103.Poole DC, Barstow TJ, McDonough P, Jones AM. Control of oxygen uptake during exercise. Med Sci Sport Exerc. 2008;40(3):462–474. doi: 10.1249/MSS.0b013e31815ef29b. [DOI] [PubMed] [Google Scholar]
- 104.Kushmerick MJ, Conley KE. Energetics of muscle contraction: the whole is less than the sum of its parts. Biochem Soc Trans. 2002;30(2):227–231. doi: 10.1042/bst0300227. [DOI] [PubMed] [Google Scholar]
- 105.Boone J, Koppo K, Barstow TJ, Bouckaert J. Effect of exercise protocol on deoxy[Hb + Mb]: incremental step versus ramp exercise. Med Sci Sport Exerc. 2010;42(5):935–942. doi: 10.1249/MSS.0b013e3181c0ecea. [DOI] [PubMed] [Google Scholar]
- 106.Hamaoka T, Mizuno M, Katsumura T, Osada T, Shimomitsu T, Quistorff B. Correlation between indicators determined by near infrared spectroscopy and muscle fiber types in humans. Jpn J Appl Physiol. 1998;28(5):243–248. [Google Scholar]
- 107.McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J Gerontol. 1994;49(3):B128–B134. doi: 10.1093/geronj/49.3.B128. [DOI] [PubMed] [Google Scholar]
- 108.Hanada A, Okita K, Yonezawa K, Ohtsubo M, Kohya T, Murakami T, Nishijima H, Tamura M, Kitabatake A. Dissociation between muscle metabolism and oxygen kinetics during recovery from exercise in patients with chronic heart failure. Heart. 2000;83(2):161–166. doi: 10.1136/heart.83.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107(2):294–299. doi: 10.1161/01.CIR.0000044914.42696.6A. [DOI] [PubMed] [Google Scholar]
- 110.Puente-Maestu L, Tena T, Trascasa C, Pérez-Parra J, Godoy R, García MJ, Stringer WW. Training improves muscle oxidative capacity and oxygenation recovery kinetics in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol. 2003;88(6):580–587. doi: 10.1007/s00421-002-0743-9. [DOI] [PubMed] [Google Scholar]
- 111.Ichimura S, Murase N, Osada T, Kime R, Homma T, Ueda C, Nagasawa T, Motobe M, Hamaoka T, Katsumura T. Age and activity status affect muscle reoxygenation time after maximal cycling exercise. Med Sci Sport Exerc. 2006;38(7):1277–1281. doi: 10.1249/01.mss.0000227312.08599.f1. [DOI] [PubMed] [Google Scholar]
- 112.Kime R, Hamaoka T, Sako T, Murakami M, Homma T, Katsumura T, Chance B. Delayed reoxygenation after maximal isometric handgrip exercise in high oxidative capacity muscle. Eur J Appl Physiol. 2003;89(1):34–41. doi: 10.1007/s00421-002-0757-3. [DOI] [PubMed] [Google Scholar]
- 113.McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Jr, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol. 1994;77(1):5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- 114.Willingham TB, Southern WM, McCully KK. Measuring reactive hyperemia in the lower limb using near-infrared spectroscopy. J Biomed Opt. 2016;21(9):091302. doi: 10.1117/1.JBO.21.9.091302. [DOI] [PubMed] [Google Scholar]
- 115.McCully KK, Landsberg L, Suarez M, Hofmann M, Posner JD. Identification of peripheral vascular disease in elderly subjects with optical spectroscopy. J Gerontol A Biol Sci Med Sci. 1997;52(3):B159–B165. doi: 10.1093/gerona/52A.3.B159. [DOI] [PubMed] [Google Scholar]