Abstract
The pattern of respiratory activity in abdominal muscles was studied in anesthetized, spontaneously breathing, vagotomized neonatal rats at postnatal days 0–3. Anesthesia (2.0% isoflurane, 50% O2) depressed breathing and resulted in hypercapnia. Under this condition, abdominal muscles showed discharge late in the expiratory phase (E2 activity) in most rats. As the depth of anesthesia decreased, the amplitude of discharges in the diaphragm and abdominal muscles increased. A small additional burst frequently occurred in abdominal muscles just after the termination of diaphragmatic inspiratory activity (E1 or postinspiratory activity). Since this E1 activity is not often observed in adult rats, the abdominal respiratory pattern likely changes during postnatal development. Anoxia-induced gasping after periodic expiratory activity without inspiratory activity, and in most rats, abdominal expiratory activity disappeared before terminal apnea. These results suggest that a biphasic abdominal motor pattern (a combination of E2 and E1 activity) is a characteristic of vagotomized neonatal rats during normal respiration.
Keywords: Control of breathing, Motor pattern, Expiration
Introduction
Many mammalian species, including humans, recruit abdominal muscles under conditions of high respiratory drive in order to increase expiratory airflow and sustain adequate lung ventilation [1]. Recently, rats have become the most popular animal model for in vivo and in vitro studies of central ventilatory control. However, the respiratory motor pattern of the abdominal muscles in neonatal rats in vivo has not yet been fully described [2–4]. Two of the relevant publications are abstracts and do not provide details of the experimental conditions (e.g., body temperature, and kind and depth of anesthesia) [2, 3]. Both abstracts report that asphyxia resulted in a change in abdominal muscle activity to a two-burst pattern, with one burst before and one after the inspiratory phase (E2 and E1 activity, respectively). Similar biphasic abdominal muscle activity was also observed under the opioid-induced respiratory suppression in the vagotomized ketamine-anesthetized neonatal rat [4]. Recently, we studied abdominal expiratory activity in the adult rat [5]. Under hypercapnic acidosis, the abdominal motor nerve showed two motor patterns, low-amplitude expiratory activity that persisted throughout the expiratory phase (E-all activity), and late-expiratory, high-amplitude activity superimposed on this steady activity (E2 activity). Thus, E1 activity was not often observed in the adult rat. From these studies, I hypothesized that the E1 activity of the abdominal muscles is a characteristic of neonatal rats in vivo under hypercapnic acidosis. The first purpose of the present study was to clarify the pattern of abdominal expiratory activity under hypercapnia in neonatal rats, and compare the results with those of our previous study using adult rats [5]. Because the vagal afferents arising from pulmonary mechanoreceptors affect the breathing pattern of neonatal rats [6], we subjected neonatal rats to bilateral vagotomy in order to establish the basic motor pattern organized by the respiratory center.
Neonatal mammals can survive for considerable period in the absence of oxygen [7]. Correlated with this, gasping was sustained for a longer period in neonatal rats than in adults [8, 9]. One of the characteristics of gasping in the adult mammal is a minimal level or absence of abdominal expiratory activity [10]; however, few studies have examined abdominal expiratory motor activity during gasping in neonates [2, 3]. Janczewski and Aoki [2] reported that “After termination of occlusion the rats started to gasp and the EMG expiratory activity was suppressed”. In another abstract, however, the same researchers reported that “In adults gasping follows ‘primary apnea’, but in neonatal rats eupneic like activity reappeared” [3]. Using whole-body plethysmography, other researchers studied respiratory-related pressure changes during anoxia-induced gasping in detail in the neonatal rat [9]. Two types of gasping were observed. Type I gasping was characterized by an expiratory pressure change preceding an inspiratory pressure change and followed by a second expiratory change. In type II gasping, there was an initial inspiratory pressure change followed by a small expiratory change. During anoxia, the frequency of type I gasping decreased and was replaced by type II gasping before terminal apnea. It is possible that Janczewski and Aoki [3] have reported in one abstract that the initial phase of gasping consisted of many type I gasps, and in another abstract, that the later phase of gasping consisted of many type II gasps [2]. Therefore, the second purpose of this study was to describe how abdominal activity changed during anoxia-induced gasping in neonatal rats.
Methods
The experiments performed were approved by the Animal Research Committee of the Ibaraki Prefectural University of Health Sciences.
Animals
Eighteen neonatal Wistar rats aged 0–3 days were used. The rats were anesthetized with 2.0% isoflurane in 50% O2 and 50% N2. The gas mixture was administered at the rate of 45–60 ml/min through a mask that loosely covered the face. The rats were placed in the supine position and breathed spontaneously. Noxious pinches applied to the tail did not evoke the withdrawal reflex in any rat at this concentration of isoflurane. Body temperature was monitored with a thermometer placed in the rectum (BAT-12; Physitemp Instruments, Clifton, USA) and maintained at 37 ± 1°C with a silicone rubber heating pad. Under deep anesthesia induced in the manner described, the vagus nerves were sectioned bilaterally at the mid-cervical level. The wounds were covered with saline-immersed cotton. Blood gases were examined under 2% isoflurane and 50% O2 in 10 of the 18 rats. After more than 30 min of anesthesia, the common carotid artery was sectioned. Arterial blood (0.085 ml) was sampled using a capillary tube. Then, arterial PO2 (PaO2), arterial PCO2 (PaO2), and arterial pH (pHa) were determined using a blood gas analyzer (ABL505; Radiometer, Copenhagen, Denmark).
Recordings
To obtain an electromyogram (EMG) of the diaphragm, we used a method similar to that described by Basmajian and Stecko [11], except that one wire was inserted into one needle. Briefly, two fine polyurethane-coated copper wires (0.05 mm) were inserted percutaneously using two 31 G needles. Only the cut end of the wire was unsealed. The tip was bent to a hook shape (about 0.5 mm). Similarly, activity in the abdominal muscle was obtained by insertion of two wires into the lateral abdominal wall. The position of the tip of the wire was adjusted to minimize cross-contamination from other nonrespiratory and respiratory muscles. The signals obtained were amplified (gain, 500–10,000; band pass filter, 150–3,000 Hz) using a high-gain AC-coupled amplifier (AB-610 J; Nihon Kohden, Tokyo, Japan). Recordings were stored on a pulse-code modulation data recorder (PC208A; Sony, Tokyo, Japan) for further off-line analysis.
Experimental protocols
Electromyograms of the diaphragm and abdominal muscles were recorded in eight rats. After the inspired gas mixture was switched from 2% isoflurane to 0%, body movements were carefully observed and the isoflurane concentration in the inspired air was switched back to 2% as soon as spontaneous struggling movements were detected. The period from removal of isoflurane to the first detectable movement was dependent on the rat and averaged 533 ± 264 s (n = 8). To examine the pattern of anoxia-induced gasping in vagotomized rats, 100% N2 gas was administered immediately after the first detectable struggling movement.
The raw EMG from the diaphragm was used to obtain the respiratory cycle period and the inspiratory and expiratory durations. All variables were obtained from 20 continuous respiratory cycles under 2% isoflurane and 5 min after withdrawal of isoflurane. From 20 cycles under 0% isoflurane, the ratio of E1 activity amplitude to E2 activity amplitude was also obtained using the integrated EMG of the abdominal muscle (time constant, 0.1 s). An E2 activity was used as a standard immediately before an E1 activity. Since the amplitude of the E2 activity was large or the time lag between the E2 and E1 activities was short, the integrated wave did not decline to baseline before the E1 activity (Fig. 1D). Therefore, the amplitude of the E1 activity was defined as the difference between trough and peak values (Fig. 1D). All values are presented as mean ± standard deviation. The Wilcoxon signed rank test was used to test the significance of differences in paired data. The level of statistical significance was set at P < 0.05.
Fig. 1.
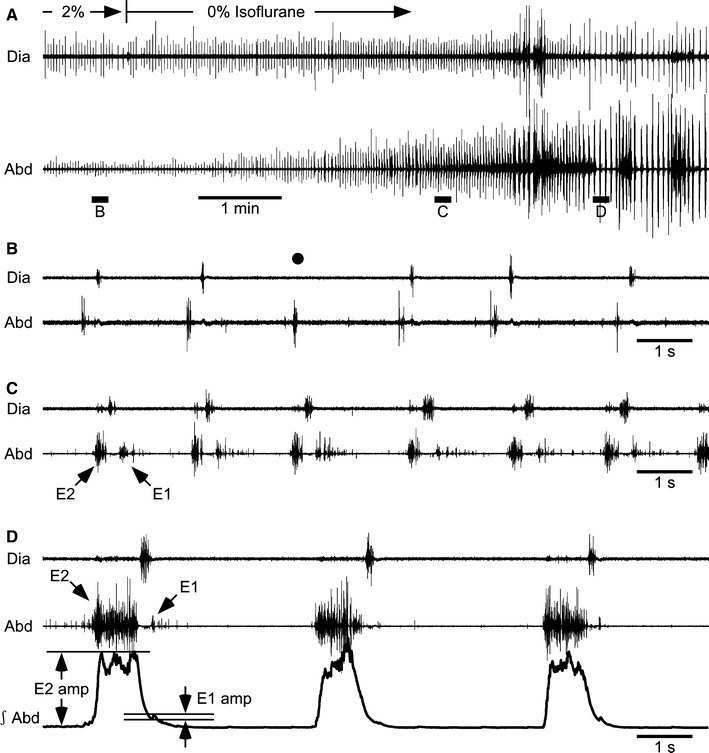
A–D Respiratory activity in a vagotomized neonatal rat. Electromyograms were obtained from the diaphragm (Dia) and abdominal muscles (Abd). The integrated electromyogram of Abd (∫Abd, time constant 0.1 s) is also shown as the third trace in panel D. The concentration of isoflurane was decreased from 2 to 0% at the time indicated in panel A. B–D Portions of recording A above the horizontal bars marked B–D are shown on an expanded time scale. The vertical gain in Abd in B is higher by a factor of four compared with other records. As indicated by the black circle in B, the inspiratory burst was absent in some respiratory cycles under 2% isoflurane. The arrows indicate E1 and E2 activities. The amplitudes of E2 and E1 activities (E2 amp, E1 amp) were measured from the baseline or trough to the peak
Results
Respiratory motor activity in vagotomized neonatal rats
Isoflurane (2%) depressed breathing and resulted in hypercapnia (Table 1). Typical responses to reduction in the depth of anesthesia by removing isoflurane from the inspired gas are shown in Fig. 1. Expiratory bursts late in the expiratory phase (E2 activity) were observed under 2% isoflurane in seven of the eight vagotomized rats. The eighth rat showed an identical pattern of abdominal expiratory activity under light anesthesia, as described below. Either the inspiratory (black circle in Fig. 1B) or the expiratory (not shown) burst was skipped in some respiratory cycles in four and two of the eight rats, respectively. After removal of isoflurane, the abdominal expiratory bursts increased in amplitude, and additional bursts appeared immediately after the termination of the inspiratory bursts (E1 activity) (Fig. 1C, D). In most respiratory cycles, E1 activity had a decrementing shape and was smaller than E2 activity (Fig. 1C, D). This biphasic expiratory burst—the combination of E2 and E1 activity—occurred in all eight rats when the depth of anesthesia decreased. The E1 amplitude was significantly smaller than the E2 amplitude (P < 0.05, n = 8), and the amplitude ratio (E1/E2) was 0.23 ± 0.16 (n = 8). As shown in Fig. 2, the respiratory cycle period, inspiratory duration, and expiratory duration were significantly prolonged when anesthesia had become light (P < 0.05, n = 8).
Table 1.
Mean ± standard deviation of arterial pH, PCO2, and PO2 in vagotomized neonatal rats (n = 10)
| Value | |
|---|---|
| pHa | 7.07 ± 0.07 |
| PaCO2 (mm Hg) | 118.6 ± 24.4 |
| PaO2 (mm Hg) | 139.5 ± 20.3 |
Fig. 2.
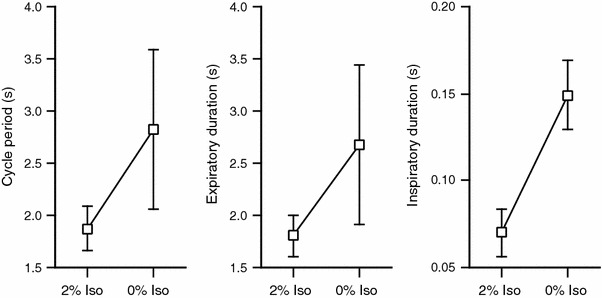
Effects of the depth of anesthesia on respiratory parameters. The cycle period, expiratory duration, and inspiratory duration were measured under 2 and 0% isoflurane (Iso). For 0% isoflurane, data were obtained about 5 min after the concentration of isoflurane was switched from 2 to 0%. All three parameters were significantly prolonged by removal of isoflurane (P < 0.05, n = 8)
Effects of anoxia
Typical responses to 100% nitrogen exposure are shown in Fig. 3. During this anoxic stimulation, the rats typically passed through five stages: (1) slight enhancement of respiration; (2) periodic expiratory activity without inspiratory activity; (3) combination of expiratory and inspiratory activity; (4) inspiratory burst with weak or no expiratory activity; and (5) terminal apnea (Fig. 3A). Similar to a previous study [6], vagotomized neonatal rats showed mouth-opening, gasp-like respiratory movements before anoxic stimulation. During stages 1, 3, and 4, the rats also showed mouth-opening respiratory movements synchronous with inspiratory activity. However, this movement was not observed during stage 2. At the time of the first inspiratory burst occurring after stage 2, defined as the onset of gasping (Fig. 3C, black circle), the latency of gasping was 70.8 ± 28.1 s after the last inspiratory burst in stage 1. During stage 3, the inspiratory activity occurred immediately after the abdominal expiratory burst (Fig. 3Da, b). The occurrence of inspiratory activity often resulted in a biphasic pattern of abdominal expiratory activity in all eight rats. This respiratory motor activity pattern was similar to the normal respiratory pattern in stage 1 or before anoxic stimulation (compare Fig. 3B, Da, b). Then, the abdominal expiratory activity gradually decreased, while the inspiratory activity in the diaphragm increased. During the disappearance of expiratory activity, E1 activity with no corresponding E2 activity was observed in five of eight rats (Fig. 3Dc, d). Expiratory bursts completely disappeared in seven of eight rats before terminal apnea. In one case, only three gasps occurred and periodic expiratory activity continued after termination of the inspiratory gasping activity. The duration of gasping (from the first inspiratory burst occurring after stage 2 to the last inspiratory burst before terminal apnea) ranged from 75.0 to 1,310.6 s among individual rats, with a mean ± standard deviation of 714.9 ± 478.8 s.
Fig. 3.
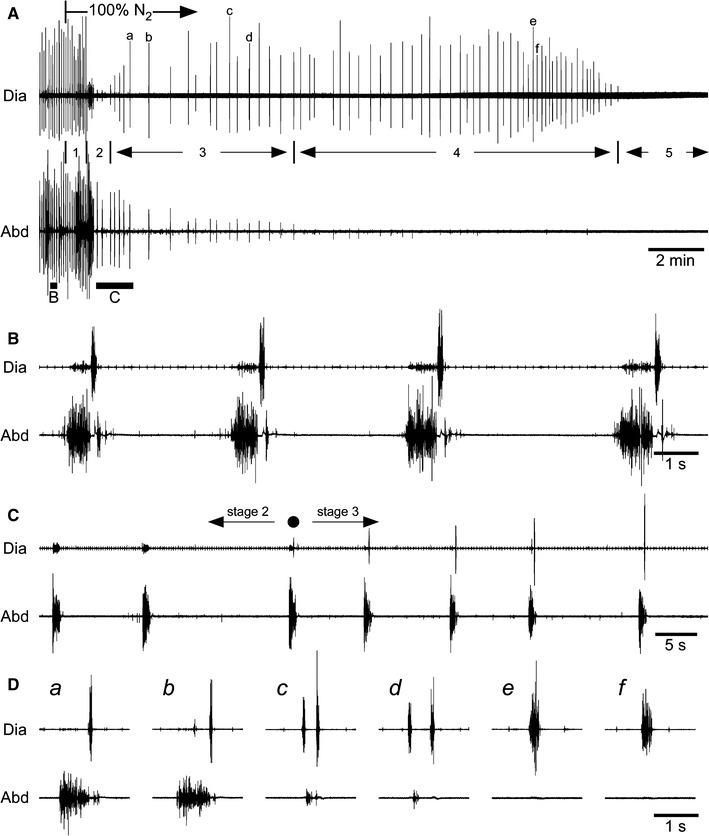
Effects of anoxia. A–D Electromyograms obtained from the diaphragm (Dia) and abdominal muscles (Abd). Numbers 1–5 in A indicate the stages following anoxic stimulation (100% N2) referred to in the text. In B and C, the portions of recording A above the horizontal bars marked B and C are shown on an expanded time scale. D The portions of recording A indicated by the letters a–f are shown on an expanded time scale. After the inspired gas was switched to 100% N2, the inspiratory bursts in the diaphragm were depressed transiently and then increased gradually (C). The first inspiratory activity that occurred after the transient disappearance is indicated by a black circle, and this time was defined as the onset of gasping. The expiratory activity disappeared before terminal apnea (e and f in panel D)
Discussion
Basic pattern of abdominal respiratory activity in vagotomized neonatal rats
Under deep anesthesia, abdominal muscles showed activity just before the inspiratory activity of the diaphragm (E2 activity). When the depth of anesthesia was gradually reduced; however, additional activity occurred just after the termination of the inspiratory bursts (E1 activity). E1 activity had a decrementing shape, and was significantly smaller than E2 activity. Since E1 activity is not observed frequently in adult rats [5], it seems likely that this biphasic activity in abdominal muscles is a characteristic of neonatal rats. A recent study in ketamine-anesthetized, midbrain-decerebrate, vagotomized, spontaneously breathing rats at postnatal days 7–13 showed that abdominal muscle activity had two components: decrementing or tonic activity during the E1 phase and phasic burst activity during the E2 phase [12]. This tonic component during the E1 phase may correspond to the E-all activity in adult rats [5] and may be different from the decrementing E1 activity. Although that study did not refer to the relationship between age and the type of activity during the E1 phase, it is possible that the motor pattern matured during postnatal days 7–13.
In an in vitro system prepared from neonatal rats, the abdominal muscles showed biphasic burst activity, one before and one after the inspiratory phase [4, 13]. This motor pattern appears similar to that observed in the neonatal rat in vivo. However, the E2 activity was smaller and often absent in the in vitro preparation [13], whereas the E2 activity was significantly larger than the E1 activity in the neonatal rat in vivo. The reason for this difference remains unknown. Differences in the experimental conditions might explain it. First, the in vitro preparation is kept at a low temperature (around 25°C). However, Fukuda [8] found that under such hypothermia, the phrenic nerve showed eupneic nerve discharges in anesthetized neonatal rats. Therefore, a difference in the temperature would not explain the difference in the expiratory motor pattern. Second, the in vitro preparation lacked blood circulation, and the tissue deep in the brainstem became hypoxic [14]. Such an abnormal condition may lead to reorganization of respiratory networks and forced expression of the respiratory motor pattern in vitro.
The importance of E1 activity is not clear. In general, the E1 phase is thought to be a passive expiratory phase effected by the elasticity of the ribcage [15]. During the E1 phase, the expiratory airflow is controlled (slowed) by the resistive action of the activated adductor muscles in the larynx [16, 17]. The presence of activity during the E1 phase could indicate that passive expiration is not enough for appropriate lung ventilation in neonatal rats. The passive mechanical time constant of the respiratory system in neonatal rats was found to be 0.148 ± 0.012 s [18], slightly greater than that in adult rats [19]. However, Mortola et al. [20] found that the dynamic expiratory time constant of the respiratory system in neonatal rats was 0.109 ± 0.013 s. Because the late portion of the expiratory flow volume curve was linear in most neonates, Mortola et al. suggested that the respiratory muscles were relaxed, and this may be explained by a dynamic reduction in lung compliance. When neonatal rats need greater minute ventilation, abdominal E1 activity may be important in providing appropriate lung ventilation.
Abdominal activity during anoxia-induced gasping in vagotomized neonatal rats
The present study extends a previous study that used whole-body plethysmography and examined anoxia-induced gasping in neonatal rats [9]. In general, the respiratory response of mammals to anoxia displays four characteristic stages: hyperpnea, primary apnea, gasping, and terminal apnea [21, 22]. The rats used in the present study typically passed through five stages during anoxia: (1) slight enhancement of respiration; (2) periodic expiratory activity without inspiratory activity; (3) a combination of expiratory and inspiratory activity; (4) an inspiratory burst with weak or no expiratory activity; and (5) terminal apnea. Although the rats did not show mouth-opening gasp-like movements during stage 2, periodic abdominal activity was observed. It remains unknown whether stage 2 corresponds to primary apnea. Stages 3 and 4 correspond to gasping. Gozal et al. [9] described two different types of gasping. Type I gasping was characterized by an expiratory pressure change preceding an inspiratory pressure change, and then a second expiratory change. In type II gasping, there was an initial inspiratory pressure change followed by a small expiratory change. Type I and II gasps are consistent with the gasps observed during stages 3 (Fig. 3Da, b) and 4 (Fig. 3De, f), respectively, in the present study. Thus, the present study confirms that the expiratory pressure change in type I gasping was partially executed by active contraction of the abdominal muscles.
The period of anoxia-induced gasping in the present study was shorter than that in a previous study [9]. It is well documented that lower ambient or body temperature prolongs the period of gasping [7, 23, 24]. Although the body temperature was maintained at 37°C in the present study, the ambient temperature was maintained at 30, 3–4°C below the thermoneutral range of the animals used in the previous study [9]. We surmise that the difference in body temperature accounts for the difference in the period of anoxia-induced gasping.
In conclusion, the E1 activity in the abdominal muscle is a characteristic of the neonatal rat and would be expected to decrease during postnatal development, since the E1 activity is not often observed in adult rats [5]. The biphasic expiratory motor pattern is not the same as in the in vitro preparation from neonatal rats, since the E2 activity was greater than the E1 activity in neonatal rats in vivo.
Acknowledgments
The author wishes to thank Dr. Sei-Ichi Sasaki for the constructive discussion during the course of this study.
References
- 1.Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/S0301-0082(98)00046-X. [DOI] [PubMed] [Google Scholar]
- 2.Janczewski WA, Aoki M. Expiratory activity in the 1–4 day old rat. Jpn J Physiol. 1999;49(Suppl):S84. [Google Scholar]
- 3.Janczewski WA, Aoki M. Expiratory muscle activity in neonatal rats. Soc Neurosci Abst. 1999;25:279. [Google Scholar]
- 4.Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol. 2007;157:196–205. doi: 10.1016/j.resp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Fedorko L, Kelly EN, England SJ. Importance of vagal afferents in determining ventilation in newborn rats. J Appl Physiol. 1988;65:1033–1039. doi: 10.1152/jappl.1988.65.3.1033. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas JF, Alexander FAD, Himwich HE. Tolerance of the newborn to anoxia. Am J Physiol. 1941;134:281–287. [Google Scholar]
- 8.Fukuda Y. Respiratory neural activity responses to chemical stimuli in newborn rats: reversible transition from normal to ‘secondary’ rhythm during asphyxia and its implication for ‘respiratory like’ activity of isolated medullary preparation. Neurosci Res. 2000;38:407–417. doi: 10.1016/S0168-0102(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D, Torres JE, Gozal YM, Nuckton TJ. Characterization and developmental aspects of anoxia-induced gasping in the rat. Biol Neonate. 1996;70:280–288. doi: 10.1159/000244377. [DOI] [PubMed] [Google Scholar]
- 10.St John WM, Zhou D, Fregosi RF. Expiratory neural activities in gasping. J Appl Physiol. 1989;66:223–231. doi: 10.1063/1.343910. [DOI] [PubMed] [Google Scholar]
- 11.Basmajian JV, Stecko G. A new bipolar electrode for electromyography. J Appl Physiol. 1962;17:849. [Google Scholar]
- 12.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iizuka M. GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat. J Physiol. 2003;551:617–633. doi: 10.1113/jphysiol.2003.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada Y, Muckenhoff K, Holtermann G, Acker H, Scheid P. Depth profiles of pH and PO2 in the isolated brain stem-spinal cord of the neonatal rat. Respir Physiol. 1993;93:315–326. doi: 10.1016/0034-5687(93)90077-N. [DOI] [PubMed] [Google Scholar]
- 15.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett D, Jr, Remmers JE, Gautier H. Laryngeal regulation of respiratory airflow. Respir Physiol. 1973;18:194–204. doi: 10.1016/0034-5687(73)90050-9. [DOI] [PubMed] [Google Scholar]
- 17.Gautier H, Remmers JE, Bartlett D., Jr Control of the duration of expiration. Respir Physiol. 1973;18:205–221. doi: 10.1016/0034-5687(73)90051-0. [DOI] [PubMed] [Google Scholar]
- 18.Mortola JP. Comparative aspects of the dynamics of breathing in newborn mammals. J Appl Physiol. 1983;54:1229–1235. doi: 10.1152/jappl.1983.54.5.1229. [DOI] [PubMed] [Google Scholar]
- 19.Rezzonico R, Gleed RD, Mortola JP. Respiratory mechanics in adult rats hypercapnic in the neonatal period. J Appl Physiol. 1990;68:2274–2279. doi: 10.1152/jappl.1990.68.6.2274. [DOI] [PubMed] [Google Scholar]
- 20.Mortola JP, Magnante D, Saetta M. Expiratory pattern of newborn mammals. J Appl Physiol. 1985;58:528–533. doi: 10.1152/jappl.1985.58.2.528. [DOI] [PubMed] [Google Scholar]
- 21.Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56:1371–1377. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson EE, Thach BT. Respiratory patterns during progressive asphyxia in newborn rabbits. J Appl Physiol. 1977;43:468–474. doi: 10.1152/jappl.1977.43.3.468. [DOI] [PubMed] [Google Scholar]
- 23.Miller JA. Factors in neonatal resistance to anoxia I. Temperature and survival of newborn guinea pigs under anoxia. Science. 1949;110:113–114. doi: 10.1126/science.110.2848.113. [DOI] [PubMed] [Google Scholar]
- 24.Serdarevich C, Fewell JE. Influence of core temperature on autoresuscitation during repeated exposure to hypoxia in normal rat pups. J Appl Physiol. 1999;87:1346–1353. doi: 10.1152/jappl.1999.87.4.1346. [DOI] [PubMed] [Google Scholar]


