Abstract
The objective of this paper was to systematically evaluate the potential preventive and therapeutic effects of exercise in attenuating stress-induced memory impairment. A systematic review was employed, searching PubMed, PsychInfo, Sports Discus and Google Scholar databases. For eligibility, studies had to be published in English, employ an experimental design, have the acute or chronic bout of exercise occur prior to, during or after the stressor, implement a psychophysiological stressor, and have an assessment of memory function occurring after the stressor. In total, 23 studies were evaluated, all of which were conducted among animal models. All 23 studies employed a chronic exercise protocol and a chronic stress protocol. Eight studies evaluated a preventive model, three employed a concurrent model, ten studies employed a therapeutic model, and two studies evaluated both a preventive and therapeutic model within the same study. Among the eight studies employing a preventive model, all eight demonstrated that the stress regimen impaired memory function. In all eight of these studies, when exercise occurred prior to the stressor, exercise attenuated the stress-induced memory impairment effect. Among the ten studies employing a therapeutic model, one study showed that the stress protocol enhanced memory function, one showed that the stress protocol did not influence memory, and eight demonstrated that the stress regimen impaired memory function. Among the eight studies showing that the stress protocol impaired memory function, all eight studies demonstrated that exercise, after the stressor, attenuated stress-induced memory impairment. Within animal models, chronic stress is associated with memory impairment and chronic exercise has both a preventive and therapeutic effect in attenuating stress-induced memory impairment. Additional experimental work in human studies is needed. Such work should also examine acute exercise and stress protocols.
Keywords: Cognition, Exercise, Memory, Physical activity, Preventive, Psychological, Psychophysiological, Rescue, Stress, Therapeutic, Treatment
Introduction
The prophylactic and treatment effects of exercise on various chronic diseases is well established [1]. Additionally, exercise can also help to prevent a host of cardiometabolic-related conditions (e.g., diabetes, early mortality) [2, 3]. Emerging work also demonstrates that, exercise, prior to a psychophysiological stressor [herein focused on toxic stress (not eustress)] [4], can mitigate the negative effects of the stressor. For example, we showed that acute exercise, occurring immediately before viewing emotionally charged, negatively valenced images, helped facilitate emotional regulation [5]. This “exercise preventive paradigm” has also been corroborated with other emotional regulation studies [6, 7].
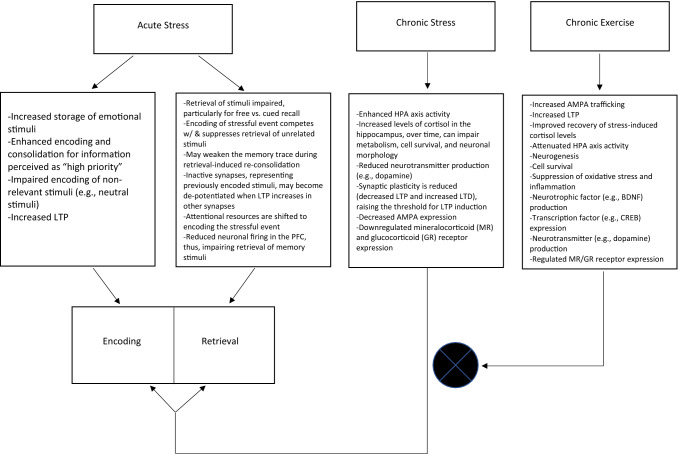
This exercise preventive paradigm effect may also have implications in memory function. Psychophysiological stressors, such as forced social participation in a verbal presentation task, may have a negative effect on memory function [8–14]. Notably, this stress-memory relationship is thought to follow an inverted U-shaped relationship [15]. See Fig. 1 (and the Discussion section) for a schematic on the potential underlying mechanisms through which stress (both acute and chronic) may influence memory function. To our knowledge, however, there are no published reviews comprehensively evaluating the literature regarding the potential protective and/or therapeutic effects of exercise on mitigating stress-induced memory impairment [16], which was the purpose of this brief systematic review. The plausibility for exercise to attenuate stress-induced memory impairment is also shown in Fig. 1 (and further addressed in the Discussion section).
Fig. 1.
Schematic indicating potential mechanisms through which acute stress may enhance memory, detrimental effects of chronic stress on memory, and how exercise may attenuate stress-induced memory impairment. The circular crossed symbol denotes that chronic exercise attenuates the chronic stress mechanisms
Methods
Studies were identified using electronic databases, including PubMed, PsychInfo, Sports Discus, and Google Scholar. We employed the computerized searches on April 25, 2018, identifying articles published prior to this date (no restriction was placed on how far back the study was published). The search terms included exercise, physical activity, stress, psychophysiological, rescue, preventive, treatment, therapeutic, psychological, memory, and cognition. To be eligible for inclusion in this systematic review, studies had to be published in English, employ an experimental design (cross-sectional designs on this topic were not eligible) [17], have the acute or chronic bout of exercise occur prior to, during, or after the stressor, implement a toxic psychophysiological stressor (pharmaceutical agent or ischemia-induction were not eligible [18–22], mild forms of the stressor were not eligible [23], and evaluating individuals without experimentally manipulating stress were not eligible) [24], and have an assessment of memory function that occurred after the stressor. To provide a comprehensive assessment on this topic, we applied no restriction on whether the study was conducted in humans or animal models. In total, 24 studies met our criteria. However, two appeared to be duplicate studies [25, 26], and thus, one was removed. As noted in Table 1, 23 studies were evaluated herein.
Table 1.
Extraction table of the evaluated studies
| Study | Animal/human | Subject characteristics | Study design | Preventive/therapeutic | Stress protocol | Exercise protocol | Memory assessment | Main findings | Speculated mechanisms |
|---|---|---|---|---|---|---|---|---|---|
| Grace (2009) [56] | Animal | Sprague-Dawley rats | Experimental | Therapeutic | Deprived from their mothers for 3 h/day for 12 days | Voluntary access to running wheels for 20 days after the maternal separation | Morris water maze, object recognition | Maternal separation did not impair memory. Exercise, however, improved memory function | N/A |
| Mello (2009) [57] | Animal | Wistar rats (3–4 months) | Experimental | Therapeutic | Deprived from their mothers for 3 h/day during first 10 days of life | At day 45, engaged in forced treadmill exercise; 50 min/day, 5 days/week, 8 total weeks | Morris water maze, object recognition, inhibitory avoidance | Exercise reversed the deficit of inhibitory avoidance and reduced the deficit of spatial memory | Exercise may attenuate HPA-axis activity |
| Makena (2012) [58] | Animal | Sprague-Dawley rats | Experimental | Therapeutic | Deprived from their mothers for 3 h/day for 12 days | Voluntary access to running wheels for ~ 20 days after the maternal separation | Objective recognition task | Maternal separation enhanced memory function. Maternal separation also prevented exercise-induced MAPK/ERK signaling | N/A |
| Kim (2013) [59] | Animal | Sprague-Dawley rats | Experimental | Therapeutic | 95 dB supersonic machine sound (1 h/day) during pregnancy | After delivery, rat pups exercise on the treadmill for 30 min/day for 7 days, starting 4 weeks after birth | Radial 8-arm maze test | Stress protocol suppressed neurogenesis in the offspring and also impaired memory. Exercise attenuated these effects. Mild-intensity exercise was more effective than high-intensity exercise | Exercise-induced neurogenesis |
| Kim (2013) [60] | Animal | Sprague-Dawley rats | Experimental | Therapeutic | Foot shocks, 3 times/day, for 7 consecutive days | 4 weeks of treadmill exercise, 30 min/day | Radial 8-arm maze test | Stress protocol impaired memory, suppressed cell proliferation in the hippocampus, which was attenuated with exercise | Exercise-induced cell proliferation in the dentate gyrus |
| Radahmadi (2013) [25] | Animal | Wistar rats | Experimental | The exercise training and stress protocol occurred concurrently | Restrained in Plexiglass cylinder for 6 h/day for 21 days | Treadmill running, 1 h/day, for 21 days | Passive avoidance learning test | Although exercise was effective in enhancing memory, exercise was not effective in improving passive avoidance acquisition and retention when exposed to the stress protocol | N/A |
| Castilla-Ortega (2014) [61] | Animal | C57BL/6 J | Experimental | Preventive | Chronic intermittent restraint stress; restrained for 13 days for 3.5 h/day | 6 days of daily exercise | What-When-Where task | Stress impaired neurogenesis and the “when” memory task, while exercise promoted neurogenesis and improved the “where” memory | The stressed exercising animals showed a larger increase in cell survival, maturation of new neurons in the dentate gyrus |
| Patki (2014) [62] | Animal | Sprague-Dawley rats | Experimental | Therapeutic | Social defeat model; seven encounters for 7 consecutive days | After stress exposure, engaged in treadmill exercise for 2 weeks (30 min/day) | Radial arm water maze | Stress impaired long-term memory (not short term), which was attenuated with exercise | Suppression of oxidative stress and inflammation. Modulation of deacetylation processes. Regulation of BDNF |
| Patki (2014) [63] | Animal | Wistar rats | Experimental | Therapeutic | Single stress exposure (2 h restraint, 20 min forced swimming, 15 min rest, and 1–2 min diethyl ether exposure) | After stress exposure, exercised on treadmill for 2 weeks (30 min/day) | Radial arm water maze | Stress impaired memory, which was attenuated with exercise | Increase in BDNF and attenuation of HPA axis |
| Neves (2015) [64] | Animal | Wistar rats | Experimental | Preventive | Maternal deprivation, 3 h/day, 10 days | 5 days/week of exercise, 50 min/day, for 8 weeks | Object recognition test, inhibitory avoidance test | Exercise prevented stress-induced memory impairment, for both short- and long-term memory | Exercise may attenuate stress-induced oxidative damage. The stress protocol increased lipid peroxidation, which was attenuated with exercise. Dopamine is metabolized by monoamine oxidase, producing hydrogen peroxide. Thus, increased dopamine turnover may induce oxidative stress, which may lead to cell death |
| Dief (2015) [65] | Animal | Wistar rats | Experimental | Preventive | Chronic immobilization stress for 10 days | 6 weeks of swimming, 5 days/week | T-maze for spatial memory | Exercise attenuated stress-induced impairment in spatial memory | Stress protocol decreased BDNF levels; exercise increased BDNF levels, which may have prevented the stress-induced impairments. Exercise also increased Ach levels |
| Kang (2015) [66] | Animal | C57BL/6 mice | Experimental | Preventive and therapeutic | 6 h daily restraint for 3 weeks. Restraint occurred during weeks 5–8 | Treadmill exercise (60 min/day, 5 days/week) occurred from week 1 to 8 | Water maze task | Stress induced memory impairment, which was counter-regulated by exercise | Stress markedly reduced hippocampal CREB/BDNF signaling, which was reversed by 8 weeks of treadmill exercise |
| Ozbeyli (2015) [67] | Animal | Wistar rats | Experimental | Preventive | Exposure to cat odor | 6 weeks of swimming, 5 days/week, 1 h/day | Object recognition task | Exercise had a protective effect against stress-induced memory decline | Decreasing oxidative damage parameters, such as lipid peroxidation, neutrophil infiltration and lucigenin activity |
| Radahmadi (2015) [68] | Animal | Wistar rats | Experimental | Preventive and therapeutic | 21-day restraint stress, 6 h/day | Treadmill running 1 h/day for 21 days | Passive avoidance task | Exercise had both a preventive and therapeutic effect on stress-induced memory function, but a greater therapeutic effect was observed | Increased antioxidant enzymes, regulation of glucocorticoid receptors, increased neurotrophic factors, increased muscarinic receptor density, and increased acetylcholine release |
| Leem (2016) [69] | Animal | C57BL/6 mice | Experimental | Therapeutic | 21-day restraint stress, 6 h/day | 3 weeks of treadmill exercise, 1 h/day, 6 days/week | Y-maze and water maze task | Restraint stress produced learning and memory deficits, which were reversed with the 3-week exercise protocol | Exercise-induced expression of BDNF |
| Wearick-Silva (2016) [70] | Animal | Balb/c mice | Experimental | Therapeutic | Maternal separation during first 2 weeks of life | 3-week running protocol, 60 min/day, 5 days/week | Object recognition task | Maternal separation impaired memory, which was reversed with exercise | Exercise-induced expression of BDNF |
| Chen (2017) [71] | Animal | Thy1-H | Experimental | Therapeutic | Restraint stress; 1 h/day for 14 days | Treadmill exercise, 1 h/day, 14 days | Novel discrimination task | Stress protocol induced dendritic spine loss and memory impairment, which was rescued with exercise | Dendritic spine density and BDNF expression |
| dos Santos (2017) [72] | Animal | Wistar rats | Experimental | Preventive | Chronic variable stress; 24-h water deprivation, 1-3 h restraint, 24-h food deprivation, forced swimming, isolation, inclination of home cage, and damp bedding | 20 min/day, 3 times/week, for 2 months | Inhibitory avoidance task | Stress protocol induced oxidative stress and impaired memory. Exercise prevented memory impairment | Exercise prevented stress-induced oxidative damage |
| Kochi (2017) [73] | Animal | Long-Evans rats | Experimental | Preventive | Social defeat paradigm | 30 min of treadmill exercise for 14 days | Radial arm water maze | Exercise, prior to the trauma experience, mitigated memory impairment | Exercise prior to the stressor reduced anxiety levels from the stressor, which may have preserved memory function |
| Lapmanee (2017) [74] | Animal | Wistar rats | Experimental | Preventive | Restraint stress (varied, 1–8 weeks) | Voluntary wheel running for 4 weeks | Morris water maze and object recognition task | Exercise prevented impairments in memory | Exercise-induced BDNF expression |
| Leem (2017) [75] | Animal | C57BL/6 mice | Experimental | Concurrent; exercise and stress occurring together | Restraint stress; 6 h/day for 21 days | Treadmill running for 4 weeks | Morris water maze and object recognition task | Stress protocol impaired memory, which was attenuated with exercise | AMPA-receptor mediated mechanisms |
| da Silva (2018) [76] | Animal | Wistar rats | Experimental | Preventive | Restraint stress with cylindrical acrylic tube | 30 days of treadmill exercise, 30 min/day | Object recognition test | Exercise, coupled with virgin coconut oil, ameliorated the effects of stress on memory impairment | Preventive effects may occur from the antioxidant capabilities of exercise and coconut oil |
| Miller (2018) [77] | Animal | C57BL/6 mice | Experimental | Preventive and concurrent | 5-min cold water swim on day 1, 30-min elevated platform stress on day 2, and 60-min restraint on day 3 | 4 weeks of voluntary wheel access | Radial arm maze | Stress alone impaired LTP and exercise alone increased LTP. Exercise with stress increased LTP more than stress only group. Exercise group made fewer errors in the memory task | Modulation of BDNF, TrkB, glucocorticoid, mineralo-corticoid, and dopamine |
Results
Table 1 displays the extraction table for the evaluated studies. All were experimental studies conducted in an animal model. The stress protocol across the studies varied, including maternal separation, loud noise exposure, immobilization/restraint, social defeat/competition and exposure to cat odor. All studies, except one (single session acute stress protocol), employed a chronic stress protocol (e.g., multiple repeated exposures over 1–2 weeks). All 23 studies employed a chronic exercise protocol (e.g., daily exercise from 2 to 8 weeks; either forced treadmill exercise or voluntary wheel access). Among the 23 studies, the commonly assessed memory tasks included the Morris water maze, object recognition test, or inhibitory avoidance task.
Eight studies evaluated a preventive model (i.e., exercise occurring prior to stress-induction), three employed a concurrent model (exercise bout occurred during or around the same time as the stress protocol), ten studies employed a therapeutic model (i.e., exercise occurring after stress-induction), and two studies evaluated both a preventive and therapeutic model within the same study.
Among the eight studies employing a preventive model, all eight demonstrated that the stress regimen impaired memory function. In all eight of these studies, when exercise occurred prior to the stressor, exercise attenuated the stress-induced memory impairment effect.
Among the ten studies employing a therapeutic model, one study showed that the stress protocol enhanced memory function, one showed that the stress protocol did not influence memory, and eight demonstrated that the stress regimen impaired memory function. Among the eight studies showing that the stress protocol impaired memory function, all eight studies demonstrated that exercise, after the stressor, attenuated stress-induced memory impairment.
Among the three concurrent models, and the two studies that evaluated both preventive and therapeutic effects, all showed that the stress protocol impaired memory function. Among the three concurrent models, two demonstrated a beneficial effect of exercise in mitigating stress-induced memory impairment. Among the two studies employing both a preventive and therapeutic model, both demonstrated attenuation effects of exercise on stress-induced memory impairment.
Discussion
The objective of this systematic review was to evaluate the potential preventive and therapeutic effects of exercise in attenuating stress-induced memory impairment. There was consistent evidence that chronic exercise had both a preventive and therapeutic effect in mitigating chronic stress-induced memory impairment. The narrative that follows will discuss these mechanistic pathways, as displayed in Fig. 1. For additional discussion on these mechanisms, the reader is referred elsewhere [16, 27, 28].
Acute stress and memory
Acute moderate levels of stress may enhance memory, particularly emotional-based information (vs neutral stimuli). Specifically, enhanced encoding and consolidation of stimuli is more likely to occur for information perceived as “high priority” [29–33]. The stressor, occurring prior to the memory task, may help to augment attentional resources (via, for example, the prefrontal and parietal structures) to the memory stimuli and, in turn, enhance encoding of the information [34, 35]. In addition to psychological stress, emerging work also suggests that exercise-induced physiological arousal may help to subserve stress-related memory function (emotional memory) [36]. Additional work is needed to determine whether there is an additive effect of exercise and acute stress on memory function.
Additionally, the stressor (including exercise) [36], occurring before, or shortly after, the memory task can help to facilitate the consolidation of the memory trace. For example, cortisol crosses the blood–brain barrier and binds to mineralocorticoid or glucocorticoid receptors. After which, PKA activation may help to facilitate exocytosis of AMPA receptors (and activation of NMDA receptors) [37], subserving hippocampal LTP [38]. Acute stress may also induce levels of epinephrine, activating the vagus nerve and, in turn, facilitating LTP via neurotransmitter (e.g., norepinephrine, dopamine, serotonin, and acetylcholine) production to the hippocampus [39–42]. To illustrate, the vagus nerve may stimulate the production of norepinephrine from the locus coeruleus, which then binds to adrenergic receptors, ultimately facilitating a cascade of intracellular signaling to induce synaptic plasticity [43]. Moreover, cortisol may augment endocannabinoid levels, binding to CB1 receptors in GABA interneurons and, ultimately, inhibiting GABA neurotransmitter levels [44]. This, in turn, may help to preserve memory, as GABA inhibition may help facilitate LTP [45] and GABA receptor activation may impair memory [46].
Although acute stress, occurring before encoding or during the early stages of consolidation, can facilitate encoding and consolidation of the prioritized stimuli, it can have the opposite effect on non-prioritized stimuli. The encoding of the stressful event may compete with the encoding of non-relevant or non-prioritized stimuli. Further, if the stressor occurs around the period of retrieving a memory, this memory retrieval process can be impaired, as attentional resources are shifted away from retrieval processes to encoding the stressful event. Additionally, inactive synapses, representing previously encoded stimuli, may become de-potentiated when LTP increases in other synapses [28]. Moreover, during the stressor, reduced neuronal firing may occur in the prefrontal cortex, which may impair memory retrieval since the prefrontal cortex plays an important role in such retrieval processes [47]. It would be worthwhile to investigate whether acute exercise can attenuate these effects by, for example, attenuating the stress response and facilitating emotional regulation [5].
Taken together, acute moderate levels of stress may help to facilitate encoding and consolidation of prioritized stimuli (particularly emotional stimuli), whereas extreme acute stressors may detrimentally influence retrieval of memories when the stressor occurs around the time of retrieving an unrelated memory. Notably, and as discussed next, chronic elevations in cortisol, lasting more than a few hours, can impair memory function (inducing LTD) [38].
Chronic stress and memory
Chronic stress may detrimentally influence stress through various mechanisms, including enhanced HPA axis activity. Over time, this may impair cell survival and neuronal morphology (e.g., loss of spines, shrinkage of dendrites) [4]. Regarding cell survival, astrocytes, which support the survival of neurons, possess glucocorticoid receptors and are significantly affected by chronic psychosocial stress [48]. Considering neuronal morphology, reduced synaptic firing, via LTD for example, causes actin loss and dendritic spine shrinkage [49]. Further, chronic stress may reduce BDNF levels [50], which play an important role in facilitating signaling pathways (e.g., RAC1) that stabilize dendritic spines [51]. Additionally, chronic stress may reduce neurotransmitter levels (e.g., dopamine) [52], decrease AMPA receptor expression [53], and downregulate mineralocorticoid and glucocorticoid receptor expression [54]. This downregulation and desensitization of these receptors may prevent activation of some of the above-mentioned cellular pathways (e.g., PKA) that may facilitate LTP. Further, chronic stress may inhibit neurogenesis, and ultimately, hippocampal volume loss, via, for example, apoptosis of progenitor cells to cell cycle arrest [49].
Exercise mitigates negative effects of chronic stress
Exercise may attenuate the memory-related consequences of chronic stress via various pathways. Ultimately, exercise may help to facilitate LTP through induced neuronal excitability, via stimulation of the vagus nerve as well as muscle afferent nerve fibers [55], which have direct projections to the brainstem and, ultimately, the hippocampus. Further, exercise-induced alterations in hormones (e.g., epinephrine and cortisol) can also influence neuronal excitability. Facilitating these effects, exercise has been shown to enhance neurotrophic factors (e.g., BDNF), induce transcription factors (e.g., CREB) expression, and increase AMPA trafficking [55]. Exercise may also help attenuate chronic stress-induced memory impairment via attenuation of HPA axis activity, suppress oxidative stress, facilitate neurogenesis, and regulate mineralocorticoid and glucocorticoid receptor expression [27].
Conclusion
This review demonstrates that, within animal models, chronic stress is associated with memory impairment and chronic exercise has both a preventive and therapeutic effect in attenuating stress-induced memory impairment. Given the paucity of work among human studies, future work on this topic among humans should investigate, specifically, whether exercise has a preventive and therapeutic effect in mitigating memory impairment caused from psychophysiological stress. Such work should also consider models that evaluate acute exercise and acute stress protocols. Further, work should also evaluate varying parameters of exercise, such as the intensity, duration, and type of exercise, as variations of these dimensions may have a unique influence on potentially attenuating stress-induced memory impairment.
Compliance with ethical standards
Conflict of interest
Author PL declares no conflict of interest. Author EF declares no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
No consent was needed as this is a review paper.
References
- 1.Kujala UM. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med. 2009;43(8):550–555. doi: 10.1136/bjsm.2009.059808. [DOI] [PubMed] [Google Scholar]
- 2.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–556. doi: 10.1097/HCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017 doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards MK, Rhodes RE, Loprinzi PD. A randomized control intervention investigating the effects of acute exercise on emotional regulation. Am J Health Behav. 2017;41(5):534–543. doi: 10.5993/AJHB.41.5.2. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein EE, McNally RJ. Acute aerobic exercise helps overcome emotion regulation deficits. Cogn Emot. 2017;31(4):834–843. doi: 10.1080/02699931.2016.1168284. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein EE, McNally RJ. Acute aerobic exercise hastens emotional recovery from a subsequent stressor. Health Psychol. 2017;36(6):560–567. doi: 10.1037/hea0000482. [DOI] [PubMed] [Google Scholar]
- 8.de Quervain DJ, Henke K, Aerni A, et al. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17(6):1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- 9.de Quervain DJ, Roozendaal B, Nitsch RM, et al. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3(4):313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 10.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30(8):771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394(6695):787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 12.Diamond DM, Park CR, Heman KL, et al. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Park CR, Zoladz PR, Conrad CD, et al. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15(4):271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guez J, Saar-Ashkenazy R, Keha E, et al. The effect of Trier social stress test (TSST) on item and associative recognition of words and pictures in healthy participants. Front Psychol. 2016;7:507. doi: 10.3389/fpsyg.2016.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17(10):522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- 16.Leem YH. The potential role of exercise in chronic stress-related changes in AMPA receptor phenotype underlying synaptic plasticity. J Exerc Nutr Biochem. 2017;21(4):11–15. doi: 10.20463/jenb.2017.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head D, Singh T, Bugg JM. The moderating role of exercise on stress-related effects on the hippocampus and memory in later adulthood. Neuropsychology. 2012;26(2):133–143. doi: 10.1037/a0027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schimidt HL, Vieira A, Altermann C, et al. Memory deficits and oxidative stress in cerebral ischemia-reperfusion: neuroprotective role of physical exercise and green tea supplementation. Neurobiol Learn Mem. 2014;114:242–250. doi: 10.1016/j.nlm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Shih PC, Yang YR, Wang RY. Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLoS One. 2013;8(10):e78163. doi: 10.1371/journal.pone.0078163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Xu Z, Tang J, et al. Voluntary exercise counteracts Abeta25-35-induced memory impairment in mice. Behav Brain Res. 2013;256:618–625. doi: 10.1016/j.bbr.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Cechetti F, Worm PV, Elsner VR, et al. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem. 2012;97(1):90–96. doi: 10.1016/j.nlm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Gozal D, Nair D, Goldbart AD. Physical activity attenuates intermittent hypoxia-induced spatial learning deficits and oxidative stress. Am J Respir Crit Care Med. 2010;182(1):104–112. doi: 10.1164/rccm.201001-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adlard PA, Engesser-Cesar C, Cotman CW. Mild stress facilitates learning and exercise improves retention in aged mice. Exp Gerontol. 2011;46(1):53–59. doi: 10.1016/j.exger.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Zlomuzica A, Woud ML, Machulska A, et al. Deficits in episodic memory and mental time travel in patients with post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:42–54. doi: 10.1016/j.pnpbp.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Radahmadi M, Alaei H, Sharifi MR, et al. The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. Int J Prev Med. 2013;4(4):430–437. [PMC free article] [PubMed] [Google Scholar]
- 26.Radahmadi M, Alaei H, Sharifi MR, et al. The effect of synchronized forced running with chronic stress on short, mid and long-term memory in rats. Asian J Sports Med. 2013;4(1):54–62. doi: 10.5812/asjsm.34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Nakagawa S, An Y, et al. The exercise-glucocorticoid paradox: how exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front Neuroendocrinol. 2017;44:83–102. doi: 10.1016/j.yfrne.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon SA, Wagner AD. Acute stress and episodic memory retrieval: neurobiological mechanisms and behavioral consequences. Ann N Y Acad Sci. 2016;1369(1):55–75. doi: 10.1111/nyas.12996. [DOI] [PubMed] [Google Scholar]
- 29.Clewett DV, Huang R, Velasco R, et al. Locus coeruleus activity strengthens prioritized memories under arousal. J Neurosci. 2018;38(6):1558–1574. doi: 10.1523/JNEUROSCI.2097-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clewett D, Sakaki M, Huang R, et al. Arousal amplifies biased competition between high and low priority memories more in women than in men: the role of elevated noradrenergic activity. Psychoneuroendocrinology. 2017;80:80–91. doi: 10.1016/j.psyneuen.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TH, Itti L, Mather M. Evidence for arousal-biased competition in perceptual learning. Front Psychol. 2012;3:241. doi: 10.3389/fpsyg.2012.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TH, Sakaki M, Cheng R, et al. Emotional arousal amplifies the effects of biased competition in the brain. Soc Cogn Affect Neurosci. 2014;9(12):2067–2077. doi: 10.1093/scan/nsu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect Psychol Sci. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA. 1998;95(24):14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daffner KR, Scinto LF, Weitzman AM, et al. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. J Cogn Neurosci. 2003;15(2):294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- 36.Loprinzi PD, Frith E, Edwards MK. Exercise and emotional memory: a systematic review. J Cogn Enhanc. 2018 doi: 10.1007/s41465-018-0086-z. [DOI] [Google Scholar]
- 37.Whitehead G, Jo J, Hogg EL, et al. Acute stress causes rapid synaptic insertion of Ca2+-permeable AMPA receptors to facilitate long-term potentiation in the hippocampus. Brain. 2013;136(Pt 12):3753–3765. doi: 10.1093/brain/awt293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joels M, Krugers HJ. LTP after stress: up or down? Neural Plast. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marzo A, Bai J, Otani S. Neuroplasticity regulation by noradrenaline in mammalian brain. Curr Neuropharmacol. 2009;7(4):286–295. doi: 10.2174/157015909790031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morcom AM, Bullmore ET, Huppert FA, et al. Memory encoding and dopamine in the aging brain: a psychopharmacological neuroimaging study. Cereb Cortex. 2010;20(3):743–757. doi: 10.1093/cercor/bhp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mlinar B, Stocca G, Corradetti R. Endogenous serotonin facilitates hippocampal long-term potentiation at CA3/CA1 synapses. J Neural Transm (Vienna) 2015;122(2):177–185. doi: 10.1007/s00702-014-1246-7. [DOI] [PubMed] [Google Scholar]
- 42.Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman CA, Perez Y, Lacaille JC. Effects of GABA(A) inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8(3):289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 46.Krebs-Kraft DL, Wheeler MG, Parent MB. The memory-impairing effects of septal GABA receptor activation involve GABAergic septo-hippocampal projection neurons. Learn Mem. 2007;14(12):833–841. doi: 10.1101/lm.809407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czeh B, Simon M, Schmelting B, et al. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31(8):1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 49.Krugers HJ, Lucassen PJ, Karst H, et al. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci. 2010;2:24. doi: 10.3389/fnsyn.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami S, Imbe H, Morikawa Y, et al. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53(2):129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14(8):536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alghasham A, Rasheed N. Stress-mediated modulations in dopaminergic system and their subsequent impact on behavioral and oxidative alterations: an update. Pharm Biol. 2014;52:368–377. doi: 10.3109/13880209.2013.837492. [DOI] [PubMed] [Google Scholar]
- 53.Kallarackal AJ, Kvarta MD, Cammarata E, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci. 2013;33(40):15669–15674. doi: 10.1523/JNEUROSCI.2588-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer U, van Kampen M, Isovich E, et al. Chronic psychosocial stress regulates the expression of both GR and MR mRNA in the hippocampal formation of tree shrews. Hippocampus. 2001;11(3):329–336. doi: 10.1002/hipo.1047. [DOI] [PubMed] [Google Scholar]
- 55.Loprinzi PD, Edwards MK, Frith E. Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. Eur J Neurosci. 2017;46(5):2067–2077. doi: 10.1111/ejn.13644. [DOI] [PubMed] [Google Scholar]
- 56.Grace L, Hescham S, Kellaway LA, et al. Effect of exercise on learning and memory in a rat model of developmental stress. Metab Brain Dis. 2009;24(4):643–657. doi: 10.1007/s11011-009-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mello PB, Benetti F, Cammarota M, et al. Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiol Learn Mem. 2009;92(3):364–369. doi: 10.1016/j.nlm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Makena N, Bugarith K, Russell VA. Maternal separation enhances object location memory and prevents exercise-induced MAPK/ERK signalling in adult Sprague-Dawley rats. Metab Brain Dis. 2012;27(3):377–385. doi: 10.1007/s11011-012-9298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim TW, Shin MS, Park JK, et al. Treadmill exercise alleviates prenatal noise stress-induced impairment of spatial learning ability through enhancing hippocampal neurogenesis in rat pups. J Exerc Rehabil. 2013;9(5):451–456. doi: 10.12965/jer.130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim BK, Seo JH. Treadmill exercise alleviates post-traumatic stress disorder-induced impairment of spatial learning memory in rats. J Exerc Rehabil. 2013;9(4):413–419. doi: 10.12965/jer.130058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castilla-Ortega E, Rosell-Valle C, Pedraza C, et al. Voluntary exercise followed by chronic stress strikingly increases mature adult-born hippocampal neurons and prevents stress-induced deficits in ‘what-when-where’ memory. Neurobiol Learn Mem. 2014;109:62–73. doi: 10.1016/j.nlm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Patki G, Solanki N, Atrooz F, et al. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol Behav. 2014;130:135–144. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patki G, Li L, Allam F, et al. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014;130:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neves BH, Menezes J, Souza MA, et al. Physical exercise prevents short and long-term deficits on aversive and recognition memory and attenuates brain oxidative damage induced by maternal deprivation. Physiol Behav. 2015;152(Pt A):99–105. doi: 10.1016/j.physbeh.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Dief AE, Samy DM, Dowedar FI. Impact of exercise and vitamin B1 intake on hippocampal brain-derived neurotrophic factor and spatial memory performance in a rat model of stress. J Nutr Sci Vitaminol. 2015;61:1–7. doi: 10.3177/jnsv.61.1. [DOI] [PubMed] [Google Scholar]
- 66.Kang JS. Exercise copes with prolonged stress-induced impairment of spatial memory performance by endoplasmic reticulum stress. J Exerc Nutr Biochem. 2015;19(3):191–197. doi: 10.5717/jenb.2015.15080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozbeyli D, Gokalp AG, Koral T, et al. Protective effect of exercise and sildenafil on acute stress and cognitive function. Physiol Behav. 2015;151:230–237. doi: 10.1016/j.physbeh.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 68.Radahmadi M, Alaei H, Sharifi MR, et al. Preventive and therapeutic effect of treadmill running on chronic stress-induced memory deficit in rats. J Bodyw Mov Ther. 2015;19(2):238–245. doi: 10.1016/j.jbmt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Kim DM, Leem YH. Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience. 2016;324:271–285. doi: 10.1016/j.neuroscience.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Wearick-Silva LE, Marshall P, Viola TW, et al. Running during adolescence rescues a maternal separation-induced memory impairment in female mice: potential role of differential exon-specific BDNF expression. Dev Psychobiol. 2017;59(2):268–274. doi: 10.1002/dev.21487. [DOI] [PubMed] [Google Scholar]
- 71.Chen K, Zhang L, Tan M, et al. Treadmill exercise suppressed stress-induced dendritic spine elimination in mouse barrel cortex and improved working memory via BDNF/TrkB pathway. Transl Psychiatry. 2017;7(3):e1069. doi: 10.1038/tp.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dos Santos TM, Kolling J, Siebert C, et al. Effects of previous physical exercise to chronic stress on long-term aversive memory and oxidative stress in amygdala and hippocampus of rats. Int J Dev Neurosci. 2017;56:58–67. doi: 10.1016/j.ijdevneu.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Kochi C, Liu H, Zaidi S, et al. Prior treadmill exercise promotes resilience to vicarious trauma in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:216–221. doi: 10.1016/j.pnpbp.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, et al. Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PLoS One. 2017;12(11):e0187671. doi: 10.1371/journal.pone.0187671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leem YH, Chang H. Arc/Arg3.1 protein expression in dorsal hippocampal CA1, a candidate event as a biomarker for the effects of exercise on chronic stress-evoked behavioral abnormalities. J Exerc Nutr Biochem. 2017;21(4):45–51. doi: 10.20463/jenb.2017.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.da Silva DC, Tavares MG, do Nascimento CKB, et al. Can coconut oil and treadmill exercise during the critical period of brain development ameliorate stress-related effects on anxiety-like behavior and episodic-like memory in young rats? Food Funct. 2018;9(3):1492–1499. doi: 10.1039/c7fo01516j. [DOI] [PubMed] [Google Scholar]
- 77.Miller RM, Marriott D, Trotter J, et al. Running exercise mitigates the negative consequences of chronic stress on dorsal hippocampal long-term potentiation in male mice. Neurobiol Learn Mem. 2018;149:28–38. doi: 10.1016/j.nlm.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]